Abstract
Pseudomonas aeruginosa is an important opportunistic pathogen that causes infections that can be extremely difficult to treat due to its high intrinsic antibiotic resistance and broad repertoire of virulence factors, both of which are highly regulated. It is demonstrated here that the psrA gene, encoding a transcriptional regulator, was upregulated in response to subinhibitory concentrations of cationic antimicrobial peptides. Compared to the wild type and the complemented mutant, a P. aeruginosa PAO1 psrA::Tn5 mutant displayed intrinsic supersusceptibility to polymyxin B, a last-resort antimicrobial used against multidrug-resistant infections, and the bovine neutrophil antimicrobial peptide indolicidin; this supersusceptibility phenotype correlated with increased outer membrane permeabilization by these agents. The psrA mutant was also defective in simple biofilm formation, rapid attachment, and swarming motility, all of which could be complemented by the cloned psrA gene. The role of PsrA in global gene regulation was studied by comparing the psrA mutant to the wild type by microarray analysis, demonstrating that 178 genes were up- or downregulated ≥2-fold (P ≤ 0.05). Dysregulated genes included those encoding certain known PsrA targets, those encoding the type III secretion apparatus and effectors, adhesion and motility genes, and a variety of metabolic, energy metabolism, and outer membrane permeability genes. This suggests that PsrA might be a key regulator of antimicrobial peptide resistance and virulence.
The opportunistic gram-negative bacterium Pseudomonas aeruginosa is the most prevalent cause of life-threatening infections in the lungs of cystic fibrosis patients (37) and the third leading cause of severe hospital-acquired infections (24). P. aeruginosa can cause substantial morbidity and mortality, due in part to its wide repertoire of virulence factors, and it is extremely difficult to combat due to high intrinsic antibiotic resistance (14). The current treatment of P. aeruginosa infections often involves potent β-lactams, aminoglycosides, or fluoroquinolones, or a combination thereof, but resistance can arise nevertheless (14), and there has been a recent emergence of P. aeruginosa clinical isolates resistant to virtually all antibiotics. When multidrug resistance occurs, polymyxins have become a drug of last resort (21). Thus, it is important to understand the basis for resistance in this organism and its interrelationship with pathogenesis. For example, there is a well-noted discrepancy between in vitro antibiotic susceptibility and the clinical success of particular antibiotics for P. aeruginosa (12, 14). One possible basis for this is the induction of resistance mechanisms due to environmental factors, a process termed adaptive resistance, which is differentiated from acquired or mutational resistance because it reverts upon removal of the antibiotic.
Structurally diverse cationic antimicrobial peptides are part of the innate immune system of complex organisms and can possess direct antimicrobial activity and/or a profound ability to modulate innate immunity (16). Improved synthetic derivatives demonstrate considerable promise against infections by multiply antibiotic-resistant bacteria (13, 28). However, P. aeruginosa is able to sense the presence of peptides and to become adaptively resistant, for example, through peptide-mediated regulation of the arnBCADTEF (pmrHFIJKLM) LPS modification operon, independently of the PmrA-PmrB or PhoP-PhoQ two-component regulatory system (31, 32).
Virulence is similarly complex, representing a series of complex adaptations to growth in a host organism, including biofilm formation, swarming motility, and quorum sensing. For example, in P. aeruginosa, motility is important for biofilm formation, virulence, and colonization of different niches (17, 35). There are three basic types of motility. Type IV pili extend and retract to promote twitching motility on solid surfaces, whereas flagella power swimming motility in dilute media. On the other hand, swarming motility appears to be a coordinated and complex adaptation to moderately viscous environments and involves a number of factors that include flagella, type IV pili, quorum sensing, rhamnolipids, etc. (33, 34). There is considerable overlap in the genes utilized in swarming motility and biofilm formation (4, 34, 39), both of which have been proposed to contribute to disease pathogenesis (36) and to lead to increased resistance to several antibiotics (33, 35).
In this study, it was demonstrated that antimicrobial peptides transcriptionally upregulated the expression of psrA, a previously documented Pseudomonas regulator of RpoS and the type III secretion system, but one for which the activating signals were unknown (19, 20, 38). Detailed phenotypic studies indicated that PsrA regulated polymyxin and antimicrobial peptide resistance, motility, and biofilm formation. Microarray analysis of the psrA mutant provided insight into the basis for these observed phenotypes.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains and plasmids used in this study are described in Table 1. Cultures were routinely grown in Luria-Bertani (LB) broth containing 1.8% (wt/vol) Difco agar (Becton Dickinson Co., Oakville, Ontario, Canada), when appropriate. The defined medium used was BM2-glucose minimal medium [62 mM potassium phosphate buffer (pH 7), 7 mM (NH4)2SO4, 10 μM FeSO4, 0.4% (wt/vol) glucose] containing 2 mM (high) MgSO4. Antibiotics for selection were used at the following concentrations: tetracycline, 50 to 100 μg/ml for P. aeruginosa; ampicillin, 100 μg/ml for Escherichia coli; carbenicillin, 500 μg/ml for P. aeruginosa; and gentamicin, 30 μg/ml for P. aeruginosa and 15 μg/ml for E. coli.
TABLE 1.
P. aeruginosa strains and plasmids used in this study
| Strain or plasmid | Genotype or characteristicsa | Source or referenceb |
|---|---|---|
| Strains | ||
| P. aeruginosa strains | ||
| WT | WT P. aeruginosa PAO1; H103 | Lab collection |
| UW WT | UW WT P. aeruginosa PAO1 | UW (15) |
| UW-psrA | psrA::ISlacZ/hah-Tcr; insertion at position 46 (702 bp) in psrA; derived from UW WT | UW (15) |
| psrA mutant | psrA::ISlacZ/hah-Tcr; H103 background; Tcr | This study |
| psrA (Tn7-psrA+) | psrA mutant with Tn7-psrA+ integrated; Tcr Gmr | This study |
| PA14 | Wild-type P. aeruginosa PA14 | 22 |
| coxA | 05_2::A11; derived from PA14 | 22 |
| etfA | 04_4::A12; derived from PA14 | 22 |
| fhp | 09_1::F11; derived from PA14 | 22 |
| mexC | 01_4::H2; derived from PA14 | 22 |
| pprB | 08_3::C3; derived from PA14 | 22 |
| rhlG | 05_3::A8; derived from PA14 | 22 |
| flp | 14_1::F4; derived from PA14 | 22 |
| rcpA | 01_2::B12; derived from PA14 | 22 |
| tadA | 01_2::A7; derived from PA14 | 22 |
| tadB | 04_2::H5; derived from PA14 | 22 |
| wbpM | 03_4::E4; derived from PA14 | 22 |
| wzz | 06_1::F2l; derived from PA14 | 22 |
| PA1883 (homolog) | 12_1::A7; derived from PA14 | 22 |
| wbpI | wbpI::ISlacZ/hah-Tcr; insertion 807 (1,065 bp) | 15 |
| wbpL | wbpL::ISlacZ/hah-Tcr; insertion 302 (1,020 bp) | 15 |
| E. coli strain | ||
| TOP10 | F−mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacX74 recA1 araΔ139 Δ(ara-leu)7697 galU galK rpsL (Strr) endA1 nupG | Invitrogen |
| Plasmids | ||
| pCR-Blunt II-TOPO | PCR cloning vector; Kanr | Invitrogen |
| pCR-psrA+ | pCR-BluntII-TOPO harboring 1.12-kb psrA amplicon | This study |
| pUC18-mini-Tn7T-Gm | Suicide plasmid; Gmr Ampr | H. Schweizer (8) |
| pUC-Tn7-psrA+ | pUC18-mini-Tn7T-Gm with 1.12-kb psrA fragment from pCR-psrA | This study |
| pTNS2 | Transposition helper plasmid; Ampr | H. Schweizer (8) |
Antibiotic resistance phenotypes are indicated as follows: Ampr, ampicillin resistance for E. coli and carbenicillin resistance for P. aeruginosa; Gmr, gentamicin resistance; Kanr, kanamycin resistance; Tcr, tetracycline resistance.
UW, University Washington.
Genetic manipulations.
