Abstract
Experiments requiring strong repression and precise control of cloned genes can be difficult to conduct because of the relatively high basal level of expression of currently employed promoters. We report the construction of a family of vectors that contain a reengineered lacIq-lac promoter-operator complex in which cloned genes are strongly repressed in the absence of inducer. The vectors, all based on the broad-host-range plasmid pBBR1, are mobilizable and stably replicate at moderate copy number in representatives of the alpha- and gammaproteobacteria. Each vector contains a versatile multiple cloning site that includes an NdeI site allowing fusion of the cloned gene to the initiation codon of lacZα. In each tested bacterium, a uidA reporter fused to the promoter was not expressed at a detectable level in the absence of induction but was inducible by 10- to 100-fold, depending on the bacterium. The degree of induction was controllable by varying the concentration of inducer. When the vector was tested in Agrobacterium tumefaciens, a cloned copy of the traR gene, the product of which is needed at only a few copies per cell, did not confer activity under noninducing conditions. We used this attribute of very tight and variably regulatable control to assess the relative amounts of TraR required to activate the Ti plasmid conjugative transfer system. We identified levels of induction that gave wild-type transfer frequencies, as well as levels that induced correspondingly lower frequencies of transfer. We also used this system to show that the antiactivator TraM sets the level of intracellular TraR required for tra gene activation.
Bacterial expression vectors have been designed for two main purposes: those with promoters engineered for very strong expression, from bacteriophage SP6 (34) or T7 (39, 51) for example, are useful for overexpressing proteins for subsequent purification, and those with regulatable promoters, Plac (42) for example, are useful for studies in which controlled expression of the cloned gene at physiologically relevant levels is important. Among the latter, the lac promoter of Escherichia coli, as well as its mutant and hybrid forms lacUV5 (6), Ptac (10), and Ptrc (4), have been extensively exploited in combination with its control protein, LacI. In addition, other regulatory elements have been developed and are widely used, including the arabinose promoter of E. coli (19), the xylene (xyl) promoter of Pseudomonas putida (35), and the xylose (xyl) promoter of Caulobacter crescentus (48).
Of these options, the lac promoter has certain advantages. First, more is known about this promoter and how it is regulated by LacI and other transcription factors than perhaps any other bacterial promoter. Second, several versions of the promoter with different strengths are available. Third, a number of engineered versions of the lac promoter and its regulatory elements, which include multiple cloning sites and reporter genes, such as lacZα, have been constructed. Fourth, repression by LacI can be relieved by using several inducers, one of which, isopropyl-β-d-thiogalactopyranoside (IPTG), apparently is membrane permeable (23). This characteristic allows the use of the lac system in many bacteria, including those that do not express their own lactose transporter. The xylene promoter shares this feature; this nonpolar aromatic compound, while requiring a facilitator for passive diffusion across the outer membrane, is most probably freely diffusible across bacterial inner membranes (24). However, the xyl promoter, while strongly responsive to xylene in its host of origin and close relatives, does not exhibit regulatory properties in other, more-distantly related bacteria (25). Moreover, xylene can be toxic to the bacteria (24). The ara and xylose promoters both require that the host express a transporter that facilitates uptake of the inducer. Moreover, if the transporter itself is regulated by the inducer, predictably dose-dependent induction is problematic (23, 37, 46). The xylose promoter of C. crescentus has the added disadvantage that, until recently (33), the regulatory element, xylR, had not been identified. To our knowledge, this gene has not yet been incorporated into the available Pxyl vectors.
Most of these expression systems share an additional limitation. Although the expression of the cloned gene can be upregulated by adding inducer, basal levels of expression in the absence of inducer often are significant. This property can be particularly troublesome in studies in which the expressed protein is active in small quantities in vivo.
TraR, the quorum-sensing transcriptional activator that controls conjugative transfer of the Ti plasmids of Agrobacterium tumefaciens, is active when expressed at only a few copies per cell (49). We have need of a vector system in which we can reliably maintain repression of traR expression at levels below that which would activate the target operons yet which allows induction of the expression of this transcription factor at will. Unfortunately, when cloned in our available expression vectors, the basal, repressed level of expression of traR is sufficient to activate the Ti plasmid transfer system, making these vectors unsuitable for our purposes.
Here we report the construction of a set of broad-host-range vectors in which we have reengineered the lac promoter system to provide very tight regulation of any properly cloned gene. The vector promoter system exhibits very low basal levels of expression in six bacteria representing the alpha and gamma subdivisions of the Proteobacteriaceae. In all cases, the addition of IPTG yielded good levels of induction, and, where tested, such levels could be controlled by the inducer concentration. We also report the results of studies in which we have used this expression system to examine the relative levels of TraR required to activate conjugative transfer in a donor population and to assess the role of the antiactivator TraM in controlling the Ti plasmid quorum-sensing system.
MATERIALS AND METHODS
Bacterial strains and media.
The bacterial strains used in the study are listed in Table 1. E. coli derivatives were grown in LB medium at 37°C. A. tumefaciens was grown on nutrient agar and in ABM (9) or MG/L (5) at 28°C as described in the text. Pseudomonas fluorescens 1855-344, Rhizobium leguminosarum bv. viciae 3841, Sinorhizobium meliloti 1021, and C. crescentus CB15 were grown in ABM, YEM (52), TY (3), and PYE (12) medium, respectively, at 28°C. Brucella abortus 2308 was grown in brucella broth (Becton-Dickinson, Franklin Lakes, NJ) with aeration at 37°C or on Schaedler agar (45) supplemented with 5% defibrinated bovine blood (SBA) at 37°C under 5% CO2. Antibiotics were added as required at the following concentrations in μg per ml: ampicillin, 100; kanamycin (Km) or neomycin, 45, 50, or 100; gentamicin (Gm), 30 or 50; and tetracycline (Tc), 5 or 10.
TABLE 1.
