Abstract
Increases in the second messenger cAMP are associated with receptor-mediated ATP release from erythrocytes. In other signaling pathways, cAMP-specific phosphodiesterases (PDEs) hydrolyze this second messenger and thereby limit its biological actions. Although rabbit and human erythrocytes possess adenylyl cyclase and synthesize cAMP, their PDE activity is poorly characterized. It was reported previously that the prostacyclin analog iloprost stimulated receptor-mediated increases in cAMP in rabbit and human erythrocytes. However, the PDEs that hydrolyze erythrocyte cAMP synthesized in response to iloprost were not identified. PDE3 inhibitors were reported to augment increases in cAMP stimulated by prostacyclin analogs in platelets and pulmonary artery smooth muscle cells. Additionally, PDE3 activity was identified in embryonic avian erythrocytes, but the presence of this PDE in mammalian erythrocytes has not been investigated. Here, using Western blot analysis, we determined that PDE3B is a component of rabbit and human erythrocyte membranes. In addition, we report that the preincubation of rabbit and human erythrocytes with the PDE3 inhibitors milrinone and cilostazol potentiates iloprost-induced increases in cAMP. In addition, cilostamide, the parent compound of cilostazol, potentiated iloprost-induced increases in cAMP in human erythrocytes. These findings demonstrate that PDE3B is present in rabbit and human erythrocytes and are consistent with the hypothesis that PDE3 activity regulates cAMP levels associated with a signaling pathway activated by iloprost in these cells.
Keywords: adenylyl cyclase; cilostazol; milrinone; red blood cell; cyclic adenosine 3′,5′-monophosphate
the erythrocyte, by virtue of the hemoglobin that it contains, has long been recognized as a vehicle for oxygen (O2) transport. In addition to this well-established role for the erythrocyte in the circulation, it has been shown that this cell can also participate in the regulation of vascular resistance via the release of ATP (5, 7–9, 16, 47, 49). Erythrocyte-derived ATP has been shown to be a stimulus for nitric oxide synthesis in the circulation of the lung as well as in the striated muscle (7, 47, 49).
Human and rabbit erythrocytes release ATP when exposed to reduced O2 tension (5, 9, 28), mechanical deformation (46), or when incubated with either β-adrenergic agonists or prostacyclin analogs (28, 29, 44, 45). Importantly, it was demonstrated that increases in intracellular cyclic adenosine 3′,5′-monophosphate (cAMP) are required for ATP release from rabbit and human erythrocytes (48). Thus the regulation of the concentration of cAMP within the erythrocyte is a critical control point for erythrocyte ATP release.
The second messenger cAMP is an important component of many cellular signal transduction pathways. The level of cAMP present within any cell is the product of its synthesis by adenylyl cyclases (ACs) and hydrolysis by phosphodiesterases (PDEs) (20). Although once controversial, the presence of AC activity in the erythrocyte is now established (26, 36, 44, 50). Nakagawa et al. (26) demonstrated that the stabilization of this enzyme during the isolation of erythrocyte membranes is critical to maintain enzyme activity. Recently, with the use of Western blot analysis, AC type II was identified in rabbit and human erythrocyte membranes (44, 50). In support of a functional role for AC in erythrocytes, previous reports demonstrated that sodium fluoride, β-adrenergic agonists, or prostacyclin and its analogs stimulate cAMP synthesis in erythrocytes (17, 33, 36, 37), and this cAMP can regulate intracellular magnesium (22), hypotonic lysis (34), and the filterability of these cells (53). In addition, the incubation of erythrocytes with β-adrenergic agonists or prostacyclin analogs results in cAMP accumulation and ATP release (27, 28, 45, 48).
Despite the fact that AC activity is clearly present in rabbit and human erythrocytes (26, 36, 44, 50), the identity of PDEs that hydrolyze cAMP in these cells has not been characterized. The activity of PDE1 has been shown to be present in mammalian (human) erythrocytes; however, this PDE was shown to hydrolyze cyclic guanosine 3′,5′-monophosphate (cGMP), not cAMP (31). PDE activity associated with the hydrolysis of cAMP has been described in rat (30), human (31, 51), rabbit (1), and embryonic avian erythrocytes (2). However, only in embryonic avian erythrocytes has an individual PDE isoform (PDE3) associated with this activity been identified (2). In the latter study, norepinephrine- and forskolin-induced increases in cAMP were potentiated by the PDE3 inhibitor milrinone (2). However, the presence of the PDE3 protein was not demonstrated.
It has become increasingly clear that specific PDEs are associated with discrete signaling pathways (21, 35, 55), yet there are no reports of PDEs associated with specific signaling pathways in mammalian erythrocytes. PDE3 activity has been associated with prostacyclin signaling in other cell types. Importantly, PDE3 inhibitors have been shown to potentiate vasodilatory effects of prostacyclin analogs in the pulmonary circulation (38, 39).
Here we designed studies to establish that PDE3 is present in rabbit and human erythrocyte membranes and to determine whether this PDE participates in the regulation of receptor-mediated increases in cAMP. We report, for the first time, that PDE3B is a component of erythrocyte membranes of rabbits and humans and that inhibitors of PDE3 activity augment iloprost-induced increases in cAMP.
MATERIALS AND METHODS
Isolation of human and rabbit erythrocytes.
Rabbit blood was obtained from male New Zealand white rabbits (2 to 3 kg, 10–12 wk of age). The animals were anesthetized with ketamine (12.5 mg/kg) and xylazine (1.5 mg/kg) intramuscularly, and then intravenous pentobarbital sodium (10 mg/kg) was administered. After tracheal intubation, the animals were ventilated with room air (tidal volume, 10 ml/kg; and rate, 25 breaths/min). A catheter was placed in a carotid artery for the administration of heparin (500 units) and for blood removal. The animals were exsanguinated 10 min after heparin administration. Human blood was obtained by venipuncture using a syringe containing heparin (500 units). Blood was collected from nine females and 17 males with an average age of 39 ± 3 yr (range, 21–66 yr). Protocols used to obtain blood from rabbits and humans were approved by the Institutional Animal Care and Use Committee and by the Institutional Review Board of Saint Louis University, respectively.