Routine molecular biology techniques were performed according to standard protocols (1). Primers were synthesized by AlphaDNA Inc. (Montreal, Quebec, Canada), and their sequences are available upon request. Plasmid DNA was isolated using QIAprep spin miniprep kits (Qiagen Inc., Mississauga, Ontario, Canada), and agarose gel fragments were purified using a QIAquick gel extraction kit (Qiagen). T4 DNA ligase was from Invitrogen (Burlington, Ontario, Canada), and restriction endonucleases were from New England Biolabs (Mississauga, Ontario, Canada).
Mobilizing the UW-psrA transposon mutation into a new PAO1 background.
The UW-psrA mutation (confirmed to be correct by PCR and sequencing of the junctions of the transposon mutation) was first transferred into our laboratory wild-type (WT) P. aeruginosa PAO1 strain H103 as described previously (7). Genomic DNA was isolated from the UW-psrA mutant by the hexadecyltrimethyl ammonium bromide method (1). Approximately 1 microgram of this DNA (which contained the tetracycline resistance-encoding transposon ISlacZ/hah-Tc insertion in psrA) was electroporated into WT H103. Cells were allowed to recover for 1 hour at 37°C and then were plated onto LB agar plates containing 100 μg/ml tetracycline. After 18 h of growth at 37°C, tetracycline-resistant transformants were then analyzed by colony PCR, using a transposon-specific primer and a custom gene primer with Taq polymerase (Invitrogen), to verify that the transposon was correctly inserted into psrA. The new psrA mutant allowed better analysis of motility-related phenotypes (H103 is swarm positive under our conditions [see below]) and was therefore used for all experiments reported in this study.
Genetic complementation of psrA.
Forward and reverse primers for psrA were designed from the P. aeruginosa PAO1 genome sequence (www.pseudomonas.com), using Primer3 (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi), to clone psrA together with 347 bp of upstream DNA with the native promoter and 67 bp of downstream DNA. Amplification of psrA from P. aeruginosa WT H103 genomic DNA was carried out using high-fidelity Platinum Pfx DNA polymerase (Invitrogen) with the primers PsrA-L (5′-CGGAGCACAGAGAAAGGAGA-3′) and PsrA-R (5′-GACTTGAAGCCGAGTTCCTG-3′). The resulting PCR product was then cleaned (Qiagen PCR purification kit), and the amplicons were cloned into pCR-Blunt II-TOPO, using a Zero Blunt TOPO PCR cloning kit (Invitrogen), and transformed into One Shot TOP10 cells (Invitrogen), creating pCR-psrA+. An NsiI fragment containing the psrA gene was excised from pCR-psrA+ and subcloned into pUC18mini-Tn7T-Gm, generating pUC18-mini-Tn7T-Gm-psrA+. pUC18mini-Tn7T-Gm-psrA+ was coelectroporated with pTNS2 into the psrA mutant, using sucrose electroporation (7). As previously described, gentamicin-resistant transformants were analyzed by colony PCR, using primers PglmS-up and PTn7L, to determine the correct transposon integration of mini-Tn7 into the chromosome (8).
Killing curves.
Overnight P. aeruginosa cultures were diluted 1/100 in fresh BM2-glucose minimal medium containing 2 mM Mg2+. Upon reaching the mid-logarithmic phase of growth (optical density at 600 nm [OD600], ∼0.5), 1 ml of each culture was pelleted, resuspended in 1 ml 1× BM2 salts (buffer), and diluted 1/10 in prewarmed 1× BM2 salts. Killing was then initiated by the addition of 1 μg/ml polymyxin B sulfate (Sigma, St. Louis, MO) or 64 μg/ml indolicidin for analysis of intrinsic resistance. Flasks were shaken at 37°C, and aliquots were withdrawn at the designated times to assay for survivors by plating diluted 100-μl aliquots onto LB agar plates.
Outer membrane permeabilization assays.
P. aeruginosa outer membrane barrier function and the efficiency of the self-promoted uptake route were determined using the 1-N-phenyl naphthylamine (NPN) assay (23). Cultures were grown to mid-logarithmic phase in BM2-glucose minimal medium containing 2 mM MgSO4. The cells were then harvested, washed, and resuspended to an OD600 of 0.5 in 5 mM HEPES, pH 7.0, 5 mM glucose, and 5 μM carbonyl cyanide m-chlorophenylhydrazone (CCCP). Two milliliters of each bacterial suspension was placed in a quartz cuvette with a magnetic stir bar. NPN (Sigma) was then added to the cuvette at a concentration of 10 μM, and the fluorescence (baseline) was measured using an LS-50B fluorescence spectrophotometer (Perkin-Elmer, Inc., Waltham, MA) (excitation and emission wavelengths of 350 nm and 420 nm, respectively). Indolicidin peptide was then added to initiate the assay, and the increase in fluorescence due to peptide-mediated entry of the hydrophobic fluorophor NPN into the membrane was measured until a stable signal was observed (indicating that additional partitioning of NPN into the membrane had stopped).
Biofilm and attachment assays.
Static microtiter biofilm assays were performed as described previously (34). Overnight LB cultures were diluted 1/1,000 in fresh LB broth, and 100 μl was inoculated into wells of a 96-well polystyrene round-bottomed microtiter plate (Becton Dickinson). For PA14 strains, overnight cultures were diluted 1:500 in BM2-glucose medium containing 2 mM MgSO4 and 0.5% Casamino Acids. Plates were then incubated at 37°C without shaking. At the specified time point, medium and planktonic cells were discarded, and the wells were washed three times with distilled H2O. Surface-attached bacteria were then stained with 0.1% (wt/vol) crystal violet for 20 min, followed by ethanol solubilization of crystal violet-stained cells for quantification of A600.
Rapid attachment was assayed as described previously, with slight modifications (25). Overnight cultures were first diluted 1/100 in fresh LB medium and grown to an OD600 of ∼0.5, and 100 μl was added to each well of a 96-well polystyrene microtiter plate. Cells were allowed to attach for 30 min at room temperature prior to being stained with crystal violet as described above.
Motility assays.
Swimming motility was assayed on BM2-glucose plates containing 0.3% (wt/vol) agar. Swarming was assayed on modified BM2-glucose plates containing 0.5% (wt/vol) agar and with 0.5% (wt/vol) Casamino Acids (or 0.1% for PA14 strains) substituted for 7 mM (NH4)2SO4 (34). Swimming and swarming motilities were assayed by inoculating 1 μl of mid-logarithmic-phase liquid culture grown in BM2-glucose containing 2 mM Mg2+ onto the motility plate, incubating the plate for 16 to 18 h at 37°C, and measuring motility zone diameters. Twitching motility was assessed by toothpick inoculating cells from agar plates into thin LB-1% agar plates, down to the agar-plastic interface, and measuring the twitch zone diameter after 24 and 48 h of incubation at 37°C.
Growth curves.
Overnight cultures were grown in BM2-glucose containing 2 mM Mg2+, and 0.1 ml was diluted into 10 ml fresh medium. Flasks were shaken at 37°C, and aliquots were withdrawn periodically to determine the cell density as the OD600. Similarly, determination of planktonic growth at 37°C in static 96-well polystyrene microtiter plates (simple biofilm conditions) was assayed by monitoring the OD600.
Microarray analysis.
Detailed technical descriptions of microarray analyses were provided previously (30). In overview, for each strain, microarray analysis involved five independent cultures. P. aeruginosa WT and psrA mutant cultures were grown with shaking in BM2-glucose medium plus 2 mM MgSO4 at 37°C for 18 h and then diluted 1 in 100 in fresh medium. Cultures were grown at 37°C with shaking (250 rpm) to the mid-logarithmic phase of growth (OD600 = 0.5), and then total RNA was isolated using RNeasy Midi columns (Qiagen). Contaminating genomic DNA was removed by treatment with a DNA-free kit (Ambion Inc., Austin, TX). RNA was stored at −80°C with 0.2 U/μl of SUPERase-In RNase inhibitor (Ambion Inc.). RNA quality was assessed by agarose gel electrophoresis and spectrophotometrically. RNA was converted to cDNA, hybridized, and analyzed as previously described. P. aeruginosa PAO1 microarray slides were provided by The Institute for Genomic Research (TIGR) Pathogenic Functional Genomics Resource Center (http://pfgrc.tigr.org/). Images of slides were quantified using ImaGene 6.0, standard edition, software (BioDiscovery, Inc., El Segundo, CA). ArrayPipe, version 1.7, was used for assessment of slide quality, normalization, detection of differential gene expression, and statistical analysis, using available genome annotation from www.pseudomonas.com. Data analysis of DNA microarrays was carried out as previously described (30). The five biological replicates were averaged to obtain overall changes for the psrA mutant relative to the WT, and two-sided one-sample Student's t test was applied to determine significant changes in gene expression. Changes of ≥2-fold with a Student's t test P value of ≤0.05 were used as the cutoffs for reporting expression changes.