Strains and plasmids used in this study
| Strain | Characteristics | Source or reference |
|---|---|---|
| A. tumefaciens strains | ||
| C58 | Wild-type pathogenic strain carrying pTiC58 | Our collection |
| NT1 | Derivative of C58, cured of pTiC58 | 53 |
| NTL4 | Derivative of C58, cured of pTiC58; ΔtetRS | 31 |
| C58C1RS | Derivative of C58, cured of pTiC58; Rifr StrR | Our collection |
| E. coli strains | ||
| MG1655 | K-12 derivative; F− λ−ilvG rfb-50 rph-1 | J. Cronan, Jr. |
| DH5α | supE44 ΔlacU169(φlacZΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | Invitrogen |
| WM5979 | lacIqrrnB3 ΔlacZ4787 hsdR514 Δ(araBAD)567 Δ(rhaBAD)568 rph-1 Δatt λ::pAE12-Δ(oriR6K/cat::frt5) Δ4229(dapA)::frtΔ(endA)::frt attHK::pJK1006::Δ1/2 (ΔoriR6K-cat::frt5 ΔtrfA::frt) | W. Metcalf |
| Other strains | ||
| Rhizobium leguminosarum bv. viciae 3841 | Derivative of R. leguminosarum bv. viciae strain 300; Smr | J. A. Downie |
| Sinorhizobium meliloti 1021 | Derivative of wild-type strain SU47; Smr | S. R. Long |
| Caulobacter crescentus CB15 | Wild-type strain | J. M. Slauch |
| Pseudomonas fluorescens 1855-344 | Wild-type strain | Our collection |
| Brucella abortus 2308 | Wild-type, smooth, virulent laboratory strain | R. M. Roop II |
| Ralstonia solanacearum AW1 | Wild-type strain | T. P. Denny |
| Plasmids | ||
| pTiC58 | Wild-type Ti plasmid of strain C58; Noc+ Vir+ Trai | Our collection |
| pKPC12 | pTiC58 (traR::lacZ) TraR−; Noc+ Vir+ Tra− Kmr | 41 |
| pBluescript II SK(+) | Cloning vector; Co1E1 f1(+) Ampr | Stratagene |
| pBBR1MCS-2 | Broad-host-range vector; Kmr | 26 |
| pBBR1MCS-3 | Broad-host-range vector; Tetr | 26 |
| pBBR1MCS-5 | Broad-host-range vector; Gmr | 26 |
| pZLQ | pBBR1MCS2-derived expression vector containing trc promoter; Kmr | 30 |
| pZLQR | pZLQ containing C58 traR under the control of Ptrc | 30 |
| pSRKGm(α−) | pBBR1MCS-5 derivative; ΔlacZα | This study |
| pSRKKm(α−) | pBBR1MCS-2 derivative; ΔlacZα | This study |
| pSRKTc(α−) | pBBR1MCS-3 derivative; ΔlacZα | This study |
| pSRKGm(lacI) | pSRKGm(α−) containing lacIq and placZ | This study |
| pSRKGm | pBBR1MCS-5-derived broad-host-range expression vector containing lac promoter and lacIq, lacZα+, and Gmr | This study |
| pSRKKm | pBBR1MCS-2-derived broad-host-range expression vector containing lac promoter and lacIq, lacZα+, and Kmr | This study |
| pSRKTc | pBBR1MCS-3-derived broad-host-range expression vector containing lac promoter and lacIq, lacZα+, and Tetr | This study |
| pSRKKm::uidA | pSRKKm containing placZ::uidA | This study |
| pSRKGmtraR | pSRKGm containing traR from pTiC58 inserted between NdeI and BamHI sites | This study |
| pZLB251 | pRG970b containing traA to traC intergenic region and traA::lacZ fusion | 30 |
| pYZ1 | pRK415 containing BamHI fragment 24 of pTiC58, source of traM gene | 21 |
| pGPO3 | Derivative of pDSK519 containing traR from pTiC58 under the control of Plac; lacIq Kanr | 17 |
| pGPO3-I-gnt | pGPO3 containing lacIq and gentamicin resistance cassette Gmr; Kanr | This study |
| pKK38-I | Expression vector containing Ptrc and lacIq; Tetr | 32 |
| pKKTR2-I | pKK38-I expressing traR from pTiC58 under the control of Ptrc; lacIq Tetr | 32 |
Genetic techniques.
Standard methods of plasmid and genomic DNA preparation and restriction digestion were used (44). DNA fragments were amplified by using either Platinum Pfx-DNA polymerase (Invitrogen) or Pfu Turbo DNA polymerase (Stratagene) in a thermal cycler (MJ Scientific). DNA was transformed into E. coli by heat shock (44) and into A. tumefaciens and P. fluorescens by electroporation (5) using a Gene Pulser equipped with Pulse Controller (Bio-Rad). DNA was mobilized into R. leguminosarum bv. viciae 3841, S. meliloti 1021, and C. crescentus CB15 by biparental mating using E. coli WM5979 (Table 1) as the donor. Plasmids were transferred into B. abortus 2308 by electroporation as previously described (28).
Construction of plasmid vectors.
For the construction of pSRKGm, pBBR1MCS-5 (26) was digested with SspI and self-ligated, resulting in the loss of an internal 704-bp fragment, to give rise to pSRKGm(α−). This intermediate lacks the entire lacZα-peptide sequence and the lac promoter of pBBR1MCS-5. A 1,321-bp fragment containing a full-length lacI gene with the lacIq promoter, the lac promoter-operator complex, and the start codon of lacZ was amplified by PCR from genomic DNA of E. coli MG1655. The oligonucleotides used were 5′-CGCGTTCGAAATTGAATTCTGATTGACACCATCGAATGGTG-3′ and 5′-GCGCGTTCGAATTGCTAGCCATATGCTGTTTCCTGTGTGAAAT-3′ and contain BstBI sites at both ends. Moreover, in the amplified fragment, the start codon of lacZ is engineered as part of an NdeI site. This fragment was cloned into pSRKGm(α−) at the unique BstBI site, giving pSRKGm(lacI). Finally, the 366-bp lacZα gene of pBluescript SK(+) was amplified by using oligonucleotides 5′-GCGCGTTCGAACATATGACCATGATTACGCCAAGC-3′ and 5′-GCGCGTTCGAAGCTAGCTTACAATTTCCATTCGCC-3′ and cloned into pSRKGm(lacI) between the unique NdeI and NheI sites. In the resultant plasmid, called pSRKGm, the ATG codon of the α-peptide is part of the NdeI site.
For the construction of pSRKKm, pBBR1MCS-2 was digested with BstBI and self-ligated, resulting in the loss of an internal 1,046-bp fragment, to give rise to pSRKKm(α−). Subsequently, a 1,684-bp BstBI fragment containing the lacIq-Plac-lacZα region from pSRKGm was ligated into pSRKKm(α−), yielding pSRKKm.
pSRKTc was constructed from pBBR1MCS-3 in three steps. First, pBBR1MCS-3 was digested with EcoRI, resulting in the loss of a ca. 1.7-kb fragment that contains the tetracycline resistance gene and part of the lacZα sequence. In this intermediate vector, the Tc resistance gene was reintroduced between the EcoRI sites as a PCR-generated 1,285-bp fragment, yielding pSRKTc(m). In the second step, pSRKTc(m) was digested with SmaI and SspI, resulting in the loss of a 255-bp fragment containing the remaining segment of the α-peptide coding region. This vector, called pSRKTc(α−), was subsequently digested with BstBI, and the lacIq-Plac-lacZα region from pSRKGm was ligated into this site, yielding pSRKTc.
Construction of the reporter and traR expression clones.
The β-glucuronidase gene (uidA) was amplified from genomic DNA of E. coli MG1655 by PCR and cloned between the NdeI and NheI sites of pSRKKm (sites were generated as part of the primers), generating pSRKKm::uidA. In this construct, the start codon of uidA is synonymous with that of lacZα.