After collection, whole blood was centrifuged at 500 g at 4°C for 10 min and the plasma, buffy coat, and uppermost erythrocytes were removed by aspiration and discarded. The remaining erythrocytes were washed three times in wash buffer containing (in mM) 21.0 tris(hydroxymethyl)aminomethane, 4.7 KCl, 2.0 CaCl2, 140.5 NaCl, 1.2 MgSO4, and 5.5 glucose and 0.5% bovine albumin fraction V, final pH 7.4. Wright stains of erythrocytes prepared in this fashion reveal <1 leukocyte/50 high power fields (∼8–10 leukocytes/mm3). Cells were prepared on the day of use.
Preparation of erythrocyte membranes.
Washed erythrocytes were diluted 1:100 with ice-cold hypotonic buffer containing 5 mM Tris·HCl and 2 mM EDTA, pH 7.4, and stirred vigorously at 4°C for 20 min. The lysate was centrifuged at 23,300 g for 15 min at 4°C. The supernatant was removed and discarded. The pellet containing the erythrocyte membranes was washed two times with ice-cold buffer and centrifuged. The membranes were resuspended in ice-cold buffer and frozen at −80°C. Membrane protein concentrations were determined using the BCA protein assay (Pierce, Rockford, IL).
Preparation of platelet membranes.
Whole heparinized blood was centrifuged at 400 g for 10 min at 4°C. The supernatant containing platelets was collected, 0.5 ml heparin and 1 mg/ml EDTA were added, and the supernatant was recentrifuged for 40 min at 200 g at 4°C. The platelet-rich plasma was collected and centrifuged at 1,400 g for 20 min at 4°C. The supernatant was discarded and 200 ml of Western lysis buffer containing 25 mM HEPES, 300 mM NaCl, 10 mM EDTA, 1.5 mM MgCl2·6H2O, 20 mM β-glycerophosphate, 0.1 mM sodium vanadate, and 1% Triton X-100 was added to the pellet and followed by sonication (10 s). After 15 min on ice, samples were centrifuged at 14,000 g for 20 min at 4°C. The supernatant was discarded, and the protein concentration of the pellet was determined using the BCA protein assay (Pierce).
Western blot analysis.
Erythrocyte membranes were solubilized in SDS buffer containing 8% SDS, 60% glycerol, 0.25 M Tris·HCl (pH 6.8), 0.004% bromophenol blue, and 400 mM dithiothreitol, boiled, loaded onto a precast 7.5% acrylamide gel (Cambrex, East Rutherford, NJ), and subjected to electrophoresis at 150 volts for 90 min. Proteins were transferred to a polyvinylidene difluoride (PVDF) membrane (100 volts for 60 min) in buffer containing 25 mM Tris, 192 mM glycine, and 10% methanol. Membranes were blocked overnight and immunoblotted with a primary polyclonal antibody directed against an NH2 terminus (human erythrocyte membranes) or COOH terminus (rabbit erythrocyte membranes) epitope of human PDE3B (Santa Cruz Biotechnology, Santa Cruz, CA), followed by incubation with an appropriate secondary antibody. Labeled proteins were visualized using enhanced chemiluminescence (Pierce).
Purified human PDE3B (Calbiochem, San Diego, CA) served as a positive control. The protein was diluted 1:1 with Laemmli sample buffer (2× concentrate; Sigma-Aldrich, St. Louis, MO), boiled, loaded onto a precast gel, and subjected to electrophoresis. The proteins were transferred to a PVDF membrane as described above. The PVDF membrane was immunoblotted with an antibody directed against the NH2 terminus of human PDE3B (Santa Cruz Biotechnology).
Platelets possess PDE3 activity (15). To ensure that erythrocyte preparations are devoid of platelet contamination and confounding PDE3 activity from these cells, platelets were prepared for Western blot analysis along with erythrocyte membrane preparations. Both membrane preparations were probed with a platelet-specific marker (see Western blot analysis) (24). Rabbit and human erythrocyte membranes were prepared as described above. Platelet membranes were solubilized and diluted 1:10 in SDS buffer (described above), boiled, loaded onto 7.5% precast gel (Cambrex), and subjected to electrophoresis along with erythrocyte membranes. The proteins were transferred to a PVDF membrane as described above. The PVDF membrane was immunoblotted with a monoclonal antibody directed against a platelet marker human integrin αIIb (CD41; Chemicon, Temecula, CA) and followed by incubation with an appropriate secondary antibody and visualization by chemiluminescence (Pierce).
Incubation of erythrocytes with pharmacological agents.
Washed erythrocytes diluted to a 50% hematocrit (1 ml) were preincubated with a PDE inhibitor or its vehicle, N′N-dimethylformamide (Sigma-Aldrich), for 30 min. The PDE inhibitors studied were the nonselective PDE inhibitor 3-isobutyl-1-methylxanthine (IBMX; 10 μM; Sigma-Aldrich), the selective PDE1 inhibitor vinpocetine (30 μM; Sigma-Aldrich), and the selective PDE3 inhibitors milrinone (20 μM; Tocris, Ellisville, MO), cilostazol (10, 30, or 100 μM; Sigma-Aldrich), or cilostamide (30 μM; Tocris). Following preincubation with the PDE inhibitor, iloprost (1 μM; Cayman, Ann Arbor, MI) was added to the cell suspension for 15 min. The reaction was stopped by the addition of 4 ml of ice-cold ethanol containing 1 mM HCl. Samples were then prepared for the determination of cAMP concentration.
Measurement of cAMP.
After the addition of ice-cold ethanol-HCl, the samples were centrifuged at 14,000 g for 10 min at 4°C. The supernatant was removed and stored overnight at −20°C to precipitate the remaining proteins. This supernatant was centrifuged at 3,700 g for 10 min at 4°C. The final supernatant was removed and dried under vacuum centrifugation. The dried sample was reconstituted in assay buffer. The concentration of cAMP was determined using an enzyme immunoassay (GE Healthcare, Piscataway, NJ). Cell counts were obtained from erythrocytes at a hematocrit of 50%. The amounts of cAMP measured were normalized to 1010 cells/ml.
Data analysis.
Statistical significance between groups was determined using ANOVA. In the event that the F ratio indicated that a change had occurred, a Fisher's least-significant difference test was performed to identify individual differences. Results were reported as means ± SE.
RESULTS
Identification of PDE3B as a component of rabbit and human erythrocyte membranes.