Real-time qPCR.
Total RNA was isolated, using RNeasy Midi columns (Qiagen), from P. aeruginosa grown in BM2-glucose minimal medium containing 2 mM Mg2+, with or without 2 μg/ml indolicidin, to the mid-logarithmic phase of growth. DNase treatment of RNA samples, cDNA synthesis, and real-time quantitative PCR (qPCR) were carried out as described previously (30). cDNA was diluted 1/1,000, and 1 μl was used as a template for real-time PCR, using 1× SYBR green PCR master mix (Applied Biosystems, Foster, CA) and an ABI Prism 7000 instrument (Applied Biosystems). Forward and reverse primers were designed internal to psrA, using PrimerExpress (Applied Biosystems). All reactions were normalized to the rpsL gene, encoding the 30S ribosomal protein S12.
Microarray accession number.
The MIAMExpress accession number is E-FPMI-14.
RESULTS
Activation of psrA transcription in response to antimicrobial peptides.
Preliminary studies indicated that the psrA (PA3006) gene was induced 2.5-fold (P < 0.05) by a subinhibitory concentration (2 μg/ml; one-eighth the MIC) of the bovine antimicrobial peptide indolicidin (11, 31), based on microarray analyses of cultures grown to mid-logarithmic phase under high-Mg2+ (2 mM MgSO4) conditions. Independent real-time qPCR experiments confirmed that the transcription of psrA was upregulated 3.0- ± 0.3-fold in the presence of indolicidin, with similar induction by the indolicidin variant peptide CP11CN (data not shown). As a positive control, in agreement with previous studies (31), arnB (pmrF; PA3552), the first gene of the aminoarabinose lipopolysaccharide (LPS) modification operon (PA3552 to -9), was confirmed here by qPCR to be upregulated 54.3- ± 8.1-fold under these conditions, and microarray data confirmed that the indolicidin-regulated pmrA-pmrB operon was also upregulated in the presence of a subinhibitory concentration of indolicidin, but the Mg2+-regulated oprH-phoP-phoQ operon was not (data not shown).
Contribution of psrA to intrinsic antimicrobial peptide and polymyxin B resistance.
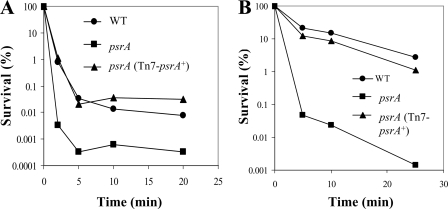
The influence of the psrA gene on intrinsic resistance to peptides was examined. Intrinsic resistance was assayed by growing cells under high (2 mM)-Mg2+ conditions to suppress the possibility of induction by limiting Mg2+. The psrA mutant exhibited an increased susceptibility to the polycationic lipopeptide polymyxin B, as shown by kill curves (Fig. 1A). This supersusceptibility phenotype could be complemented to WT polymyxin B susceptibility by introducing a single WT psrA+ allele into the chromosome of the mutant, using mini-Tn7 integration technology (Fig. 1A). Similarly, the psrA mutant demonstrated supersusceptibility to the cationic antimicrobial peptide indolicidin, which could be complemented back to WT susceptibility (Fig. 1B). Thus, the psrA gene product appeared to be essential for normal intrinsic resistance.
FIG. 1.
Intrinsic polymyxin B and antimicrobial peptide supersusceptibility in psrA mutants. Intrinsic sensitivity was analyzed by growing cells to mid-log phase in BM2-glucose with 2 mM Mg2+, exposing them to 1 μg/ml polymyxin B (A) or 64 μg/ml indolicidin (B), and plating diluted aliquots to check for survivors. For each condition, one representative experiment is shown of four independent experiments that produced identical trends.
psrA mutation affects permeabilization of the outer membrane.
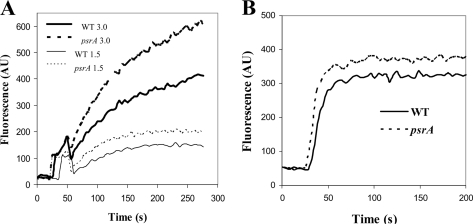
Polycationic molecules such as polymyxin B and antimicrobial peptides pass across the outer membrane by self-promoted uptake. The first stage of self-promoted uptake involves the interaction of the polycation with divalent cation binding sites on surface polyanionic LPS, causing a disruption of the permeability barrier and subsequent uptake of the permeabilizing polycationic antibiotic (16, 23). To address the possibility that altered outer membrane permeability was the basis for peptide supersusceptibility in the psrA mutant, NPN was used as a probe for outer membrane permeabilization by indolicidin (Fig. 2). The hydrophobic fluorophor NPN is normally excluded from entering cells due to its inability to penetrate the outer membrane. Upon permeabilization of the outer membrane (as occurs during self-promoted uptake), NPN is taken up and becomes strongly fluorescent in the hydrophobic environment of cell membranes (23). There was no obvious difference in the abilities of the psrA mutant and the WT to exclude NPN. However, indolicidin, at concentrations of 3.0 and 1.5 μg/ml, was able to permeabilize the outer membranes of the psrA mutant to a greater extent than those of WT cells (Fig. 2A). Thus, the supersusceptibility of the psrA mutant to indolicidin correlated with an outer membrane that was more easily permeabilized by this antimicrobial peptide. Similarly, polymyxin B also preferentially permeabilized the psrA mutant (Fig. 2B).
FIG. 2.
PsrA mutation effect on outer membrane permeabilization by peptides. Cells from mid-logarithmic-phase cultures of WT and psrA mutant strains were exposed to 1.5 or 3 μg/ml of indolicidin (A) or 0.2 μg/ml polymyxin B (B) and the increase in fluorescence due to peptide-stimulated partitioning of NPN into the outer membrane was measured. One representative experiment is shown of three independent experiments, each of which showed reproducible trends. AU, arbitrary units.
Contribution of psrA to biofilm formation and attachment.
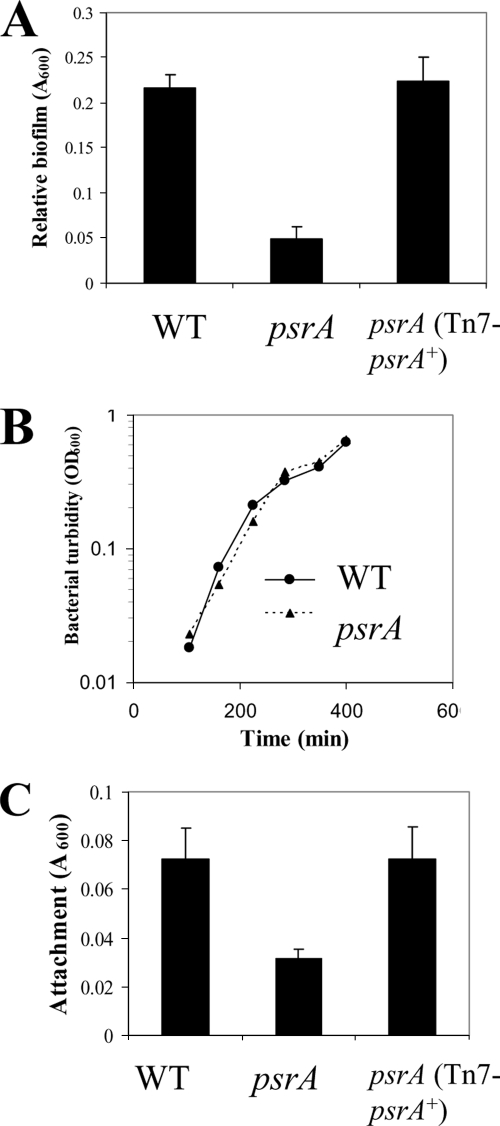
Other genes, such as the PhoQ gene, that regulate antimicrobial peptide resistance also regulate biofilm formation and motility. To assess the ability of the psrA mutant to form simple biofilms, static microtiter biofilm assays were employed to demonstrate that the psrA mutant displayed significant (>4-fold; P < 0.05 by Student's t test) impairment in biofilm formation at 18 h (Fig. 3A). Biofilm impairment could be complemented successfully by introducing the WT psrA allele into the mutant (Fig. 3A). No observable growth differences were observed when the OD600 of planktonic cells was measured as a function of time during the period of growth in the microtiter wells (Fig. 3B). Similarly, assessment of growth in defined medium in shaking flasks revealed no differences between the mutant and WT strains (data not shown), indicating no primary growth defect.