The traR gene of pTiC58 was excised from pZLQR (30) as an NdeI-BamHI fragment and cloned into pSRKGm, giving rise to pSRKGmtraR. We also constructed another lacI-lac promoter-based vector expressing traR as follows. The lacIq gene, obtained as an EcoRI fragment from pKK38-I (Table 1) was cloned into pGPO3, a derivative of pDSK519 in which traR is expressed from the lac promoter (17) to give pGPO3-I. Subsequently, pGPO3-I was partially digested with EcoRI and ligated with a gentamicin resistance cassette obtained from pQKK-gnt, yielding pGPO3-I-gnt.
β-Galactosidase and β-glucuronidase assays.
The β-galactosidase or β-glucuronidase activity was assessed qualitatively by patching cultures onto solid medium containing 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) or 5-bromo-4-chloro-3-indolyl-β-d-glucuronoside (X-Gluc), respectively, using both at a concentration of 50 μg per ml. The colony color and intensity were visually assessed after 24 to 96 h of growth at appropriate temperatures. β-Galactosidase activity was quantified according to Miller (36), while β-glucuronidase activity, expressed as modified Miller units, was quantified as described by Jefferson et al. (22).
Conjugative transfer of Ti plasmids.
The conjugative transfer of pTiC58 derivatives was measured by drop-plate mating as described previously (49). Briefly, 5-μl volumes of 10-fold serial dilutions of cultures of donor strains were spotted onto lawns of A. tumefaciens C58C1RS (Table 1) spread on medium selective for transconjugants only. The progeny arising from the transfer of the Ti plasmid to the recipients were counted, and the frequency of transfer was calculated and expressed as the number of transconjugants obtained per donor cell in each spot on the lawn.
RESULTS
Construction and properties of the pSRK family of vectors.
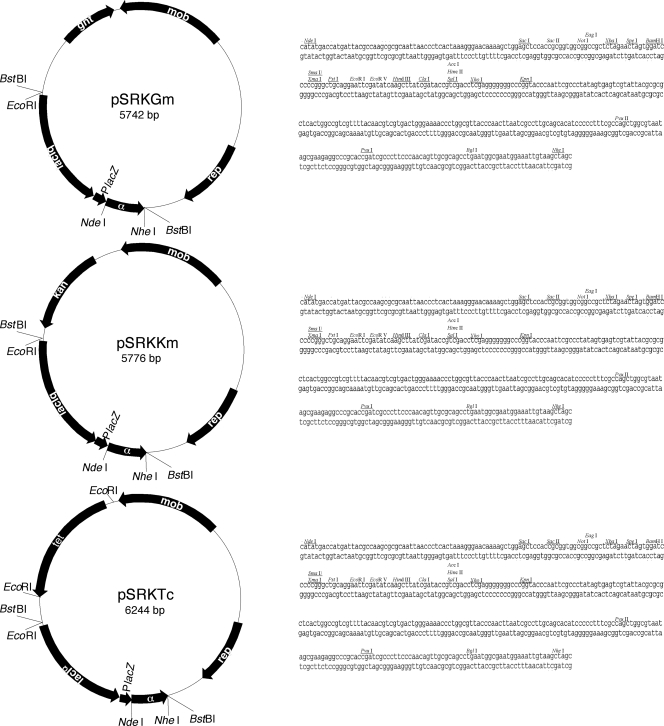
Although there are a number of broad-host-range vectors with expression systems based on lacIq-Plac (2, 7, 16, 18, 30), cloned genes often are expressed at unacceptably high levels in the absence of induction. Full repressional control of the lac operon by LacI requires cooperative interaction of the tetrameric repressor at two of the three operators, O1, O2, and O3, that are located in the operon (38). The lacI-Plac vectors that we know of have been constructed without regard to the relative location of O3, encoded within the 3′ region of lacI, with respect to O1 in the lac promoter region (7, 30). We reasoned that the spacing between these two operators may be critical for maximum repression by tetrameric LacI (27, 38). With this in mind we set out to construct a series of vectors in which the region encompassing lacI-O3 and the lac promoter with its adjacent operator, O1, is as similar to that of the native lac operon as possible. The three new vectors, pSRKKm, pSRKGm, and pSRKTc, all based on the broad-host-range pBBR1MCS vector family (26), contain a segment that codes for lacIq with O3, the native lac promoter with O1, and lacZα containing the multiple cloning site of pBluescript II SK(+) (Fig. 1). While the segment lacks O2, which is located in the 5′ half of lacZ, the spacing and sequence are virtually identical to those of lacI and the promoter-control region of the lac operon of E. coli K-12. Like the parent vector, these plasmids replicate stably at moderate copy number in many gram-negative bacteria and are mobilizable by IncP1 conjugative transfer systems (26). Because of differences in the antibiotic resistance cassettes, not all cleavage sites of the pBluescriptII SK(+) multiple cloning sites are unique in the three vectors. However, in all three vectors, properly designed insertions at the unique NdeI site can be used to generate a translational fusion to the initiation codon of lacZα. Moreover, the entire interval encompassing the lacIq promoter through the 3′ end of lacZα can be excised as a BstB1 fragment (Fig. 1), allowing this segment, either alone or containing a cloned gene, to be moved to other plasmid backbones.
FIG. 1.
Structures of the pSRK family of broad-host-range expression vectors. The physical maps for each of the three vectors show the locations of the key genetic determinants, including replication (rep), antibiotic resistance (gentamicin [gnt, Gm], kanamycin [kan, Km], and tetracycline [tet, Tc]), and mobilization (mob). The expression cassette, composed of lacIq, the lac promoter-O1 complex (PlacZ), and the lacZα (α) coding sequence, is identical in the three vectors. The sequence of the multiple cloning site, located between the NdeI and NheI sites, is shown for each vector. While the sequence of the multiple cloning site is identical among the three vectors, the complements of unique restriction sites (underlined) differ for each.
Regulated expression of lacZα in E. coli.
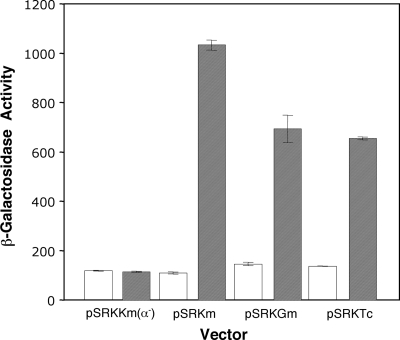
We tested the regulatory properties of the three vectors in E. coli DH5α by assessing the expression of β-galactosidase by α-complementation. On L agar plates containing X-Gal, colonies were white with no hint of blue in the absence of IPTG but grew with a deep blue color on plates containing 1 mM inducer (data not shown). When tested in L broth without IPTG, β-galactosidase activities in strains harboring each of the vectors were not detectable at levels above that observed in cells in which the vector lacks lacZα (Fig. 2). The addition of IPTG resulted in levels of β-galactosidase activity that were 5- to 10-fold higher than the background level (Fig. 2). Adding glucose to the medium resulted in the failure to induce, even in the presence of 1 mM IPTG (data not shown). Thus, the promoter complex is subjected to glucose-mediated catabolite repression in E. coli hosts.
FIG. 2.