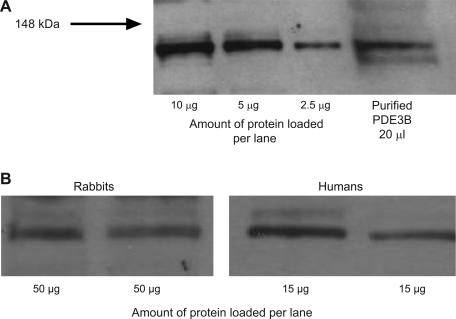
Although PDE3 activity was identified in embryonic avian erythrocytes (2), there are no reports of this PDE in mammalian erythrocytes. As a first step in establishing a role for PDE3 in mammalian erythrocytes, we determined that PDE3 protein is present in rabbit and human erythrocytes. Cells were lysed, and the membrane fractions were isolated and resolved by Western blot analysis. Membranes were probed with one of two polyclonal antibodies directed against human PDE3B, the membrane-bound isoform of PDE3 (41, 42). With the use of an antibody directed against the NH2 terminus of human PDE3B, a band with the predicted molecular weight of PDE3B (135 kDa) (41) was identified in human erythrocyte membranes (Fig. 1, A and B). A band at the same molecular weight was identified in rabbit erythrocyte membranes using an antibody directed against the COOH terminus of human PDE3B (Fig. 1B). Importantly, we were able to identify PDE3B in both rabbit and human erythrocyte membrane preparations using either antibody (data not shown). The fact that two antibodies directed to two different epitopes of the PDE3B protein provides strong support that PDE3B is present in rabbit and human erythrocyte membranes.
Fig. 1.
Identification of phosphodiesterase 3B (PDE 3B) in rabbit and human erythrocyte membranes. A: human erythrocyte membranes and purified human PDE3B were probed with a polyclonal antibody generated against the NH2 terminus of human PDE3B. B: rabbit and human erythrocyte membranes were probed with either a polyclonal antibody directed against the COOH terminus of human PDE3B (rabbit erythrocyte membranes, representative of 6 studies) or a polyclonal antibody generated against the NH2 terminus of human PDE3B (human erythrocyte membranes, representative of 14 studies).
Monocytes, lymphocytes, and eosinophils also possess PDE3B (12). Using manual counting, we concluded that our erythrocyte preparations contain <10 leukocytes/mm3 (data not shown). This finding, along with the fact that monocytes, lymphocytes, and eosinophils make up only 35% of the total leukocyte population (54), precludes the interpretation that the presence of PDE3 observed in Western blot analyses of the erythrocyte membrane is due to leukocyte contamination.
Effect of a nonselective PDE inhibitor on iloprost-induced increases in cAMP in rabbit and human erythrocytes.
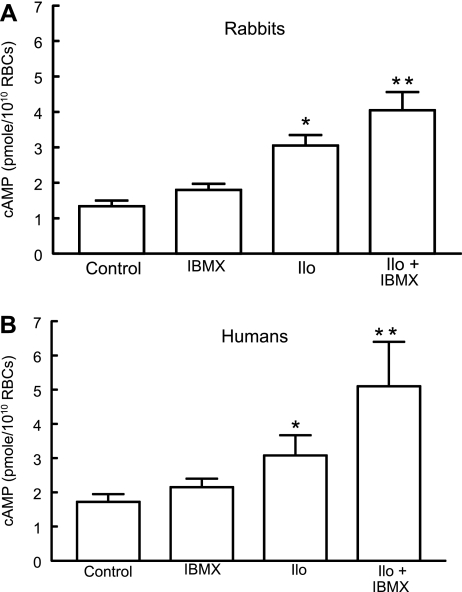
Although PDE activity responsible for the hydrolysis of 3′5′-cAMP has been reported to be present in rabbit and human erythrocytes (1, 31, 51), the isoform(s) responsible for this activity has not been identified. To determine whether the inhibition of PDE activity potentiates iloprost-induced increases in cAMP in erythrocytes, cells were incubated with iloprost (1 μM) in the presence and absence of a nonselective PDE inhibitor, IBMX (10 μM). Importantly, this concentration of IBMX was chosen because it did not affect basal cAMP levels. As shown in Fig. 2, treatment with IBMX potentiated the effect of iloprost in rabbit (Fig. 2A) and human (Fig. 2B) erythrocytes, demonstrating that PDE activity is present in these cells and regulates iloprost-induced increases in cAMP. However, these studies do not provide insights into which PDE isoform(s) are involved in this pathway.
Fig. 2.
Effect of 3-isobutyl-1-methylxanthine (IBMX; 10 μM) on iloprost (Ilo)-induced levels of cAMP in rabbit and human erythrocytes. A: washed erythrocytes from rabbits (n = 5) were incubated with Ilo (1 μM) in the presence of 10 μM IBMX or its vehicle [N,N-dimethylformamide (DMF)]. B: washed erythrocytes from humans (n = 8) were incubated with Ilo (1 μM) in the presence of 10 μM IBMX or its vehicle (DMF). In both A and B, cells were incubated with IBMX or its vehicle (DMF) for 30 min, and then cAMP increases were stimulated by the addition of Ilo for 15 min. Values are means ± SE. P < 0.05; *different from control and inhibitor alone; **greater than all other values. RBCs, red blood cells.
Effect of selective inhibitors of PDE1 or PDE3 on iloprost-induced increases in cAMP in rabbit erythrocytes.
The only PDE activity previously identified in mammalian erythrocytes was that of PDE1, but this PDE was shown to metabolize cGMP (31). To investigate the possible contribution of PDE1 activity to the regulation of iloprost-induced increases in cAMP in rabbit erythrocytes, cells were incubated with iloprost (1 μM) in the presence and absence of a selective PDE1 inhibitor, vinpocetine (30 μM; n = 8) (20, 25). Vinpocetine had no effect on either basal or iloprost-stimulated increases in cAMP (Table 1). These results suggest that PDE1 does not regulate iloprost-induced cAMP increases in rabbit erythrocytes.
Table 1.
Effect of 30 μM PDE1 inhibitor vinpocetine on 1 μM iloprost-induced increases in rabbit and human erythrocyte cAMP
| Group | Control | Vinpocetine | Iloprost | Iloprost + Vinpocetine |
|---|---|---|---|---|
| Rabbits | 0.94±0.1 | 0.95±0.1 | 2.3±0.2* | 2.2±0.3* |
| Humans | 1.7±0.2 | 1.4±0.18 | 2.5±0.3* | 2.7±0.4* |
Values are means ± SE; n = 8 rabbits and 6 humans. P < 0.05;
different from respective control and vinpocetine alone. PDE, phosphodiesterase.