FIG. 3.
Defects in biofilm formation and attachment in psrA mutants. (A) Requirement for psrA in static biofilm formation. Cells were grown at 37°C for 18 h in polystyrene microtiter plates containing LB. Adherent biofilm cells were stained with crystal violet, followed by ethanol solubilization of the crystal violet and quantification (A600) of stained wells. (B) Planktonic growth of the psrA mutant under these biofilm conditions was unaffected. Planktonic cells were grown as in biofilm microtiter assays, and turbidity was measured (OD600). (C) Requirement for psrA for rapid attachment. Rapid attachment was assayed using mid-log-phase cells for 30 min. Adherent cells were stained with crystal violet, followed by ethanol extraction of the crystal violet for quantification as the A600. Results shown are means with standard deviations for three biological experiments, each with eight technical repeats.
To determine whether this biofilm formation phenotype occurred during the initial attachment stage or later during biofilm development, a rapid (30 min) attachment assay was performed. The psrA mutant displayed impaired attachment (>2-fold; P < 0.05 by Student's t test), and this defect could be complemented with the psrA+ gene (Fig. 3C).
Requirement for PsrA for normal swarming.
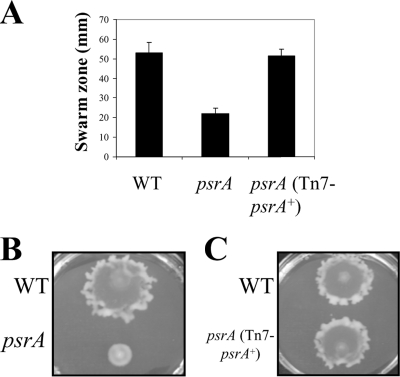
Mutant studies have revealed an intricate relationship between motility and biofilm formation in P. aeruginosa (17). Therefore, the psrA mutant was assessed for the ability to undergo swimming, twitching, and swarming motility. Neither flagellum-mediated swimming motility nor type IV pilus-mediated twitching motility was significantly affected in the psrA mutant. However, the psrA mutant demonstrated a severe impairment in swarming motility, with a significant (>2.5-fold; P < 0.05 by Student's t test) decrease in swarming zone size, and this could be complemented by introducing the WT psrA allele (Fig. 4).
FIG. 4.
Swarming motility defect in psrA mutants. (A) Swarming motility was evaluated by spot inoculating cells onto BM2 swarm plates containing 0.5% agar, followed by incubation at 37°C for 18 h. Diameters of the characteristic circular PAO1 swarm zones were measured, and means with standard deviations are reported for three biological repeats, each with three technical repeats. (B) Representative WT (top) and psrA mutant (bottom) swarming morphologies. (C) Complemented psrA mutant swarming morphology. WT (top) and psrA (Tn7-psrA+) (bottom) morphologies are shown.
Microarray analysis.
The above-described complex phenotype indicated that PsrA might control the expression of a substantial regulon. To assess this and to identify candidate genes that might explain the observed psrA mutant phenotypes, microarray analysis was performed, comparing the psrA mutant to the WT after growth to mid-logarithmic phase in BM2-glucose minimal medium containing 2 mM Mg2+. There were a total of 178 genes that were significantly (P ≤ 0.05) dysregulated ≥2-fold, among which 70 were upregulated and 108 were downregulated in the mutant relative to the WT (see the table in the supplemental material). A selection of these genes is shown in Tables 2 to 4. Independent quantitative reverse transcription-PCR (qRT-PCR) analysis confirmed the regulation of six of these genes (indicated by asterisks in Tables 2 to 4) and thus provided validation for our psrA microarray data. Most previously identified genes with predicted PsrA binding sites in their promoters (18) were observed to be dysregulated in this microarray analysis (Table 2).
TABLE 2.
| Gene identifierb | Gene name | Change (fold)c | Descriptiona |
|---|---|---|---|
| PA0506 | 16.2* | Probable acyl-CoA dehydrogenase | |
| PA2673 | hplV | −27.9 | Probable type II secretion system protein |
| PA2951 | etfA | 3.3 | Electron transfer flavoprotein alpha subunit |
| PA2952 | etfB | 3.8 | Electron transfer flavoprotein beta subunit |
| PA2953 | 6.4* | Electron transfer flavoprotein-ubiquinone oxidoreductase | |
| PA3013 | foaB | 15.2* | Fatty acid oxidation complex beta subunit |
| PA3014 | faoA | 11.6 | Fatty acid oxidation complex alpha subunit |
| PA3622 | rpoS | −2.3* | Alternative sigma factor RpoS |
Only genes showing a ≥2-fold change in the psrA mutant are depicted. The P value was <0.0001 in all cases.
According to the P. aeruginosa genome website (http://www.pseudomonas.com/).
Regulation of genes differentially expressed in the psrA mutant relative to the WT. A positive number indicates transcript upregulation in the psrA mutant. *, confirmation of gene regulation by qRT-PCR.
TABLE 4.
Other known genes significantly dysregulated in psrA mutants, determined using a microarraya
| Gene identifierb | Gene name | Change (fold)c | P value | Description |
|---|---|---|---|---|
| PA0106 | coxA | −3.4 | 0.01 | Cytochrome c oxidase, subunit I |
| PA0217 | mdcR | −4.8 | 0.007 | Transcriptional regulator |
| PA0459 | clpC | −3.5 | 0.05 | ClpA/B protease ATP binding subunit |
| PA0507 | −3.2 | 0.001 | Probable acyl-CoA dehydrogenase | |
| PA0511 | nirJ | 4.8 | 0.007 | Heme d1 biosynthesis protein |
| PA0512 | nirH | 2.3 | 0.04 | Conserved hypothetical protein |
| PA0513 | nirG | 4.5 | 0.008 | Probable transcriptional regulator |
| PA0517 | nirC | −2.6 | 0.001 | c-type cytochrome |
| PA0530 | −4.3 | 0.04 | Pyridoxal phosphate-dependent aminotransferase | |
| PA0588 | yeaG | −3.3 | 0.001 | Conserved hypothetical protein |
| PA0707 | toxR | −4.2 | 0.009 | ToxR/RegA transcriptional regulator |
| PA0719 | −4.1 | 0.009 | Bacteriophage Pf1 protein | |
| PA0724 | coaA | 2.8 | 0.02 | Coat protein A of bacteriophage Pf1 |
| PA0840 | −8.9 | 0.006 | Probable oxidoreductase | |
| PA0852 | cbpD | 2.2 | 0.04 | Chitin-binding protein CbpD |
| PA1041 | −12.9 | 0.005 | OmpA-family outer membrane protein | |
| PA1173 | napB | −2.6 | 0.02 | Cytochrome c-type protein NapB |
| PA1187 | lcaD | −2.1 | 0.04 | Acyl-CoA dehydrogenase |
| PA1399 | −2.0 | 0.004 | Probable LysR-family transcriptional regulator | |
| PA1648 | −3.0 | 0.002 | Probable oxidoreductase | |
| PA1649 | −6.3 | 0.0003 | Probable short-chain dehydrogenase | |
| PA1650 | −2.0 | 0.008 | Probable transporter | |
| PA1828 | −3.1 | 0.006 | Probable short-chain dehydrogenase | |
| PA1881 | −2.2 | 0.03 | Probable oxidoreductase | |
| PA1883 | −10.2 | 0.002 | NADH-ubiquinone/plastoquinone oxidoreductase | |
| PA1927 | metE | 2.1 | 0.007 | Methionine synthase |
| PA1976 | −2.2 | 0.0009 | Two-component sensor kinase | |
| PA1978 | agmR | −2.9 | 0.003 | Two-component response regulator |
| PA1982 | exaA | −2.7 | 0.03 | Quinoprotein ethanol dehydrogenase |
| PA1983 | exaB | −2.7 | 0.02 | Cytochrome c550 |
| PA1984 | exaC | −3.6 | 0.05 | Aldehyde dehydrogenase |