Levels of expression of lacZα from the three pSRK vectors under repressed and induced conditions. Cultures of E. coli DH5α harboring one of the three pSRK vectors or a derivative of pSRKKm in which the lacZα coding region has been deleted (pSRKKm(α−) were grown in LB medium with (filled bars) or without (open bars) IPTG (1 mM final concentration) for 4 h. The cells were harvested and assayed for β-galactosidase activity, expressed as Miller units, as described in Materials and Methods. Error bars show standard deviations.
Regulated expression of a cloned gene in other bacteria.
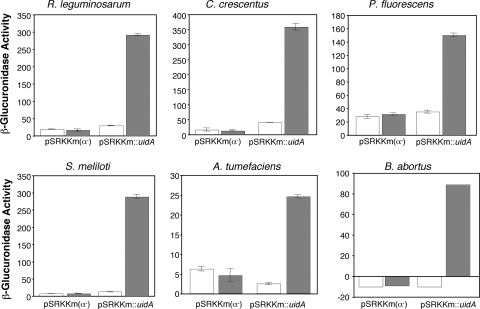
We assessed the expression properties of pSRKKm in several bacteria representing the alpha, beta, and gamma families of the proteobacteria. As a reporter useable in all tested strains, we cloned the uidA gene of E. coli into pSRKKm as an NdeI-NheI fragment, generating a 5′ translational fusion to the initiation codon of lacZα, all as described in Materials and Methods. The recombinant clones were then mobilized into the seven tester strains. We also introduced into these strains pSRKKm(α−), a derivative of pSRKKm that lacks lacZα and uidA, as a control. Each pair of strains was grown in the appropriate medium with and without IPTG. Cultures of A. tumefaciens, R. leguminosarum, S. meliloti, C. crescentus, P. fluorescens, and Ralstonia solanacearum were grown for 12 h following induction, while the culture of B. abortus was grown for 24 h after the addition of IPTG. Cells were harvested and assayed for β-glucuronidase activity as described in Materials and Methods. We were unable to detect expression of the uidA gene in R. solanacearum under any conditions tested, and this strain was dropped from the study. In the remaining six strains, levels of β-glucuronidase activity were at or below the background level of the assay in cells grown in the absence of IPTG (Fig. 3). Growth with inducer resulted in levels of expression ranging from a low of 30 U in A. tumefaciens to around 300 U in C. crescentus, R. leguminosarum, and S. meliloti (Fig. 3). These levels represent induction values of 6- to 100-fold, depending upon the tester strain, over the levels in uninduced cells.
FIG. 3.
The lac promoter of the pSRK vector family is strongly controlled in diverse bacteria. Cultures of A. tumefaciens C58, B. abortus 2308, C. crescentus CB15, P. fluorescens 1855-344, R. leguminosarum 3841, and S. meliloti 1021, each harboring pSRKKm::uidA or pSRKKm(α−), were grown in the appropriate medium with (filled bars) or without (open bars) IPTG (1 mM final concentration) as described in Materials and Methods and Results. The cells were harvested and assayed for β-glucuronidase activity, expressed as modified Miller units, as described in Materials and Methods. Error bars show standard deviations.
Response of the promoter to inducer.
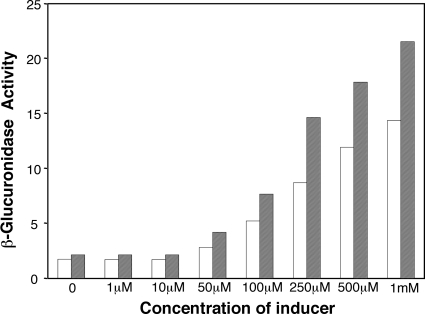
We assessed the response of the promoter to the inducer concentration by using pSRKGm::uidA in A. tumefaciens strain C58. The strain was grown in MG/L to about 107 CFU per ml, the culture was divided into eight subcultures, and IPTG was added to seven of these at concentrations ranging from 1 μM to 1 mM. The eight subcultures were reincubated, samples were taken 4 and 8 h after the addition of inducer, and the collected cells were assayed for β-glucuronidase activity. Cells not exposed to IPTG or exposed to inducer at concentrations of 1 and 10 μM showed background levels of activity at both sampling times (Fig. 4). In cells grown with IPTG at concentrations of 50 μM and higher, the levels of β-glucuronidase activity rose in proportion to the concentration of inducer (Fig. 4).
FIG. 4.
Levels of expression from the lac promoter of the pSRK vector family can be controlled by inducer concentration. A culture of A. tumefaciens C58 harboring pSRKGm::uidA was grown in MG/L medium to early exponential phase and split into eight equal subcultures. IPTG at the indicated final concentrations was added to seven of the subcultures. The eight cultures were sampled after 4 h (open bars) and 8 h (filled bars) of additional incubation and assayed for β-glucuronidase activity, expressed as modified Miller units, as described in Materials and Methods.
Regulating the expression of a transcriptional activator: a test of the system.
In A. tumefaciens, conjugative transfer of its Ti plasmids is controlled by a quorum-sensing system involving the transcriptional activator TraR and its acyl-homoserine lactone (acyl-HSL) ligand, N-(3-oxo-octanoyl)-l-HSL (3-oxo-C8-HSL) (54). The expression of traR, in turn, is controlled by factors called conjugative opines that are produced by the crown gall tumors induced by the pathogen (14, 40, 41). Conjugative transfer thus requires the opine signal from the plant to induce transcription of traR and the quorum-sensing signal to convert TraR to its active form. As with many such regulatory factors, only a few molecules of active, ligand-bound TraR are required to activate the expression of the Ti plasmid tra regulon (49).
Transfer of the classical nopaline-type Ti plasmid pTiC58 is induced by agrocinopines A and B, a family of sugar phosphodiester opines produced by tumors induced by strain C58 (11, 41). These opines are not commercially available, are very difficult to synthesize (29), and are not easily obtained from tumors in useable amounts. To circumvent this problem, we tried expressing traR from a LacIq-regulated lac-based promoter system in two of our expression vectors, pGPO3-I (Table 1) and pKKTR2-I (32). In each vector, lacIq is functional but is not positioned as the gene is in the native lac operon. When tested in A. tumefaciens NTL4(pKPC12) (pTiC58traR::lacZ) (Table 1), in which the resident traR is inactive (41), both clones of the activator conferred high levels of transfer even when the donor cells were grown without IPTG (Table 2). Donors lacking a cloned copy of traR failed to transfer the Ti plasmid at detectable frequencies. Clearly, the lac promoter in these two vectors is not sufficiently repressed by LacI to keep the expression of traR below levels that will activate the tra regulon.
TABLE 2.
Regulation of traR expression from different vectors
| Vectora | traR promoterb | Frequency of transferc
|
|
|---|---|---|---|
| − IPTG | + IPTGd | ||
| None | NA | <10−7 | <10−7 |
| pGP03-I-gnt | Plac | 5.5 × 10−3 | 8.6 × 10−3 |
| pKKTR2-I | Ptrc | 6.4 × 10−3 | 7.9 × 10−3 |
| pSRKGmtraR | Plac | <5 × 10−6 | 5.6 × 10−2 |
Vectors were tested in donor strain A. tumefaciens NTL4(pKPC12).