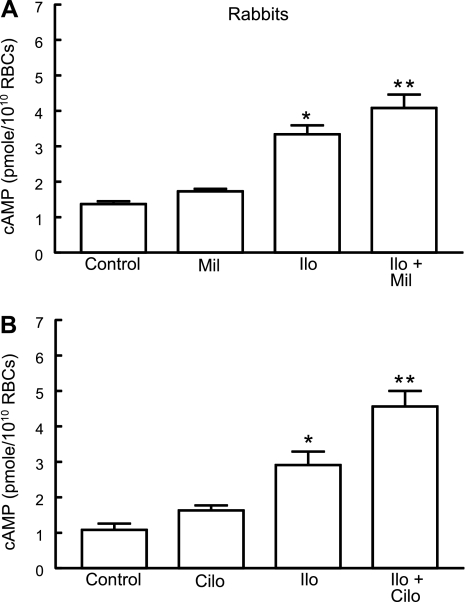
To determine whether PDE3 activity is involved in the regulation of cAMP increases stimulated by iloprost in rabbit erythrocytes, cells were incubated with iloprost (1 μM) in the presence or absence of the PDE3 inhibitor milrinone (20 μM; n = 6) (19, 20). The concentration of milrinone chosen had no effect on basal cAMP levels. As depicted in Fig. 3A, milrinone potentiated iloprost-induced cAMP increases by 22 ± 5% (P < 0.05).
Fig. 3.
Effect of inhibitors of PDE3 on Ilo-induced increases in cAMP in rabbit erythrocytes. A: washed erythrocytes from rabbits (n = 6) were incubated with Ilo (1 μM) in the presence of 20 μM milrinone (Mil) or its vehicle (DMF). B: washed erythrocytes from rabbits (n = 6) were incubated with Ilo (1 μM) in the presence of 10 μM cilostazol (Cilo) or its vehicle (DMF). Cells were incubated with Mil, Cilo, or DMF for 30 min, and then cAMP increases were stimulated by the addition of Ilo for 15 min. Values are means ± SE. P < 0.05; *different from control and inhibitor alone; **greater than all other values.
Milrinone has been reported to be a selective PDE3 inhibitor (19, 20, 41). However, recent evidence from studies in cardiomyocytes indicates that milrinone can also inhibit PDE4 activity at low micromolar concentrations (40, 52). Indeed, the greater increases in intracellular cAMP in response to treatment with milrinone compared with cilostazol in these cells were attributed to the ability of milrinone to inhibit both PDE3 and PDE4 (40). To confirm that the inhibition of PDE3 potentiates iloprost-induced increases in cAMP, rabbit erythrocytes were preincubated with cilostazol, a chemically dissimilar selective PDE3 inhibitor, followed by iloprost (19, 20). The concentrations of cilostazol used in these studies (10 to 100 μM) have been reported to inhibit the activity of PDE3, but not PDE4 (6, 19). At these concentrations, cilostazol had no effect on basal cAMP. Pretreatment with cilostazol (10 μM; n = 6) potentiated iloprost (1 μM)-induced increases in cAMP produced by the incubation in rabbit erythrocytes (Fig. 3B). Thus two dissimilar inhibitors of PDE3 activity, milrinone and cilostazol, augment iloprost-induced increases in cAMP in rabbit erythrocytes.
Effect of selective inhibitors of PDE1 or PDE3 on iloprost-induced increases in cAMP in human erythrocytes.
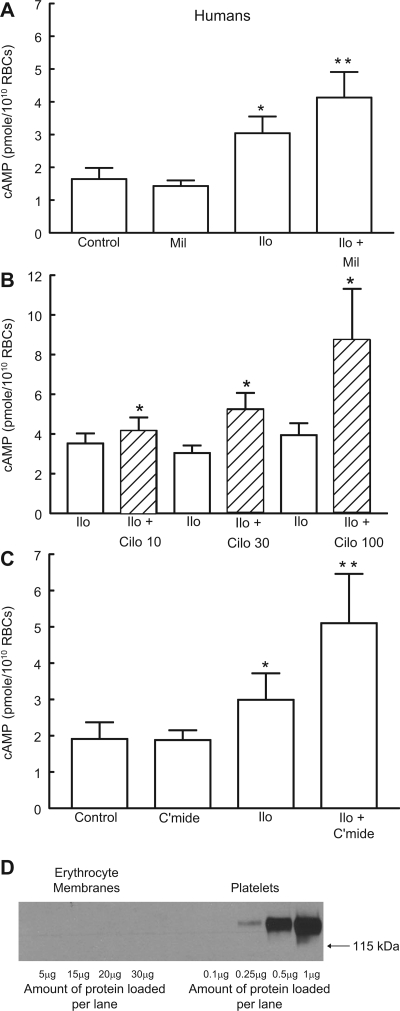
As was the case with rabbit erythrocytes, the PDE1 selective inhibitor vinpocentine (30 μM; n = 6) (13, 25, 31) had no effect on either basal or iloprost-induced increases in cAMP in human erythrocytes (Table 1). Since the PDE3 protein is present in membranes of both rabbit and human erythrocytes and inhibitors of this PDE potentiated iloprost-induced increases in cAMP in rabbit cells, we determined the effect of the PDE3 inhibitors milrinone and cilostazol on iloprost-induced increases in cAMP in human erythrocytes. Similar to the results with rabbit erythrocytes, 20 μM milrinone potentiated iloprost-induced cAMP increases by 34 ± 10% (n = 6, P < 0.05; Fig. 4A). Cilostazol potentiated iloprost-induced increases in cAMP in human erythrocytes at concentrations of 10, 30, and 100 μM (Fig. 4B). Importantly, at none of these concentrations did cilostazol produce increases in basal cAMP concentration (data not shown). In four additional studies with human erythrocytes, pretreatment with cilostamide (30 μM; n = 4), the parent compound of cilostazol (19), augmented iloprost-induced increases in cAMP (Fig. 4C) and had no effect on basal cAMP levels. Taken together, these results provide strong support for the hypothesis that PDE3 activity modulates iloprost-induced increases in cAMP in both rabbit and human erythrocytes.