| PA1985 | pqqA | −4.5 | 0.002 | Pyrroloquinoline quinone biosynthesis protein A |
| PA2124 | 3.2 | 0.01 | Probable dehydrogenase | |
| PA2277 | arsR | 0.05 | Transcriptional regulator | |
| PA2278 | arsB | 0.02 | Ion transport membrane protein | |
| PA2339 | mtlF | −2.1 | 0.05 | Maltose/mannitol transport protein |
| PA2350 | 3.1 | 0.02 | Probable ATP-binding component of ABC transporter | |
| PA2352 | 2.4 | 0.03 | Probable glycerophosphoryl diester phosphodiesterase | |
| PA2371 | clpV3 | −3.0 | 0.02 | Probable ClpA/B-type protease |
| PA2396 | pvdF | 2.1 | 0.05 | Pyoverdine synthetase F |
| PA2398 | fpvA | 2.5 | 0.03 | Ferripyoverdine outer membrane receptor |
| PA2432 | 5.8 | 0.03 | Probable transcriptional regulator | |
| PA2469 | −4.1 | 0.01 | Probable transcriptional regulator | |
| PA2522 | czcC | −3.7 | 0.01 | Outer membrane efflux protein |
| PA2535 | −2.3 | 0.05 | Probable oxidoreductase | |
| PA2536 | ynbB | 2.2 | 0.03 | Phosphatidate cytidylyltransferase |
| PA2550 | −3.7 | 0.001 | Probable acyl-CoA dehydrogenase | |
| PA2551 | −2.3 | 0.001 | Probable transcriptional regulator | |
| PA2573 | −2.9 | 0.02 | Probable chemotaxis transducer | |
| PA2664 | fhp | −83.1 | 0.0004 | Flavohemoprotein |
| PA2892 | −2.2 | 0.04 | Probable short-chain dehydrogenase | |
| PA2893 | −2.4 | 0.03 | Probable very-long-chain acyl-CoA synthetase | |
| PA2939 | pepB | −4.9 | 0.007 | Secreted aminopeptidase |
| PA3077 | 2.5 | 0.03 | Two-component response regulator | |
| PA3145 | wbpL | −2.5 | 0.03 | WbpL rhamnosyltransferase in LPS biosynthesis |
| PA3148 | wbpI | −4.4 | 0.04 | UDP-N-acetylglucosamine 2-epimerase WbpI |
| PA3150 | wbpG | −2.0 | 0.05 | LPS biosynthesis protein WbpG |
| PA3152 | hisH2 | −2.3 | 0.03 | Glutamine amidotransferase |
| PA3277 | −4.2 | 0.0003 | Probable short-chain dehydrogenase | |
| PA3327 | −2.7 | 0.02 | Probable nonribosomal peptide synthetase | |
| PA3387 | rhlG | −2.7 | 0.0002 | Beta-ketoacyl reductase |
| PA3409 | −2.1 | 0.05 | Probable transmembrane sensor | |
| PA3418 | ldh | −3.0 | <0.0001 | Leucine dehydrogenase |
| PA3427 | −2.6 | 0.002 | Probable short-chain dehydrogenases | |
| PA3454 | −2.1 | 0.002 | Probable acyl-CoA thiolase | |
| PA3630 | 2.2 | 0.05 | Probable transcriptional regulator | |
| PA3723 | yqiM | −3.0 | 0.005 | FMN oxidoreductase |
| PA3877 | narK1 | −3.8 | 0.0008 | Nitrite extrusion protein 1 |
| PA3957 | 3.7 | 0.01 | Probable short-chain dehydrogenase | |
| PA4135 | −2.9 | 0.02 | Probable transcriptional regulator | |
| PA4497 | −4.1 | 0.01 | Binding protein component of ABC transporter | |
| PA4599 | mexC | −2.0 | 0.008 | RND multidrug efflux membrane fusion protein |
| PA4654 | −5.6 | 0.02 | Major facilitator superfamily transporter | |
| PA4911 | −6.6 | 0.004 | Probable permease of ABC amino acid transporter | |
| PA5020 | −3.7 | 0.003 | Probable acyl-CoA dehydrogenase | |
| PA5097 | hutT | 2.7 | 0.02 | Amino acid permease |
| PA5141 | hisA | 2.8 | 0.02 | Histidine biosynthesis protein |
| PA5187 | −3.2 | 0.0004 | Probable acyl-CoA dehydrogenase | |
| PA5188 | −2.0 | 0.0004 | Probable 3-hydroxyacyl-CoA dehydrogenase | |
| PA5234 | −2.2 | 0.005 | Probable oxidoreductase | |
| PA5302 | dadX | −3.3 | 0.02 | Catabolic alanine racemase |
Dysregulated hypothetical or unclassified open reading frames are not included. Only genes showing a ≥2-fold change in the psrA mutant are depicted.
According to the P. aeruginosa genome website (http://www.pseudomonas.com/).
Regulation of genes differentially expressed in the psrA mutant relative to the WT. A positive number indicates transcript upregulation in the psrA mutant.
In addition, we observed dysregulation of the entire type III secretion apparatus and its effectors (Table 3), certain adhesion and motility genes, 17 regulators (rpoS, pcrH, mdcR, toxR, arsA, PA0513, PA1399, PA1976, PA1978, PA2432, PA2469, PA2551, PA3077, PA3409, PA3630, PA4135, and PA4296), and a variety of metabolic and energy metabolism genes (Tables 3 and 4).
TABLE 3.
Type III secretion system, adhesion (tad), motility, and type II secretion genes significantly dysregulated in psrA mutants, determined using a microarray
| Gene identifiera | Gene name | Change (fold)b | P value | Description |
|---|---|---|---|---|
| Type III secretion genes | ||||
| PA0044 | exoT | 4.6 | 0.0005 | Exoenzyme T; type III secretion system effector |
| PA1695 | pscP | 3.0 | <0.0001 | Translocation protein in type III secretion |
| PA1697 | pscN | 2.1 | 0.004 | ATP synthase in type III secretion system |
| PA1698 | popN | 2.6 | 0.004 | Outer membrane protein PopN |
| PA1699 | 2.3 | 0.003 | Conserved hypothetical protein in type III secretion | |
| PA1700 | 2.0 | 0.0009 | Conserved hypothetical protein in type III secretion | |
| PA1701 | 4.5 | 0.0003 | Conserved hypothetical protein in type III secretion | |
| PA1702 | 2.0 | 0.0002 | Conserved hypothetical protein in type III secretion | |
| PA1703 | pcrD | 2.0 | 0.01 | Type III secretory apparatus protein PcrD |
| PA1705 | pcrG | 3.8 | 0.002 | Regulator in type III secretion |
| PA1706 | 2.1 | 0.001 | Type III secretion protein PcrV | |
| PA1707 | pcrH | 2.4 | 0.0002 | Regulatory protein PcrH |
| PA1708 | popB | 3.1 | 0.005 | Translocator protein PopB |
| PA1709 | popD | 2.7 | 0.002 | Translocator protein PopD |
| PA1710 | exsC | 2.1 | <0.0001 | Exoenzyme S synthesis protein C |
| PA1712 | exsB | 2.0 | 0.001 | Exoenzyme S synthesis protein B |
| PA1715 | pscB | 2.5 | 0.001 | Type III export apparatus protein |
| PA1717 | pscD | 2.8 | 0.01 | Type III export protein PscD |
| PA1721 | pscH | 2.0 | 0.01 | Type III export protein PscH |
| PA1723 | pscJ | 2.1 | 0.01 | Type III export protein PscJ |
| PA2191 | exoY | 2.1 | 0.0004 | Adenylate cyclase ExoY; type III secretion effector |
| PA3841 | exoS | 2.6 | 0.001 | Exoenzyme S; type III secretion effector |
| PA3842 | orf1 | 3.5 | 0.001 | Chaperone for ExoS secretion |
| Adhesion and motility genes | ||||
| PA0176 | aer2 | −4.2 | 0.04 | Aerotaxis methyl-accepting chemotaxis protein |
| PA1803 | lon | −2.0 | 0.05 | ATP-dependent Lon protease |
| PA4296 | pprB | −4.9* | 0.05 | PprB two-component response regulator |
| PA4300 | tadC | −2.2 | 0.04 | Flp pilus assembly protein, PilC-like |
| PA4302 | tadA | −4.1* | 0.01 | TadA traffic ATPase in Flp pilus assembly |
| PA4303 | tadZ | −2.7 | 0.02 | Flp pilus assembly protein |
| PA4305 | rcpC | −2.1 | 0.05 | Flp pilus assembly protein |
| Type II secretion genes | ||||
| PA0683 | hxcY | −4.4 | 0.008 | Hxc type II secretion system membrane protein |
| PA1871 | lasA | 10.5 | 0.002 | LasA protease |
| PA2672 | hplW | −2.5 | 0.003 | Type II secretion system prepilin peptidase substrate |
According to the P. aeruginosa genome website (http://www.pseudomonas.com/).