NA, not applicable.
Expressed as number of transconjugants obtained per input donor.
Supplemented at 1 mM final concentration.
We then tested a clone of traR in pSRKGm, constructed as described in Materials and Methods, in which the activator gene is fused at its 5′ end to the translational start codon of lacZα. Donors harboring this clone, when grown without inducer, did not transfer the Ti plasmid at detectable frequencies (Table 2). However, donors grown with IPTG at 1 mM transferred the Ti plasmid at frequencies similar to that of the Trac mutant pTiC58ΔaccR (∼10−2; 40) (Table 2).
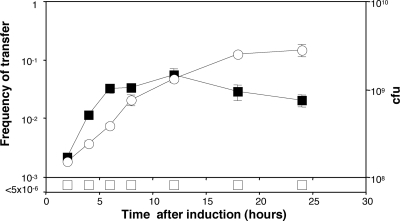
We examined the kinetics of induction and maintenance of transfer of pTiC58 by traR expressed from pSRKGmtraR. Donors harboring pKPC12 and the traR clone were grown to mid-exponential phase. The culture was split in two, IPTG was added to a final concentration of 1 mM to one subculture, and the two cultures were reincubated. Samples of each culture were removed at intervals, and the donors tested for conjugative competence in matings with A. tumefaciens C58C1RS as described in Materials and Methods. Donors incubated without IPTG failed to transfer the Ti plasmid at detectable frequencies at any time tested (Fig. 5). However, donors incubated with IPTG yielded detectable transconjugants at the first time point, 2 h after induction. Conjugative competence, measured as the number of transconjugants arising per donor, increased rapidly for an additional 4 h and remained high for the duration of the experiment. Consistent with the results in our previous report (49), donors continued to transfer the Ti plasmid at high frequency following entry into stationary phase (Fig. 5).
FIG. 5.
traR, expressed from pSRKGm, is tightly controlled in Agrobacterium tumefaciens. A culture of A. tumefaciens NTL4 harboring pKPC12 (traR::lacZ) and pSRKGmtraR was grown in ABM minimal medium to early exponential phase, and the culture was split into two equal subcultures, to one of which IPTG (1 mM final concentration) was added. Incubation was continued, each subculture (▪, with IPTG; □, without IPTG) was sampled at the indicated times, and the cells were tested for donor competency in matings with A. tumefaciens C58C1RS as described in Materials and Methods. Growth of the culture, expressed as CFU per ml (○), was monitored by viable-cell counts of samples taken at each time point. Transfer frequencies are expressed as the number of transconjugants obtained per input donor. Error bars show standard deviations.
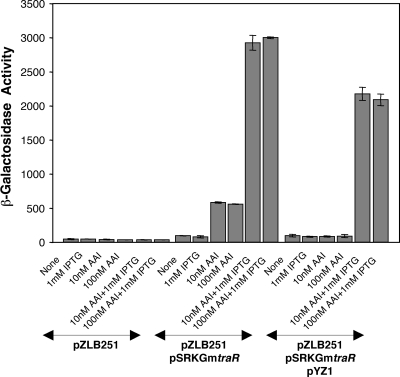
We also assessed the degrees of repression and induction of traR expressed from pSRKGmtraR by monitoring the activation of transcription of a TraR-dependent lacZ fusion carried by pZLB251 (30). Cells harboring only the reporter clone failed to express the lacZ reporter at levels above the background level when grown with IPTG, 3-oxo-C8-HSL, or both (Fig. 6). On the other hand, cells harboring both the reporter clone and pSRKGmtraR strongly expressed the reporter when grown with both IPTG and 3-oxo-C8-HSL. Cells grown only with IPTG failed to express the reporter, while cells grown only with 3-oxo-C8-HSL expressed the reporter at a low but detectable level (Fig. 6).
FIG. 6.
TraM inhibits basal levels of TraR and modulates the activity of induced levels of TraR in controlling Ti plasmid conjugative transfer. Cultures of A. tumefaciens NTL4 harboring one or more plasmids, as indicated, coding for traR (pSRKGmtraR), traM (pYZ1), and a TraR-dependent traA::lacZ reporter fusion (pZLB251) were grown in MG/L medium supplemented with IPTG (1 mM), N-(3-oxo-octanoyl)-l-HSL (AAI; 10 or 100 nM as noted), or both, as indicated, for 4 h; samples were harvested; and the cells were assayed for β-galactosidase activity. Enzyme activity is expressed as Miller units. Error bars show standard deviations.
In this quorum-sensing regulatory system, the activity of TraR is inhibited by an antiactivator, TraM, that also is encoded by the Ti plasmid (15, 20). TraM binds to TraR and prevents the activator from binding to its promoter recognition elements (8, 21, 43, 50). We tested the influence of TraM on the activity of TraR by using the TraR-dependent lacZ reporter. In strains expressing TraM from pYZ1 and TraR from pSRKGmtraR, no reporter activity was detected in cells grown without IPTG or without 3-oxo-C8-HSL (Fig. 6). Significantly, in contrast to cells lacking the traM clone (Fig. 6), no activity was detected in cells grown with the acyl-HSL but without IPTG. Moreover, while cells grown with both IPTG and 3-oxo-C8-HSL strongly expressed the fusion, the level of β-galactosidase activity was reduced by about one-fourth in comparison with the level in cells lacking traM grown under the same conditions (Fig. 6).
Assessing the minimum concentrations of IPTG required to induce Ti plasmid transfer.
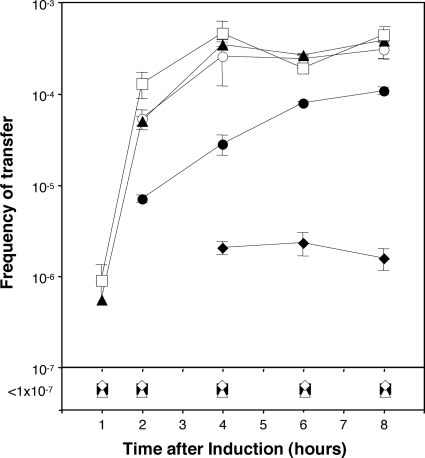
The fact that the expression of genes cloned in the pSRK vectors can be modulated by the concentration of IPTG added to the culture (Fig. 3) allowed us to assess the relative levels of expression of traR required to induce the Ti plasmid transfer system. Donors harboring pKPC12 (pTiC58traR::lacZ) and pSRKGmtraR were grown without inducer or with IPTG at concentrations ranging from 1 μM to 1 mM. At intervals after the addition of inducer, samples were removed from each subculture and donors tested for conjugative competence. Donors grown without inducer or with IPTG at 1 or 10 μM failed to transfer the Ti plasmid at detectable frequencies at any time point tested (Fig. 7). Donors grown with IPTG at 250 μM and higher concentrations transferred their Ti plasmids at maximum efficiency, while donors grown with inducer at 50 and 100 μM transferred the Ti plasmid at low to barely detectable frequencies (Fig. 7). At saturating levels of IPTG, transconjugants were first detected 1 h after the addition of inducer and rose to maximum levels by 4 h after induction. Donors grown with limiting amounts of inducer initiated transfer at later times and did not show a rapid rise in conjugative competence (Fig. 7).