Fig. 4.
Effect of PDE3 inhibitors on Ilo-induced levels of cAMP in human erythrocytes. A: washed erythrocytes from humans (n = 6) were incubated with Ilo (1 μM) in the presence of 20 μM Mil or its vehicle (DMF). B: washed erythrocytes from humans were incubated with Ilo (1 μM) in the presence of 10 μM (n = 12), 30 μM (n = 9), or 100 μM (n = 6) Cilo or its vehicle (DMF). C: washed erythrocytes from humans (n = 4) were incubated with Ilo (1 μM) in the presence of 30 μM cilostamide (C'mide) or its vehicle (DMF). Cells were incubated with Mil, Cilo, C'mide, or DMF for 30 min, and then cAMP increases were stimulated by the addition of Ilo for 15 min. Values are means ± SE. P < 0.05; *different from control and inhibitor alone (A and C) or corresponding Ilo (B); **greater than all other values. D: human erythrocyte membranes and human platelet membranes (representative of 7 studies) were probed with a monoclonal antibody generated against integrin αIIb (CD41).
It is important to note that platelets also possess PDE3 activity. However, the isoform present in platelets is PDE3A (15). To ensure that erythrocyte preparations were devoid of significant and potentially confounding platelet contamination, separate Western blot analyses of human erythrocyte membrane preparations were performed and probed with an antibody directed against a platelet marker, integrin αIIb (CD41) (24). As shown in Fig. 4D, CD41 was detected in human platelet preparations but not in human erythrocyte membrane preparations. The CD41 antibody used for human erythrocyte membrane preparations does not react with rabbit platelet CD41, making it impossible to use this antibody to study the platelet contamination of rabbit erythrocyte membrane preparations. To address this important issue, we subjected washed rabbit erythrocyte preparations to manual platelet counting. Whole rabbit blood contained 320,000 ± 13,000 platelets/mm3. No platelets were detected in washed rabbit erythrocyte preparations (n = 4).
DISCUSSION
The concentration of cAMP in cells is the product of its synthesis by ACs and the activity of PDEs that hydrolyze this important second messenger (20, 21). In many cell types, the receptor-mediated activation of G protein-coupled receptors results in the stimulation of AC and synthesis of cAMP, a critical control point in the associated signaling pathway. cAMP-specific PDEs limit the biological actions of this second messenger and regulate the response of cells to external stimuli.
Initial reports suggested that the mature human erythrocyte possessed little or no AC activity (3, 10, 18, 43). However, as the result of methodological improvements in the isolation of erythrocyte membranes and the measurement of cAMP, it was demonstrated that human and rabbit erythrocytes do indeed possess AC activity (17, 26, 29, 33, 44). In these studies, the incubation of erythrocytes with prostacyclin analogs or β-adrenergic agonists resulted in increases in cAMP. However, no role for cAMP in erythrocyte signaling pathways was demonstrated. Recently, it was shown that the incubation of rabbit and human erythrocytes with β-adrenergic agonists or prostacyclin analogs results in receptor-mediated increases in cAMP (45) as well as the release of ATP (44, 45). The finding that the incubation of rabbit and human erythrocytes with the active cell-permeable cAMP analog, Sp-cAMP, results in ATP release (48) demonstrates a relationship between intracellular cAMP and the release of ATP from these cells.
A signal transduction pathway that relates receptor-mediated increases in cAMP to ATP release from erythrocytes has been described (44, 45). This pathway includes the heterotrimeric G protein Gs, AC, cAMP, and cAMP-dependent protein kinase (PKA) (29, 44, 47, 48). The finding that increases in intracellular cAMP are required for ATP release from erythrocytes (48) suggests that the regulation of the concentration of this cyclic nucleotide would be a critical control point in this pathway. Thus the identification of the PDE(s) in erythrocytes that is associated with any specific signaling pathway would lead to a new understanding of the regulation of cAMP in this cell.
Of the 11 known PDE families, only the activity of PDE1 was reported to be present in human erythrocytes (31). PDE1 has three known subfamilies, and each can hydrolyze both cAMP and cGMP although the isoforms have different affinities for the two cyclic nucleotides (13, 20). The only study identifying PDE1 activity in human erythrocytes demonstrated that this PDE hydrolyzed cGMP (31). Here we report that the selective PDE1 inhibitor vinpocetine (13, 31) has no effect on iloprost-induced increases in cAMP in rabbit or human erythrocytes (Table 1). Thus it is unlikely that PDE1 participates in the regulation of cAMP synthesized in response to the activation of this signaling pathway.
We next examined the contribution of PDE3 activity to the regulation of iloprost-induced increases in cAMP. PDE3 was chosen for study because this PDE has been reported to be associated with prostacyclin (IP) receptor signaling pathways in platelets (11) as well as vascular smooth muscle (32, 38, 39). In addition, the PDE3 inhibitor milrinone was reported to potentiate cAMP increases stimulated by β-adrenergic agonists in avian embryonic erythrocytes (2). However, neither the expression of PDE3 nor its role in the regulation of cAMP in a mammalian erythrocyte has been previously investigated.
To establish that PDE3 is present in rabbit and human erythrocytes, isolated membranes were probed with antibodies directed against the membrane-associated isoform of that PDE, PDE3B (42). As depicted in Fig. 1, we determined that these erythrocytes possess the PDE3B protein. PDE3B has been reported to be present not only in the plasma membrane of other cell types but also in membranes of endoplasmic reticulum, adipose tissue microsomes, and cardiac sarcoplasmic reticulum (4, 41, 42, 52). However, because mature mammalian erythrocytes lack those organelles, we conclude that PDE3B is localized to the plasma membrane of erythrocytes of rabbits and humans.
Although these studies demonstrate for the first time the presence of the PDE3B protein in mammalian erythrocytes, they do not establish a function for this PDE in a signaling pathway. Clearly, PDE activity is associated with iloprost-induced increases in cAMP in rabbit and human erythrocytes, as demonstrated by the finding that the nonselective PDE inhibitor IBMX (10 μM) (20) potentiates iloprost-induced increases in cAMP in both cell types (Fig. 2). However, studies with IBMX do not permit the identification of the individual PDE family(s) that is involved.