Regulation of genes differentially expressed in the psrA mutant relative to the WT. A positive number indicates transcript upregulation in the psrA mutant. *, confirmation of gene regulation by qRT-PCR.
Additional mutant phenotypic analyses.
The list of genes dysregulated in the psrA mutant provided a useful starting point toward understanding the basis for the observed psrA mutant phenotypes. To understand the phenotypes associated with selected dysregulated genes, transposon mutants from the strain PA14 comprehensive nonredundant library (22) were utilized (Table 1).
The microarray was examined to find genes that might influence peptide susceptibility (since the microarray and time-kill experiments used similar growth conditions). The dysregulation of several genes of the wbp gene cluster (Table 4), which is involved in the biosynthesis of B band (serotype O antigen) LPS (3), suggested a possible role for B band LPS in the observed supersusceptibility of the psrA mutant. In addition, a small panel of PA14 mutants related to energy metabolism was tested, since our preliminary unpublished observations indicated a role for energy metabolism in resistance to antimicrobial peptides.
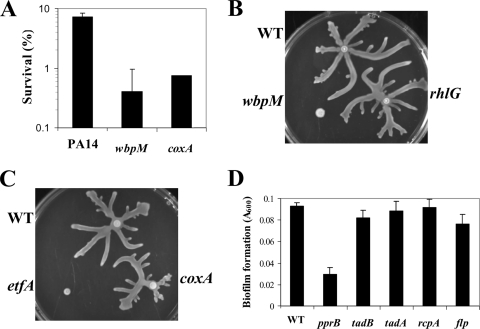
As shown in Fig. 5A, both wbpM (previously shown to lack B band LPS) (3) and coxA (cytochrome c oxidase subunit 1) mutants showed modest supersusceptibility to indolicidin relative to the WT at 25 min. No differences were seen for mutants in fhp, PA1883, mexC, and wzz (data not shown). Unfortunately, an etfA energy metabolism mutant clumped during growth, which made establishing killing curves for this mutant difficult.
FIG. 5.
Peptide susceptibility, swarming, and biofilm analyses of PA14 mutants in selected genes transcriptionally downregulated in psrA mutants. (A) Intrinsic indolicidin supersusceptibility time-kill curve analysis of PA14 coxA and wbpM mutants compared to the WT. Cells were grown to mid-logarithmic phase and exposed to 64 μg/ml indolicidin, and survival was assessed after 25 min. The means plus standard deviations for three independent experiments are shown. (B and C) PA14 mutants in wbpM and etfA cannot undergo normal swarming motility. Mid-logarithmic-phase cultures were spot inoculated onto PA14-type swarm agar plates and incubated for 18 h at 37°C. Swarming assays were performed with three independent cultures of each strain, and a representative swarm morphology was photographed. (D) Biofilm impairment of the pprB mutant. Overnight cultures were diluted 1:500 and then grown for 18 h at 37°C, followed by washing with deionized H2O and staining with crystal violet. Biofilm formation was repeated three times, each time with six technical replicates, and the data shown are the means with standard deviations for one representative experiment.
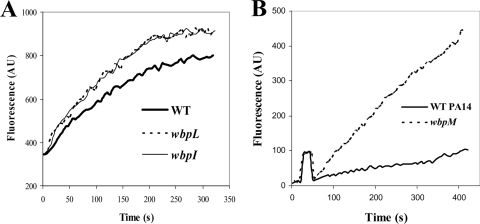
No major differences in O antigen chain length expression were seen when LPS was isolated from the WT and the psrA mutant and analyzed by sodium dodecyl sulfate-polyacryl amide gel electrophoresis and silver staining (data not shown). However, since small changes in LPS that influence functionality, such as substitution by sugars, phosphates, and fatty acids, could be affected by the psrA mutation, mutants in wbpI, wbpL (downregulated in the psrA mutant), and wbpM were analyzed for possible outer membrane permeability phenotypes. Like the psrA mutant, all three of these mutants in LPS B band biosynthetic cluster genes showed increased outer membrane permeabilization by the peptide relative to that of the WT (Fig. 6A and B), although the effect observed with the wbpM mutant was more prominent (Fig. 6B).
FIG. 6.
Mutants in the B band O antigen biosynthetic operon demonstrating altered outer membrane permeabilization by indolicidin. (A) Cells from mid-logarithmic-phase cultures of WT PAO1 and wbpI and wbpL mutants were exposed to 3.0 μg/ml indolicidin, and the increase in fluorescence due to peptide-promoted partitioning of NPN into the outer membrane was measured. (B) Cultures of WT PA14 and the wbpM mutant were exposed to 1.5 μg/ml indolicidin. Data shown are for one representative experiment of at least three independent trials, each of which produced the same trends. AU, arbitrary units.
A panel of PA14 mutants was also analyzed for possible roles in swarming motility and biofilm formation. The wbpM mutant showed a major swarming impairment phenotype, as did an etfA mutant, although in the latter mutant this might be related to its tendency to clump (Fig. 5B and C). No swarming differences were seen for the rhlG (Fig. 5B) coxA (Fig. 5C), mexC, wzz, and PA1883 mutants (data not shown).
The downregulation of certain genes of the type IVb pilus-encoding tad cluster led us to analyze mutants in these genes for possible biofilm formation phenotypes. Under simple biofilm growth conditions, none of the tad mutants analyzed displayed biofilm impairment (Fig. 5D), confirming previously reported results (9). However, a pprB mutant, encoding a two-component response regulator, located adjacent to the tad cluster, and substantially downregulated on the arrays (Table 3), demonstrated a significant (P < 0.001) biofilm impairment phenotype (threefold) (Fig. 5D).
DISCUSSION
The psrA gene of P. aeruginosa is an important regulator of both resistance to cationic antimicrobials and virulence features. It is upregulated in response to the cationic antimicrobial peptide indolicidin and mediates cationic peptide resistance and certain virulence-related processes, such as biofilm formation, rapid attachment, and swarming motility. The involvement of PsrA in these phenotypes was supported by studies of a psrA mutant and by single-copy complementation of the psrA defect.
PsrA was previously characterized by the Venturi group as a positive regulator of transcription of the alternative sigma factor RpoS (19, 20), as also confirmed here. In other Pseudomonas spp., PsrA is known to be involved in antifungal metabolite production (6) and in the regulation of quorum sensing (5). However, the direct signals that activate psrA were unknown, and the data here now demonstrate that the cationic antimicrobial peptide indolicidin is an activating signal for transcription, consistent with other studies demonstrating that peptides are key regulators of bacterial virulence and resistance (2, 10, 31).
The demonstration that psrA contributes to cationic peptide resistance adds another regulator to the increasingly complex regulatory network influencing resistance, which already includes PhoP-PhoQ and PmrA-PmrB (10, 26, 30, 32). However, unlike these two-component regulators, which mediate an increase in resistance to peptides under growth conditions with limiting Mg2+, PsrA mediates intrinsic resistance. Thus, unlike psrA mutants, pmrA and phoP mutants do not demonstrate supersusceptibility under noninducing conditions (26), and there appears to be no obvious regulatory hierarchy, since psrA was not apparently transcriptionally regulated by PmrA or PhoP (or vice versa) and there was no substantial overlap in dysregulated genes (30) (Tables 2 to 4). All three systems, however, appear to mediate resistance by influencing the ability of cationic agents to permeabilize outer membranes (31), and the increase in permeabilization by cationic agents correlated with the supersusceptibility of the psrA mutant to polymyxin B and indolicidin (Fig. 2). Microarray experiments were utilized to select candidate genes dysregulated in the psrA mutant that might contribute to psrA supersusceptibility to peptides. Three genes (wbpG, wbpI, and wbpL) (Table 4) from the LPS B band (serotype O antigen) biosynthesis operon (wbpGHIJKLM) were downregulated 2- to 4.4-fold in the psrA mutant, indicating that PsrA positively regulates this operon. The link between peptide supersusceptibility and outer membrane permeability of the psrA mutant was supported by the observation that mutants in three of these genes (wbpI, wbpL, and wbpM) displayed modest to substantially increased outer membrane permeability (Fig. 6A and B), and the wbpM mutant was further shown here to be peptide supersusceptible (Fig. 5A) and swarming deficient (Fig. 5B). This is consistent with observations in Proteus mirabilis that LPS O antigen can contribute to both antimicrobial peptide resistance and swarming motility (29). Other possible candidates to explain peptide supersusceptibility would be gene products involved in energy generation and thus, potentially, in interaction of cationic peptides with the cytoplasmic membrane. One of the tested genes, coxA, encoding a subunit of cytochrome c oxidase, was 3.4-fold downregulated in arrays (Table 4), and a mutant in this gene led to modest supersusceptibility relative to the WT (Fig. 5A).