FIG. 7.
Influence of the expression level of TraR on Ti plasmid transfer frequencies. A culture of A. tumefaciens NTL4 harboring pKPC12 (pTiC58traR::lacZ) and pSRKGmtraR was grown in ABM minimal medium to early exponential phase and divided into eight subcultures. One subculture was left unsupplemented (⋄), while IPTG was added to the remaining seven at final concentrations of 1 μM (▪), 10 μM (▵), 50 μM (♦), 100 μM (•), 250 μM (○), 500 μM (▴), and 1 mM (□). Incubation was continued, each culture was sampled at the indicated times, and the cells were tested for donor competency by mating with strain C58C1RS as described in Materials and Methods. Frequency of transfer is expressed as the number of transconjugants recovered per input donor. Error bars show standard deviations.
DISCUSSION
The three pSRK vectors described in this work, all based on the broad-host-range plasmid pBBR1, should prove useful for studies in which induction from very low basal levels of expression is essential for evaluating the roles of the respective gene products in cellular processes. The lac/LacI promoter-regulator system used in these vectors has two advantages over alternative promoters used in other vectors. First, the lac promoter and its repressor function well in a vast array of bacteria and certainly in most members of the Proteobacteriaceae in which the system has been tested. Second, although IPTG is efficiently transported by the LacY symporter, the inducer also is membrane permeable (23) and does not require a dedicated transport system for import into the cells. This being the case, the lac regulatory system can be used in virtually any cell, and the addition of IPTG to the culture medium results in synchronous derepression within the entire population. Not only is the promoter tightly regulated, but the level of induction can be controlled. The observation that expression from the lac promoter in the pSRK vectors can be precisely modulated in response to the concentration of inducer (Fig. 4) should prove particularly valuable and will allow the assessment of the level of expression of the cloned gene required for a given phenotype. In addition, the vectors have an extensive array of cloning sites (Fig. 1) and, when coupled with IPTG and X-Gal, provide a blue-white screen for cloned inserts when tested in an α-complementing strain of E. coli.
We designed the pSRK vectors to accommodate two translation strategies. In the first, cloned genes containing their own ribosomal binding sites can be translated by using such sites from transcripts initiated at the lac promoter. In this case, the gene of interest can be cloned using any of the unique sites in the polylinker. Alternatively, open reading frames can be cloned using the unique NdeI site such that the initiation codon coincides with that of the LacZ α-peptide. This placement ensures proper spacing of the start codon relative to the lac Shine-Delgarno sequence and maximizes the probability that the encoded protein will be correctly and efficiently translated.
The vector backbone itself has a number of useful characteristics. pBBR1 and its derivatives replicate with reasonable stability in a wide variety of gram-negative bacteria (1, 26). In this regard, we successfully introduced one of our vectors into representatives of the alpha-, beta- and gammaproteobacteria, including such model organisms as E. coli, A. tumefaciens, S. meliloti, and C. crescentus. In all but the betaproteobacterium R. solanacearum AW1, the cloned uidA gene was strongly repressed in cells grown in the absence of inducer and showed significant levels of induction in cells grown with IPTG (Fig. 2 and 3). We have no explanation as to why the uidA reporter was not expressed in R. solanacearum. However, a cursory examination indicated that the reporter vector is stably maintained in this bacterium (data not shown). The elements are small, facilitating efficient gene cloning, and the plasmids replicate at modest copy number, probably around 5 to 10 copies per cell (13, 26). Although of unknown classification, pBBR1 and its derivatives, including the pSRK series, are compatible with other broad-host-range vectors of the IncP, IncQ, and IncW groups, making them particularly useful in experiments that require several plasmids to be maintained in the same cell. Finally, like the rest of the pBBR1MCS family, the three pSRK vectors can be transferred to hosts of interest by conjugative mobilization using E. coli donors, such as strains S17-1 (47) and WM5979 (Table 1). In cases in which the pBBR1 backbone may not be suitable, the expression unit composed of lacIq, the lac promoter-operator complex, and lacZα with its polylinker can be excised intact from any of the three plasmids with BstBI and transferred to other appropriate vectors.
Our pSRK vectors have proved very useful in our studies of the quorum-sensing activator TraR. With only a few copies of TraR being required per cell (49), we used the exceptionally low basal levels of expression of these vectors to examine the relationship between the expression of the activator and the development of Ti plasmid conjugative competence, a phenotype directly controlled by this quorum-sensing transcription factor (41). When in trans to a Ti plasmid mutant for its own traR, in the absence of inducer the recombinant clone did not express the activator at levels high enough to phenotypically complement the mutation (Table 2).
We used this tight regulation of TraR to assess the relative levels of expression of the activator required to induce the Ti plasmid transfer system. Growth with IPTG at concentrations of 250 μM and higher yielded rapid induction to full levels of transfer (Fig. 7), while growth with concentrations of inducer between 50 and 100 μM caused the induction of transfer at considerably lower frequencies. Concentrations of IPTG of 10 μM or lower were not sufficient to activate the transfer system in a detectable number of donors (Fig. 7). As measured by β-glucuronidase activities, growth with IPTG at concentrations between 100 and 250 μM resulted in levels of induction one-third to one-half that observed in fully induced cells (Fig. 4). These results suggest that only a few molecules of active TraR are required to fully induce the conjugative transfer system, a conclusion consistent with the results of our previous studies on the amount of the acyl-HSL quormone necessary to trigger the quorum-sensing system (49).
We also used the vector to assess the role of the antiactivator TraM in controlling the functional levels of TraR. Despite the tight regulation from the lac promoter, in the absence of IPTG traR cloned in pSRKGmtraR is expressed at a basal level sufficient to weakly activate the TraR-dependent lacZ reporter fusion (Fig. 6). However, the coexpression of a cloned copy of traM from its own promoter abolished this low level of acyl-HSL-dependent activation. Moreover, in the absence of traM, the induction of traR with IPTG resulted in high levels of expression of the reporter (Fig. 7), while the coexpression of traM reduced this high-level TraR-dependent expression of the reporter by about one-quarter. Taken together, these two sets of results indicate that in the absence of TraM, even very small amounts of TraR can activate a promoter controlling the Ti plasmid conjugative transfer system, a conclusion consistent with the observation that Ti plasmids in which traM is mutant are constitutive for transfer even though the native traR remains repressed (15, 20). Clearly, TraM serves to prevent this basal level of expression of traR from prematurely activating transfer.