The fact that mature, circulating erythrocytes do not possess a nucleus and lack protein synthetic capacity limits the methods available to manipulate the expression of PDEs in these cells. Therefore, we used selective inhibitors of PDE3 activity to define a role for this PDE as a regulator of iloprost-induced increases in cAMP in rabbit and human erythrocytes.
In the case of rabbit erythrocytes, cells were incubated with iloprost in the absence and presence of one of two chemically dissimilar, selective inhibitors of PDE3 activity, milrinone and cilostazol (19). The concentrations of milrinone and cilostazol chosen had no effect on basal cAMP levels, yet both potentiated iloprost-induced increases in cAMP in rabbit erythrocytes (Fig. 3, A and B).
To determine the contribution of PDE3 activity to the regulation of iloprost-induced increases in cAMP levels in human erythrocytes, cells were incubated with milrinone, cilostazol, or the parent compound of cilostazol, cilostamide (19, 20). Pretreatment with milrinone or cilostamide augmented iloprost-induced increases in cAMP (Fig. 4, A and C). In addition, cilostazol also potentiated iloprost-induced increases in cAMP in human cells at 10, 30, and 100 μM (Fig. 4B). The ability of PDE3 inhibitors to potentiate iloprost-induced increases in cAMP in rabbit and human erythrocytes is consistent with the hypothesis that this PDE is involved in the regulation of IP receptor signaling in these cells.
Although these studies demonstrate that the PDE3B protein is a component of the membranes of rabbit and human erythrocytes and that inhibitors of the activity of PDE3 augment iloprost-induced increases in cAMP, these findings cannot establish unequivocally that PDE3B is the PDE3 isoform associated with the regulation of iloprost-induced increases in cAMP in this signaling pathway. Available PDE3 inhibitors are not isoform selective (19, 20, 41). Another isoform of PDE3, PDE3A, is a cytosolic protein (41). The large concentration of hemoglobin in the erythrocyte impairs the ability to identify cytosolic proteins by Western blot analysis. Therefore, we cannot exclude the possibility that PDE3A is also present and active in this pathway in erythrocytes. Notwithstanding, PDE3 activity clearly contributes to the regulation of iloprost-induced increases in cAMP in these cells.
In summary, in the work presented here, we demonstrate for the first time that the PDE3B protein is present in membranes of rabbit and human erythrocytes. Moreover, the results of the studies with inhibitors of PDE3 activity are consistent with the interpretation that PDE3 regulates iloprost-induced increases in rabbit and human erythrocyte cAMP. Increases in erythrocyte cAMP are associated with ATP release from this cell (48). Erythrocyte-derived ATP has been shown to produce vasodilation in isolated lungs (47, 49) and isolated arterioles (5, 8). Iloprost is used clinically in the treatment of pulmonary hypertension and peripheral vascular disease because of its vasodilator properties (14, 23). The PDE3 inhibitor cilostazol is also used in the treatment of peripheral vascular disease (19). Interestingly, PDE inhibitors, particularly those that inhibit PDE3 activity, have been shown to potentiate the ability of prostacyclin analogs to produce pulmonary vasodilation (38, 39). The results presented here suggest a heretofore unrecognized mechanism by which iloprost and PDE3 inhibitors could interact to produce vasodilation in vivo. The ability of cilostazol to potentiate iloprost-induced cAMP increases in erythrocytes could augment ATP release from these cells and, thereby, stimulate vasodilation. Therefore, the identification of the PDE isoforms present in erythrocytes that are associated with specific signaling pathways could provide new therapeutic targets in the treatment of vascular disease.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-64180 and by National Institutes of Health Training Grant 5T32 GM008306-17, along with a grant from the American Diabetes Association.
Acknowledgments
We thank J. L. Sprague for inspiration.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Babu CR, Azhar S, Krishna Murti CR. Loss of epinephrine stimulated synthesis of cyclic adenosine 3′:5′ monophosphate during maturation of rabbit and human reticulocytes. Med Biol 53: 148–155, 1975. [PubMed] [Google Scholar]
- 2.Baumann R, Blass C, Gotz R, Dragon S. Ontogeny of catecholamine and adenosine receptor-mediated cAMP signaling of embryonic red blood cells: role of cGMP-inhibited phosphodiesterase 3 and hemoglobin. Blood 94: 4314–4320, 1999. [PubMed] [Google Scholar]
- 3.Beaumont C, Piau JP, Fischer S, Delaunay J, Schapira G. Stimulation of erythroid cells adenylate cyclase by soluble factors. Biochem Biophys Res Commun 91: 1250–1257, 1979. [DOI] [PubMed] [Google Scholar]
- 4.Degerman E, Belfrage P, Manganiello VC. Structure, localization, and regulation of cGMP-inhibited phosphodiesterase (PDE3). J Biol Chem 272: 6823–6826, 1997. [DOI] [PubMed] [Google Scholar]
- 5.Dietrich HH, Ellsworth ML, Sprague RS, Dacey RG Jr. Red blood cell regulation of microvascular tone through adenosine triphosphate. Am J Physiol Heart Circ Physiol 278: H1294–H1298, 2000. [DOI] [PubMed] [Google Scholar]
- 6.Ding B, Abe J, Wei H, Huang Q, Walsh RA, Molina CA, Zhao A, Sadoshima J, Blaxall BC, Berk BC, Yan C. Functional role of phosphodiesterase 3 in cardiomyocyte apoptosis: implication in heart failure. Circulation 111: 2469–2476, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ellsworth ML The red blood cell as an oxygen sensor: what is the evidence? Acta Physiol Scand 168: 551–559, 2000. [DOI] [PubMed] [Google Scholar]