The substantial swarming impairment displayed by the psrA mutant indicated that PsrA is involved in the regulatory mechanisms controlling this complex adaptation (33, 34). Although swarming motility requires both flagella and pili, the psrA mutant did not exhibit a defect in either flagellum-mediated swimming or type IV pilus-mediated twitching motility (34), indicating that it did not control a primary motility organelle. PsrA regulation of swarming motility might reflect the downregulation of both Lon protease (Table 3), which is required for normal swarming (27), and the LPS O antigen B band biosynthetic gene cluster, since the wbpM mutant was swarming deficient (Fig. 5B).
Biofilm formation in Pseudomonas is initiated by attachment of cells to a surface, followed by complex steps leading to development of mature biofilms (35). The psrA biofilm impairment phenotype was likely related in part to the early stages of biofilm formation, as the psrA mutant displayed a significant impairment in rapid attachment to the polystyrene surface used in the simple biofilm experiments described here (Fig. 3C). Although psrA mutants were able to attach and form simple biofilms, they did this at significantly reduced levels compared to the WT. Two possible PsrA-regulated genes that might influence the regulation of biofilm formation by PsrA are lon (27) and the pprB response regulator gene (Fig. 5D) (found directly adjacent to the tad gene cluster), since mutants in both displayed impaired biofilm formation. The finding that the psrA mutant displayed both impaired biofilm formation and impaired swarming motility suggests that PsrA is an integral component of the regulatory network that controls these two separate complex adaptations and is consistent with observations that other regulators control both processes (4, 34, 39).
Our results are consistent with previous observations that RpoS is a negative regulator of the type III secretion system, since RpoS is positively regulated by psrA (18, 41) (Table 2). PsrA was previously shown to be a positive regulator of the type III secretion system in a mucoid strain of P. aeruginosa grown in complex medium (38). In contrast, the data presented here favor negative regulation by PsrA of this secretion system in the nonmucoid P. aeruginosa strain PAO1 grown in defined medium (Table 3). We presume that this is because of other underlying regulatory mutations that are known to occur in mucoid isolates of P. aeruginosa. Consistent with these observations, the psrA mutant presented here had no effect on cytotoxicity toward epithelial cells, which is partially dependent on type III secretion (data not shown).
PA0506, an acyl-coenzyme A (acyl-CoA) dehydrogenase, was highly upregulated in the psrA mutant (43-fold, according to qRT-PCR confirmation experiments). This gene was a previously characterized target of PsrA (18), and our microarray analysis confirmed PsrA as a negative regulator of this gene (Table 2). It is noteworthy that PA0506 has previously been shown to be mutated in cystic fibrosis P. aeruginosa isolates, consistent with the suggestion that mutation of this gene favors chronic infection and that this gene might be involved in adaptation to the cystic fibrosis lung (40).
Our microarray gene lists uncovered many other interesting genes as part of the PsrA regulon. The downregulation of genes of the tad (tight adherence) cluster (Table 3), involved in the assembly of extracellular cell surface Flp pilus appendages (9), was consistent with the attachment defect in psrA mutants in the face of normal piliation and twitching motility. However, no differences were seen in biofilm formation by mutants in genes of the tad cluster (Fig. 5D). Probable type II secretion system genes (PA0683, PA2672, and PA2673) also showed modest to strong repression (Tables 2 and 3), could encode adhesion-associated products (based on the similarity of pili to the components of the type II secretion system), and thus might contribute to the attachment and biofilm phenotype observed for the psrA mutant.
Biofilm formation, attachment, and swarming motility appear to be very important in P. aeruginosa colonization and virulence, while it has been strongly suggested that Pseudomonas is exposed to cationic antimicrobial peptides during infections and, occasionally, to polymyxin B during therapy. The involvement of PsrA in these processes and its inducibility by a cationic antimicrobial peptide highlight the likely importance of this enzyme in adaptation to the cystic fibrosis lung environment through regulation of virulence and antimicrobial peptide resistance. The results presented here are consistent with the massive complexity of the regulatory network influencing these processes.
Supplementary Material
Acknowledgments
Funding from the Canadian Cystic Fibrosis Foundation (CCFF) is gratefully acknowledged. W.J.G. was supported by a CCFF studentship and a Michael Smith Foundation for Health Research-Genome British Columbia junior graduate studentship. R.H. holds a Canada Research Chair. I.W. was supported by the Juergen Manchot Foundation.
We thank Michael Jacobs and Colin Manoil for providing the UW-psrA mutant and Joerg Overhage for insightful discussions.
Footnotes
Published ahead of print on 13 June 2008.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Ausubel, F. M. 1987. Current protocols in molecular biology. Greene Publishing Associates and Wiley-Interscience, New York, NY.
- 2.Bader, M. W., S. Sanowar, M. E. Daley, A. R. Schneider, U. Cho, W. Xu, R. E. Klevit, H. Le Moual, and S. I. Miller. 2005. Recognition of antimicrobial peptides by a bacterial sensor kinase. Cell 122461-472. [DOI] [PubMed] [Google Scholar]
- 3.Burrows, L. L., R. V. Urbanic, and J. S. Lam. 2000. Functional conservation of the polysaccharide biosynthetic protein WbpM and its homologues in Pseudomonas aeruginosa and other medically significant bacteria. Infect. Immun. 68931-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caiazza, N. C., J. H. Merritt, K. M. Brothers, and G. A. O'Toole. 2007. Inverse regulation of biofilm formation and swarming motility by Pseudomonas aeruginosa PA14. J. Bacteriol. 1893603-3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chatterjee, A., Y. Cui, H. Hasegawa, and A. K. Chatterjee. 2007. PsrA, the Pseudomonas sigma regulator, controls regulators of epiphytic fitness, quorum-sensing signals, and plant interactions in Pseudomonas syringae pv. tomato strain DC3000. Appl. Environ. Microbiol. 733684-3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chin, A. W. T. F., D. van den Broek, B. J. Lugtenberg, and G. V. Bloemberg. 2005. The Pseudomonas chlororaphis PCL1391 sigma regulator psrA represses the production of the antifungal metabolite phenazine-1-carboxamide. Mol. Plant-Microbe Interact. 18244-253. [DOI] [PubMed] [Google Scholar]
- 7.Choi, K. H., A. Kumar, and H. P. Schweizer. 2006. A 10-min method for preparation of highly electrocompetent Pseudomonas aeruginosa cells: application for DNA fragment transfer between chromosomes and plasmid transformation. J. Microbiol. Methods 64391-397. [DOI] [PubMed] [Google Scholar]
- 8.Choi, K. H., J. B. Gaynor, K. G. White, C. Lopez, C. M. Bosio, R. R. Karkhoff-Schweizer, and H. P. Schweizer. 2005. A Tn7-based broad-range bacterial cloning and expression system. Nat. Methods 2443-448. [DOI] [PubMed] [Google Scholar]
- 9.de Bentzmann, S., M. Aurouze, G. Ball, and A. Filloux. 2006. FppA, a novel Pseudomonas aeruginosa prepilin peptidase involved in assembly of type IVb pili. J. Bacteriol. 1884851-4860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ernst, R. K., E. C. Yi, L. Guo, K. B. Lim, J. L. Burns, M. Hackett, and S. I. Miller. 1999. Specific lipopolysaccharide found in cystic fibrosis airway Pseudomonas aeruginosa. Science 2861561-1565. [DOI] [PubMed] [Google Scholar]