The induction of Ti plasmid transfer is initiated by conjugative opines, novel metabolites produced by the crown gall tumors induced by the pathogenic agrobacteria (11, 40). Most of these natural products are not commercially available, and they can be difficult to synthesize (29), making studies on the regulation of transfer problematic. Having traR cloned behind a very tightly regulated promoter has allowed us to substitute IPTG for the conjugative opine of pTiC58 in our studies of the interactions between opine-mediated control and its downstream quorum-sensing system (49). The vectors should be equally useful in studies with other organisms that require tight regulation and controllable promoters.
Acknowledgments
Portions of this work were supported by grant no. R01 GM52465 from the NIH to S.K.F. J.G. was supported by grant no. R01 AI48499 to R.M.R. II.
We thank Yinping Qin, Vandana Chakravartty, Michael Barnhart, and Margaret Wetzel for helpful discussions.
Footnotes
Published ahead of print on 7 July 2008.
REFERENCES
- 1.Antoine, R., and C. Locht. 1992. Isolation and molecular characterization of a novel broad-host-range plasmid from Bordetella bronchiseptica with sequence similarities to plasmids from gram-positive organisms. Mol. Microbiol. 6:1785-1799. [DOI] [PubMed] [Google Scholar]
- 2.Bagdasarian, M. M., E. Amann, R. Lurz, B. Rückert, and M. Bagdasarian. 1983. Activity of the hybrid trp-lac (tac) promoter of Escherichia coli in Pseudomonas putida. Construction of broad-host-range, controlled-expression vectors. Gene 26:273-282. [DOI] [PubMed] [Google Scholar]
- 3.Beringer, J. 1974. R factor transfer in Rhizobium leguminosarum. J. Gen. Microbiol. 84:188-198. [DOI] [PubMed] [Google Scholar]
- 4.Brosius, J., M. Erfle, and J. Storella. 1985. Spacing of the −10 and −35 regions in the tac promoter. Effect on its in vivo activity. J. Biol. Chem. 260:3539-3541. [PubMed] [Google Scholar]
- 5.Cangelosi, G. A., E. A. Best, G. Martinetti, and E. W. Nester. 1991. Genetic analysis of Agrobacterium. Methods Enzymol. 204:384-397. [DOI] [PubMed] [Google Scholar]
- 6.Charnay, P., A. Louise, A. Fritsch, D. Perrin, and P. Tiollais. 1979. Bacteriophage lambda-E. coli K12 vector-host system for gene cloning and expression under lactose promoter control. II. DNA fragment insertion at the vicinity of the lacUV5 promoter. Mol. Gen. Genet. 170:171-178. [DOI] [PubMed] [Google Scholar]
- 7.Chen, C.-Y., and S. C. Winans. 1991. Controlled expression of the transcriptional activator gene virG in Agrobacterium tumefaciens by using the Escherichia coli lac promoter. J. Bacteriol. 173:1139-1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, G., P. D. Jeffrey, C. Fuqua, Y. Shi, and L. Chen. 2007. Structural basis for antiactivation in bacterial quorum-sensing. Proc. Natl. Acad. Sci. USA 104:16474-16479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chilton, M.-D., T. C. Currier, S. K. Farrand, A. J. Bendich, M. P. Gordon, and E. W. Nester. 1974. Agrobacterium tumefaciens DNA and PS8 bacteriophage DNA not detected in crown gall tumors. Proc. Natl. Acad. Sci. USA 71:3672-3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Boer, H. A., L. J. Comstock, and M. Vasser. 1983. The tac promoter: a functional hybrid derived from the trp and lac promoters. Proc. Natl. Acad. Sci. USA 80:21-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ellis, J. G., A. Kerr, A. Petit, and J. Tempé. 1982. Conjugal transfer of nopaline and agropine Ti plasmids: the role of agrocinopines. Mol. Gen. Genet. 186:269-273. [Google Scholar]
- 12.Ely, B. 1991. Genetics of Caulobacter crescentus. Methods Enzymol. 204:372-384. [DOI] [PubMed] [Google Scholar]
- 13.Elzer, P. H., R. W. Phillips, M. E. Kovach, K. M. Peterson, and R. M. Roop II. 1994. Characterization and genetic complementation of a Brucella abortus high-temperature-requirement A (htrA) deletion mutant. Infect. Immun. 62:4135-4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuqua, W. C., and S. C. Winans. 1994. A LuxR-LuxI-type regulatory system activates Agrobacterium Ti plasmid conjugal transfer in the presence of a plant tumor metabolite. J. Bacteriol. 176:2796-2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuqua, W. C., M. Burbea, and S. C. Winans. 1995. Activity of the Agrobacterium Ti plasmid conjugal transfer regulator is inhibited by the product of the traM gene. J. Bacteriol. 177:1367-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fürste, J. P., W. Pansegrau, R. Frank, H. Blöcker, P. Scholz, M. Bagdasarian, and E. Lanka. 1986. Molecular cloning of the plasmid RP4 primase region in a multi-host-range tacP expression vector. Gene 48:119-131. [DOI] [PubMed] [Google Scholar]
- 17.Gao, P. 2000. Genetic characterization of the multimerization domain of the quorum-sensing transcriptional activator TraR of Agrobacterium tumefaciens strain C58 by C-terminal deletion analysis. M.S. thesis. University of Illinois at Urbana-Champaign, Urbana, IL.