- 8.Ellsworth ML Red blood cell-derived ATP as a regulator of skeletal muscle perfusion. Med Sci Sports Exerc 36: 35–41, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Ellsworth ML, Forrester T, Ellis CG, Dietrich HH. The erythrocyte as a regulator of vascular tone. Am J Physiol Heart Circ Physiol 269: H2155–H2161, 1995. [DOI] [PubMed] [Google Scholar]
- 10.Farfel Z, Cohen Z. Adenylate cyclase in the maturing human reticulocyte: selective loss of the catalytic unit, but not of the receptor-cyclase coupling protein. Eur J Clin Invest 14: 79–82, 1984. [DOI] [PubMed] [Google Scholar]
- 11.Fujitani K, Kambayashi J, Murata K, Yano Y, Shinozaki K, Yukawa M, Sakon M, Murata T, Kawasaki T, Shiba E, Mori T. Clinical evaluation on combined administration of oral prostacyclin analogue beraprost and phosphodiesterase inhibitor cilostazol. Pharmacol Res 31: 121–125, 1995. [DOI] [PubMed] [Google Scholar]
- 12.Gantner F, Tenor H, Gekeler V, Schudt C, Wendel A, Hatzelmann A. Phosphodiesterase profiles of highly purified human peripheral blood leukocyte populations from normal and atopic individuals: a comparative study. J Allergy Clin Immunol 100: 527–535, 1997. [DOI] [PubMed] [Google Scholar]
- 13.Goraya TA, Cooper DM. Ca2+-calmodulin-dependent phosphodiesterase (PDE1): current perspectives. Cell Signal 17: 789–797, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Grant SM, Goa KL. Iloprost. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in peripheral vascular disease, myocardial ischaemia and extracorporeal circulation procedures. Drugs 43: 889–924, 1992. [DOI] [PubMed] [Google Scholar]
- 15.Haslam RJ, Dickinson NT, Jang EK. Cyclic nucleotides and phosphodiesterases in platelets. Thromb Haemost 82: 412–423, 1999. [PubMed] [Google Scholar]
- 16.Jagger JE, Bateman RM, Ellsworth ML, Ellis CG. Role of erythrocyte in regulating local O2 delivery mediated by hemoglobin oxygenation. Am J Physiol Heart Circ Physiol 280: H2833–H2839, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Kaiser G, Quiring K, Gauger D, Palm D, Becker H, Schoeppe W. Occurrence of adenyl cyclase activity in human erythrocytes. Blut 29: 115–122, 1974. [DOI] [PubMed] [Google Scholar]
- 18.Limbird LE, Gill DM, Stadel JM, Hickey AR, Lefkowitz RJ. Loss of beta-adrenergic receptor-guanine nucleotide regulatory protein interactions accompanies decline in catecholamine responsiveness of adenylate cyclase in maturing rat erythrocytes. J Biol Chem 255: 1854–1861, 1980. [PubMed] [Google Scholar]
- 19.Liu Y, Shakur Y, Yoshitake M, Kambayashi Ji J. Cilostazol (pletal): a dual inhibitor of cyclic nucleotide phosphodiesterase type 3 and adenosine uptake. Cardiovasc Drug Rev 19: 369–386, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Lugnier C Cyclic nucleotide phosphodiesterase (PDE) superfamily: a new target for the development of specific therapeutic agents. Pharmacol Ther 109: 366–398, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Manganiello VC, Degerman E. Cyclic nucleotide phosphodiesterases (PDEs): diverse regulators of cyclic nucleotide signals and inviting molecular targets for novel therapeutic agents. Thromb Haemost 82: 407–411, 1999. [PubMed] [Google Scholar]
- 22.Matsuura T, Kanayama Y, Inoue T, Takeda T, Morishima I. cAMP-induced changes of intracellular free Mg2+ levels in human erythrocytes. Biochim Biophys Acta 1220: 31–36, 1993. [DOI] [PubMed] [Google Scholar]
- 23.Meini S, De Franco V, Auteri A, Setacci C, Di Renzo M, Pieragalli D. Short-term and long-term effects of one-week treatment with intravenous iloprost in critical limb ischemia patients (Leriche-Fontaine stage III and IV). Int Angiol 24: 64–69, 2005. [PubMed] [Google Scholar]
- 24.Moroi M, Jung SM. Integrin-mediated platelet adhesion. Front Biosci 3: d719–d728, 1998. [DOI] [PubMed] [Google Scholar]
- 25.Murray F, Patel HH, Suda RY, Zhang S, Thistlethwaite PA, Yuan JX, Insel PA. Expression and activity of cAMP phosphodiesterase isoforms in pulmonary artery smooth muscle cells from patients with pulmonary hypertension: role for PDE1. Am J Physiol Lung Cell Mol Physiol 292: L294–L303, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Nakagawa M, Willner J, Cerri C, Reydel P. The effect of membrane preparation and cellular maturation on human erythrocyte adenylate cyclase. Biochim Biophys Acta 770: 122–126, 1984. [DOI] [PubMed] [Google Scholar]
- 27.Olearczyk JJ, Stephenson AH, Lonigro AJ, Sprague RS. Heterotrimeric G protein Gi is involved in a signal transduction pathway for ATP release from erythrocytes. Am J Physiol Heart Circ Physiol 286: H940–H945, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Olearczyk JJ, Stephenson AH, Lonigro AJ, Sprague RS. NO inhibits signal transduction pathway for ATP release from erythrocytes via its action on heterotrimeric G protein Gi. Am J Physiol Heart Circ Physiol 287: H748–H754, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Olearczyk JJ, Stephenson AH, Lonigro AJ, Sprague RS. Receptor-mediated activation of the heterotrimeric G-protein Gs results in ATP release from erythrocytes. Med Sci Monit 7: 669–674, 2001. [PubMed] [Google Scholar]
- 30.Patterson WD, Hardman JG, Sutherland EW. Apparent multiple forms of cyclic AMP phosphodiesterase from rat erythrocytes. Mol Cell Endocrinol 5: 51–66, 1976. [DOI] [PubMed] [Google Scholar]
- 31.Petrov V, Fagard R, Lijnen P. Human erythrocytes contain Ca2+, calmodulin-dependent cyclic nucleotide phosphodiesterase which is involved in the hydrolysis of cGMP. Methods Find Exp Clin Pharmacol 20: 387–393, 1998. [DOI] [PubMed] [Google Scholar]