- 11.Falla, T. J., D. N. Karunaratne, and R. E. W. Hancock. 1996. Mode of action of the antimicrobial peptide indolicidin. J. Biol. Chem. 27119298-19303. [DOI] [PubMed] [Google Scholar]
- 12.Flick, M. R., and L. E. Cluff. 1976. Pseudomonas bacteremia. Review of 108 cases. Am. J. Med. 60501-508. [DOI] [PubMed] [Google Scholar]
- 13.Hancock, R. E. W., and H. G. Sahl. 2006. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 241551-1557. [DOI] [PubMed] [Google Scholar]
- 14.Hancock, R. E. W., and D. P. Speert. 2000. Antibiotic resistance in Pseudomonas aeruginosa: mechanisms and impact on treatment. Drug Resist. Updat. 3247-255. [DOI] [PubMed] [Google Scholar]
- 15.Jacobs, M. A., A. Alwood, I. Thaipisuttikul, D. Spencer, E. Haugen, S. Ernst, O. Will, R. Kaul, C. Raymond, R. Levy, L. Chun-Rong, D. Guenthner, D. Bovee, M. V. Olson, and C. Manoil. 2003. Comprehensive transposon mutant library of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 10014339-14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jenssen, H., P. Hamill, and R. E. W. Hancock. 2006. Peptide antimicrobial agents. Clin. Microbiol. Rev. 19491-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klausen, M., A. Aaes-Jorgensen, S. Molin, and T. Tolker-Nielsen. 2003. Involvement of bacterial migration in the development of complex multicellular structures in Pseudomonas aeruginosa biofilms. Mol. Microbiol. 5061-68. [DOI] [PubMed] [Google Scholar]
- 18.Kojic, M., B. Jovcic, A. Vindigni, F. Odreman, and V. Venturi. 2005. Novel target genes of PsrA transcriptional regulator of Pseudomonas aeruginosa. FEMS Microbiol. Lett. 246175-181. [DOI] [PubMed] [Google Scholar]
- 19.Kojic, M., C. Aguilar, and V. Venturi. 2002. TetR family member psrA directly binds the Pseudomonas rpoS and psrA promoters. J. Bacteriol. 1842324-2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kojic, M., and V. Venturi. 2001. Regulation of rpoS gene expression in Pseudomonas: involvement of a TetR family regulator. J. Bacteriol. 1833712-3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li, J., R. L. Nation, J. D. Turnidge, R. W. Milne, K. Coulthard, C. R. Rayner, and D. L. Paterson. 2006. Colistin: the re-emerging antibiotic for multidrug-resistant gram-negative bacterial infections. Lancet Infect. Dis. 6589-601. [DOI] [PubMed] [Google Scholar]
- 22.Liberati, N. T., J. M. Urbach, S. Miyata, D. G. Lee, E. Drenkard, G. Wu, J. Villanueva, T. Wei, and F. M. Ausubel. 2006. An ordered, nonredundant library of Pseudomonas aeruginosa strain PA14 transposon insertion mutants. Proc. Natl. Acad. Sci. USA 1032833-2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loh, B., C. Grant, and R. E. W. Hancock. 1984. Use of the fluorescent probe 1-N-phenylnaphthamine to study the interactions of the aminoglycoside antibiotics with the outer membrane of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 26546-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lyczak, J. B., C. L. Cannon, and G. B. Pier. 2000. Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist. Microbes Infect. 21051-1060. [DOI] [PubMed] [Google Scholar]
- 25.Ma, L., K. D. Jackson, R. M. Landry, M. R. Parsek, and D. J. Wozniak. 2006. Analysis of Pseudomonas aeruginosa conditional psl variants reveals roles for the psl polysaccharide in adhesion and maintaining biofilm structure postattachment. J. Bacteriol. 1888213-8221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Macfarlane, E. L., A. Kwasnicka, M. M. Ochs, and R. E. W. Hancock. 1999. PhoP-PhoQ homologues in Pseudomonas aeruginosa regulate expression of the outer-membrane protein OprH and polymyxin B resistance. Mol. Microbiol. 34305-316. [DOI] [PubMed] [Google Scholar]
- 27.Marr, A. K., J. Overhage, M. Bains, and R. E. W. Hancock. 2007. The Lon protease of Pseudomonas aeruginosa is induced by aminoglycosides and is involved in biofilm formation and motility. Microbiology 153474-482. [DOI] [PubMed] [Google Scholar]
- 28.Marr, A. K., W. J. Gooderham, and R. E. W. Hancock. 2006. Antibacterial peptides for therapeutic use: obstacles and realistic outlook. Curr. Opin. Pharmacol. 6468-472. [DOI] [PubMed] [Google Scholar]
- 29.McCoy, A. J., H. Liu, T. J. Falla, and J. S. Gunn. 2001. Identification of Proteus mirabilis mutants with increased sensitivity to antimicrobial peptides. Antimicrob. Agents Chemother. 452030-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McPhee, J. B., M. Bains, G. Winsor, S. Lewenza, A. Kwasnicka, M. D. Brazas, F. S. Brinkman, and R. E. W. Hancock. 2006. Contribution of the PhoP-PhoQ and PmrA-PmrB two-component regulatory systems to Mg2+-induced gene regulation in Pseudomonas aeruginosa. J. Bacteriol. 1883995-4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McPhee, J. B., S. Lewenza, and R. E. W. Hancock. 2003. Cationic antimicrobial peptides activate a two-component regulatory system, PmrA-PmrB, that regulates resistance to polymyxin B and cationic antimicrobial peptides in Pseudomonas aeruginosa. Mol. Microbiol. 50205-217. [DOI] [PubMed] [Google Scholar]
- 32.Moskowitz, S. M., R. K. Ernst, and S. I. Miller. 2004. PmrAB, a two-component regulatory system of Pseudomonas aeruginosa that modulates resistance to cationic antimicrobial peptides and addition of aminoarabinose to lipid A. J. Bacteriol. 186575-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Overhage, J., M. Bains, M. D. Brazas, and R. E. W. Hancock. 2008. Swarming of Pseudomonas aeruginosa is a complex adaptation leading to increased production of virulence factors and antibiotic resistance. J. Bacteriol. 1902671-2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Overhage, J., S. Lewenza, A. K. Marr, and R. E. W. Hancock. 2007. Identification of genes involved in swarming motility using a Pseudomonas aeruginosa PAO1 mini-Tn5-lux mutant library. J. Bacteriol. 1892164-2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parsek, M. R., and E. P. Greenberg. 2005. Sociomicrobiology: the connections between quorum sensing and biofilms. Trends Microbiol. 1327-33. [DOI] [PubMed] [Google Scholar]
- 36.Parsek, M. R., and P. K. Singh. 2003. Bacterial biofilms: an emerging link to disease pathogenesis. Annu. Rev. Microbiol. 57677-701. [DOI] [PubMed] [Google Scholar]
- 37.Rowe, S. M., S. Miller, and E. J. Sorscher. 2005. Cystic fibrosis. N. Engl. J. Med. 3521992-2001. [DOI] [PubMed] [Google Scholar]
- 38.Shen, D. K., D. Filopon, L. Kuhn, B. Polack, and B. Toussaint. 2006. PsrA is a positive transcriptional regulator of the type III secretion system in Pseudomonas aeruginosa. Infect. Immun. 741121-1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shrout, J. D., D. L. Chopp, C. L. Just, M. Hentzer, M. Givskov, and M. R. Parsek. 2006. The impact of quorum sensing and swarming motility on Pseudomonas aeruginosa biofilm formation is nutritionally conditional. Mol. Microbiol. 621264-1277. [DOI] [PubMed] [Google Scholar]
- 40.Smith, E. E., D. G. Buckley, Z. Wu, C. Saenphimmachak, L. R. Hoffman, D. A. D'Argenio, S. I. Miller, B. W. Ramsey, D. P. Speert, S. M. Moskowitz, J. L. Burns, R. Kaul, and M. V. Olson. 2006. Genetic adaptation by Pseudomonas aeruginosa to the airways of cystic fibrosis patients. Proc. Natl. Acad. Sci. USA 1038487-8492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yahr, T. L., and M. C. Wolfgang. 2006. Transcriptional regulation of the Pseudomonas aeruginosa type III secretion system. Mol. Microbiol. 62631-640. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.