- 18.Graupner, S., and W. Wackernagel. 2000. A broad-host-range vector series including a Ptac test plasmid and its application in the expression of the dod gene of Serratia marcescens (coding for ribulose-5-phosphate 3-epimerase) in Pseudomonas stutzeri. Biomol. Eng. 17:11-16. [DOI] [PubMed] [Google Scholar]
- 19.Guzman, L. M., D. Belin, M. J. Carson, and J. Beckwith. 1995. Tight regulation, modulation, and high-level expression by vectors containing the PBAD promoter. J. Bacteriol. 177:4121-4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hwang, I., D. M. Cook, and S. K. Farrand. 1995. A new regulatory element modulates homoserine lactone-mediated autoinduction of Ti plasmid conjugal transfer. J. Bacterial. 177:449-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hwang, I., A. Smyth, Z.-Q. Luo, and S. K. Farrand. 1999. Modulating quorum-sensing by antiactivation: TraM interacts with TraR to inhibit activation of Ti plasmid conjugal transfer genes. Mol. Microbiol. 34:282-294. [DOI] [PubMed] [Google Scholar]
- 22.Jefferson, R. A., S. M. Burgess, and D. Hirsh. 1986. β-Glucuronidase from Escherichia coli as a gene-fusion marker. Proc. Natl. Acad. Sci. USA 83:8447-8451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jensen, P. R., H. V. Westerhoff, and O. Michelsen. 1993. The use of lac-type promoters in control analysis. Eur. J. Biochem. 211:181-191. [DOI] [PubMed] [Google Scholar]
- 24.Kasai, Y., J. Inoue, and S. Harayama. 2001. The TOL plasmid pWW0 xylN gene product from Pseudomonas putida is involved in m-xylene uptake. J. Bacteriol. 183:6662-6666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim, M. N., H. H. Park, W. K. Lim, and H. J. Shin. 2005. Construction and comparison of Escherichia coli whole-cell biosensors capable of detecting aromatic compounds. Microb. Methods 60:235-245. [DOI] [PubMed] [Google Scholar]
- 26.Kovach, M. E., P. H. Elzer, D. S. Hill, G. T. Robertson, M. A. Farris, R. M. Roop II, and K. M. Peterson. 1995. Four new derivatives of the broad host range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175-176. [DOI] [PubMed] [Google Scholar]
- 27.Krämer, H., M. Niemöller, M. Amouyal, B. Revet, B. von Wilcken-Bergmann, and B. Müller-Hill. 1987. Lac repressor forms loops with linear DNA carrying two suitably spaced lac operators. EMBO J. 6:1481-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lai, F., G. G. Schurig, and S. M. Boyle. 1990. Electroporation of a suicide plasmid bearing a transposon into Brucella abortus. Microb. Pathog. 9:363-368. [DOI] [PubMed] [Google Scholar]
- 29.Lindberg, M., and T. Norberg. 1988. Synthesis of sucrose 4′-(l-arabinose-2 yl phosphate) (agrocinopine A) using an arabinose 2-H-phosphonate intermediate. J. Carbohydr. Chem. 7:749-755. [Google Scholar]
- 30.Luo, Z.-Q., and S. K. Farrand. 1999. Signal-dependent DNA binding and functional domains of the quorum-sensing activator TraR as identified by repressor activity. Proc. Natl. Acad. Sci. USA 96:9009-9014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luo, Z.-Q., T. E. Clemente, and S. K. Farrand. 2001. Construction of a derivative of Agrobacterium tumefaciens C58 that does not mutate to tetracycline resistance. Mol. Plant-Microbe Interact. 14:98-103. [DOI] [PubMed] [Google Scholar]
- 32.Luo, Z.-Q., Y. Qin, and S. K. Farrand. 2000. The antiactivator TraM interferes with the autoinducer-dependent binding of TraR to DNA by interacting with the C-terminal region of the quorum-sensing activator. J. Biol. Chem. 275:7713-7722. [DOI] [PubMed] [Google Scholar]
- 33.Meisenzahl, A. C., L. Shapiro, and U. Jenal. 1997. Isolation and characterization of a xylose-dependent promoter from Caulobacter crescentus. J. Bacteriol. 179:592-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Melton, D. A., P. A. Krieg, M. R. Rebagliati, T. Maniatis, K. Zinn, and M. R. Green. 1984. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 12:7035-7036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mermod, N., J. L. Ramos, P. R. Lehrbach, and K. N. Timmis. 1986. Vector for regulated expression of cloned genes in a wide range of gram-negative bacteria. J. Bacteriol. 167:447-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller, J. H. 1972. Assay of β-galactosidase activity, p. 352-355. In J. H. Miller (ed.), Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 37.Morgan-Kiss, R. M., C. Walder, and J. E. Cronan, Jr. 2002. Long-term and homogeneous regulation of the Escherichia coli araBAD promoter by use of a lactose transporter with relaxed specificity. Proc. Natl. Acad. Sci. USA 28:7373-7377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oehler, S., E. R. Eismann, H. Krämer, and B. Müller-Hill. 1990. The three operators of the lac operon cooperate in repression. EMBO J. 9:973-979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Picaud, S., M. E. Olsson, and P. E. Brodelius. 2007. Improved conditions for production of recombinant plant sesquiterpene synthases in Escherichia coli. Protein Expr. Purif. 51:71-79. [DOI] [PubMed] [Google Scholar]
- 40.Piper, K. R., and S. K. Farrand. 2000. Quorum sensing but not autoinduction of Ti plasmid conjugal transfer requires control by the opine regulon and the antiactivator TraM. J. Bacteriol. 182:1080-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Piper, K. R., S. B. von Bodman, I. Hwang, and S. K. Farrand. 1999. Hierarchical gene regulatory systems arising from fortuitous gene associations: controlling quorum sensing by the opine regulon in Agrobacterium. Mol. Microbiol. 32:1077-1089. [DOI] [PubMed] [Google Scholar]
- 42.Pourcel, C., C. C. Marchal, A. Louise, F. A. Fritsch, and P. Tiollais. 1979. Bacteriophage lambda-E. coli K12 vector-host system for gene cloning and expression under lactose promoter control. I. DNA fragment insertion at the lacZ EcoRI restriction site. Mol. Gen. Genet. 170:161-169. [DOI] [PubMed] [Google Scholar]
- 43.Qin, Y., S. Su, and S. K. Farrand. 2007. Molecular basis of transcriptional antiactivation. TraM disrupts the TraR-DNA complex through stepwise interactions. J. Biol. Chem. 282:19979-19991. [DOI] [PubMed] [Google Scholar]
- 44.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 45.Schaedler, R. W., R. Dubos, and R. Costello. 1965. The development of the bacterial flora in the gastrointestinal tract of mice. J. Exp. Med. 122:59-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Siegele, D. A., and J. C. Hu. 1997. Gene expression from plasmids containing the araBAD promoter at subsaturating inducer concentrations represents mixed populations. Proc. Natl. Acad. Sci. USA 94:8168-81721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simon, R., U. Priefer, and A. Pühler. 1983. Vector plasmids for in vivo and in vitro manipulations of gram-negative bacteria, p. 98-106. In A. Pühler (ed.), Genetics of the bacteria-plant interaction. Springer-Verlag, Berlin, Germany.
- 48.Stephens, C., B. Christen, K. Watanabe, T. Fuchs, and U. Jenal. 2007. Regulation of d-xylose metabolism in Caulobacter crescentus by a LacI-type repressor. J. Bacteriol. 189:8828-8834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Su, S., S. R. Khan, and S. K. Farrand. 2008. Induction and loss of Ti plasmid conjugative competence in response to the acyl-homoserine lactone quorum-sensing system. J. Bacteriol. 190:4398-4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Swiderska, A., A. K. Berndtson, M. R. Cha, L. Li, G. M. Beaudoin III, J. Zhu, and C. Fuqua. 2001. Inhibition of the Agrobacterium tumefaciens TraR quorum-sensing regulator. Interactions with the TraM anti-activator. J. Biol. Chem. 276:49449-49458. [DOI] [PubMed] [Google Scholar]
- 51.Tabor, S., and C. C. Richardson. 1985. A bacteriophage T7 RNA polymerase-promoter system for controlled exclusive expression of specific genes. Proc. Natl. Acad. Sci. USA 82:1074-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vincent, J. M. 1970. A manual for the practical study of root-nodule bacteria. Blackwell Scientific Publications, Ltd., Oxford, United Kingdom.
- 53.Watson, B., T. C. Currier, M. P. Gordon, M.-D. Chilton, and E. W. Nester. 1975. Plasmid required for virulence of Agrobacterium tumefaciens. J. Bacteriol. 123:255-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.White, C. E., and S. C. Winans. 2007. Cell-cell communication in the plant pathogen Agrobacterium tumefaciens. Philos. Trans. R. Soc. Lond. B. 362:1135-1148. [DOI] [PMC free article] [PubMed] [Google Scholar]