- 32.Phillips PG, Long L, Wilkins MR, Morrell NW. cAMP phosphodiesterase inhibitors potentiate effects of prostacyclin analogs in hypoxic pulmonary vascular remodeling. Am J Physiol Lung Cell Mol Physiol 288: L103–L115, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Piau JP, Delaunay J, Fischer S, Tortolero M, Schapira G. Human red cell membrane adenylate cyclase in normal subjects and patients with hereditary spherocytosis, sickle cell disease and unidentified hemolytic anemias. Blood 56: 963–968, 1980. [PubMed] [Google Scholar]
- 34.Rasmussen H, Lake W, Allen JE. The effect of catecholamines and prostaglandins upon human and rat erythrocytes. Biochim Biophys Acta 411: 63–73, 1975. [DOI] [PubMed] [Google Scholar]
- 35.Rochais F, Abi-Gerges A, Horner K, Lefebvre F, Cooper DM, Conti M, Fischmeister R, Vandecasteele G. A specific pattern of phosphodiesterases controls the cAMP signals generated by different Gs-coupled receptors in adult rat ventricular myocytes. Circ Res 98: 1081–1088, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodan SB, Rodan GA, Sha′afi RI. Demonstration of adenylate cyclase activity in human red blood cell ghosts. Biochim Biophys Acta 428: 509–515, 1976. [DOI] [PubMed] [Google Scholar]
- 37.Sager G Receptor binding sites for beta-adrenergic ligands on human erythrocytes. Biochem Pharmacol 31: 99–104, 1982. [DOI] [PubMed] [Google Scholar]
- 38.Schermuly RT, Inholte C, Ghofrani HA, Gall H, Weissmann N, Weidenbach A, Seeger W, Grimminger F. Lung vasodilatory response to inhaled iloprost in experimental pulmonary hypertension: amplification by different type phosphodiesterase inhibitors. Respir Res 6: 76, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schermuly RT, Roehl A, Weissmann N, Ghofrani HA, Schudt C, Tenor H, Grimminger F, Seeger W, Walmrath D. Subthreshold doses of specific phosphodiesterase type 3 and 4 inhibitors enhance the pulmonary vasodilatory response to nebulized prostacyclin with improvement in gas exchange. J Pharmacol Exp Ther 292: 512–520, 2000. [PubMed] [Google Scholar]
- 40.Shakur Y, Fong M, Hensley J, Cone J, Movsesian MA, Kambayashi J, Yoshitake M, Liu Y. Comparison of the effects of cilostazol and milrinone on cAMP-PDE activity, intracellular cAMP and calcium in the heart. Cardiovasc Drugs Ther 16: 417–427, 2002. [DOI] [PubMed] [Google Scholar]
- 41.Shakur Y, Holst LS, Landstrom TR, Movsesian M, Degerman E, Manganiello V. Regulation and function of the cyclic nucleotide phosphodiesterase (PDE3) gene family. Prog Nucleic Acid Res Mol Biol 66: 241–277, 2001. [DOI] [PubMed] [Google Scholar]
- 42.Shakur Y, Takeda K, Kenan Y, Yu ZX, Rena G, Brandt D, Houslay MD, Degerman E, Ferrans VJ, Manganiello VC. Membrane localization of cyclic nucleotide phosphodiesterase 3 (PDE3). Two N-terminal domains are required for the efficient targeting to, and association of, PDE3 with endoplasmic reticulum. J Biol Chem 275: 38749–38761, 2000. [DOI] [PubMed] [Google Scholar]
- 43.Sheppard H, Burghardt C. Adenyl cyclase in non-nucleated erythrocytes of several mammalian species. Biochem Pharmacol 18: 2576–2578, 1969. [DOI] [PubMed] [Google Scholar]
- 44.Sprague R, Bowles E, Stumpf M, Ricketts G, Freidman A, Hou WH, Stephenson A, Lonigro A. Rabbit erythrocytes possess adenylyl cyclase type II that is activated by the heterotrimeric G proteins Gs and Gi. Pharmacol Rep 57: 222–228, 2005. [PubMed] [Google Scholar]
- 45.Sprague RS, Bowles EA, Hanson MS, Dufaux EA, Sridharan M, Adderley S, Ellsworth ML, Stephenson AH. Prostacyclin analogs stimulate receptor-mediated cAMP synthesis and ATP release from rabbit and human erythrocytes. Microcirculation 15: 461–471, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sprague RS, Ellsworth ML, Stephenson AH, Kleinhenz ME, Lonigro AJ. Deformation-induced ATP release from red blood cells requires CFTR activity. Am J Physiol Heart Circ Physiol 275: H1726–H1732, 1998. [DOI] [PubMed] [Google Scholar]
- 47.Sprague RS, Ellsworth ML, Stephenson AH, Lonigro AJ. ATP: the red blood cell link to NO and local control of the pulmonary circulation. Am J Physiol Heart Circ Physiol 271: H2717–H2722, 1996. [DOI] [PubMed] [Google Scholar]
- 48.Sprague RS, Ellsworth ML, Stephenson AH, Lonigro AJ. Participation of cAMP in a signal-transduction pathway relating erythrocyte deformation to ATP release. Am J Physiol Cell Physiol 281: C1158–C1164, 2001. [DOI] [PubMed] [Google Scholar]
- 49.Sprague RS, Olearczyk JJ, Spence DM, Stephenson AH, Sprung RW, Lonigro AJ. Extracellular ATP signaling in the rabbit lung: erythrocytes as determinants of vascular resistance. Am J Physiol Heart Circ Physiol 285: H693–H700, 2003. [DOI] [PubMed] [Google Scholar]
- 50.Sprague RS, Stephenson AH, Bowles EA, Stumpf MS, Lonigro AJ. Reduced expression of Gi in erythrocytes of humans with type 2 diabetes is associated with impairment of both cAMP generation and ATP release. Diabetes 55: 3588–3593, 2006. [DOI] [PubMed] [Google Scholar]
- 51.Suzuki K, Terao T, Osawa T. Studies on adenosine 3′,5′-monophosphate phosphodiesterase of human erythrocyte membranes. Biochim Biophys Acta 602: 78–86, 1980. [DOI] [PubMed] [Google Scholar]
- 52.Thompson PE, Manganiello V, Degerman E. Re-discovering PDE3 inhibitors—new opportunities for a long neglected target. Curr Top Med Chem 7: 421–436, 2007. [DOI] [PubMed] [Google Scholar]
- 53.Tuvia S, Moses A, Gulayev N, Levin S, Korenstein R. Beta-adrenergic agonists regulate cell membrane fluctuations of human erythrocytes. J Physiol 516: 781–792, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wheater PR, Burkitt HG. Functional Histology: a text and colour atlas. Ediburgh: Churchill Livingstone, 1987.
- 55.Zaccolo M, Di Benedetto G, Lissandron V, Mancuso L, Terrin A, Zamparo I. Restricted diffusion of a freely diffusible second messenger: mechanisms underlying compartmentalized cAMP signalling. Biochem Soc Trans 34: 495–497, 2006. [DOI] [PubMed] [Google Scholar]