Abstract
Lymph nodes (LNs) are important sentinel organs where antigen-presenting cells interact with T cells to induce adaptive immune responses. In cutaneous infection of mice with Leishmania major, resistance depends on the induction of a T-helper-cell-1 (Th1)-mediated cellular immune response in draining, peripheral LNs. We investigated whether draining, peripheral LNs are absolutely required for resistance against L. major infection. We investigated the course of experimental leishmaniasis in wild-type (wt) mice lacking peripheral LNs (pLNs), which we generated by in utero blockade of membrane-bound lymphotoxin, and in mice lacking pLNs or all LNs due to genetic deletion of lymphotoxin ligands or receptors. wt mice of the resistant C57BL/6 strain without local skin-draining LNs were still able to generate specific T-cell responses, but this yielded Th2 cells. This switch to a Th2 response resulted in severe systemic infection. We also confirmed these results with mice lacking pLNs due to genetic depletion of lymphotoxin-β. The complete absence of LNs due to a genetic depletion of the lymphotoxin-β receptor also resulted in a marked deterioration of disease and a Th2 response. Thus, in the absence of pLNs, an L. major-specific Th2 response is induced in the remaining secondary lymphoid organs, such as the spleen and non-skin-draining LNs. This indicates a critical requirement for pLNs to induce protective Th1 immunity and suggests that whether Th1 or Th2 priming to the same antigen occurs depends on the site of the primary antigen recognition.
Secondary lymphoid organs serve as sites of contact between T cells, B cells, antigen-presenting cells (APCs), and follicular dendritic cells (DC) within a highly structured and organized environment. Antigen-specific T-cell responses are generated within this environment. The spleen, LNs, Peyer's patches (PPs), and mucosa-associated lymphoid tissues are examples of secondary lymphoid organs.
Cutaneous Leishmania major infection in mice (experimental leishmaniasis) is widely used as an example to study the innate and adaptive immune responses toward infectious disease (25, 32, 39). A number of studies have demonstrated the induction of a specific immune response after L. major infection in local skin-draining LNs (6, 20, 22, 31).
Resistance to L. major infection is linked to the ability to mount an L. major-specific Th1 cell response, which leads to the activation of macrophages for elimination of the obligatory intracellular parasite. Thus, in resistant mice, such as C57BL/6 mice, infection is transient and is controlled at the site of primary infection. Susceptibility in BALB/c mice, on the other hand, is associated with the development of an L. major-specific Th2 response, which results in a failure to control parasite growth and subsequent fatal visceral spread.
Lymphotoxin (LT) is a member of the tumor necrosis factor (TNF) cytokine family. While soluble LTα3 homotrimers interact with the TNF receptors I (p55) and II (p75), the LTα1β2 heterodimer interacts exclusively with the membrane-bound LTβ receptor (LTβR). There is another ligand for the LTβR, called LIGHT (for lymphotoxin-like, exhibits inducible expression, competes with herpes simplex virus glycoprotein D for HVEM, a receptor expressed by T lymphocytes), which can also bind to the receptor HVEM (herpes virus entry mediator) (40). LTβR-mediated signaling is crucial for the development of LNs and PPs during gestation (12, 27, 29).
LT-β gene-deficient (−/−) mice lack most LNs and all PPs except cervical and mesenteric LNs. The phenotype of these mice includes additional alterations in the immune system, which lead to disruption of splenic germinal center formation and antibody responses (e.g., loss of splenic marginal zones [MZ] and of follicular DC networks in spleen and LNs) (10, 11, 17, 18, 21).
LTβR−/− mice (12) lack PPs and LNs and have a profoundly altered splenic architecture with ill-defined T- and B-cell areas.
In experimental leishmaniasis, peripheral LNs (pLNs) are considered the organ where priming of L. major-specific T cells occurs. We addressed the question of whether pLNs are absolutely required for protective immunity against L. major in genetically resistant C57BL/6 mice.
Therefore, we generated wild-type (wt) mice deficient in pLNs. Signaling via LTβR is crucial during gestation for the development of LNs and PPs (12, 27, 29). Thus, blockade of membrane LT in utero during a certain time interval can irreversibly prevent development of LNs and PPs.
These organogenic defects in LN development are irreversible, while the architecture of the remaining secondary lymphoid organs, including B-cell localization, integrity of splenic MZ populations, and the expression of the addressins MAdCAM-1 and peripheral node addressin, is restored in the mesenteric, sacral, lumbar, and cervical LNs of the adult progeny (26, 27).
In order to investigate whether the non-skin-draining LNs present in wt mice lacking pLNs contribute to the development of an L. major-specific immune response, we also studied the course of L. major infection in LTβR−/− mice completely lacking all LNs.
These models, together with the previously analyzed LTβ−/− mouse model and a transient blockade of LTβR-mediated signaling by LTβR-immunoglobulin G (IgG) fusion protein in wt mice, allowed us to distinguish between the roles of LNs and of the LTβR signaling pathway in the development of the host defense against L. major.
Our data demonstrate that protection against L. major is abrogated in wt C57BL/6 mice deficient in pLNs (and also in C57BL/6 mice lacking all LNs) and that this abrogation is associated with the generation of an L. major-specific Th2 response. In addition, the presence of mesenteric LNs was not sufficient to induce resistance against L. major. Our results show that local draining LNs are indispensable for the generation of an L. major-specific Th1 response.
MATERIALS AND METHODS
Mice.
C57BL/6 wt mice were purchased from Jackson Laboratories (Bar Harbor, ME). C57BL/6 LTβ−/− mice were provided by N. Ruddle (Yale University, New Haven, CT). C57BL/6 LTβR−/− mice were provided by K. Pfeffer (University of Düsseldorf, Düsseldorf, Germany). All mice were kept under sterile conditions in microisolator cages in the animal facilities of the Münster University Department of Dermatology with unlimited access to food and water according to federal animal protection regulations (permits G5/99 and G78/2000). Moribund mice were euthanized.
Generation of LTβR-IgG fusion protein and treatment with LTβR-IgG.
LTβR-IgG was purified from supernatants of an LTβR-IgG-expressing CHO cell line using protein A affinity chromatography. Mice were intraperitoneally injected with 100 μg LTβR-IgG once or twice weekly. In all experiments, mice were injected with LTβR-IgG 24 h prior to infection. Purified human immunoglobulin (huIgG) was purchased from Biotest (Dreieich, Germany). The biological efficiency of this treatment protocol in blocking LTβR-mediated signaling has previously been demonstrated with C57BL/6 mice infected with Citrobacter rodentium (36) and was also tested using an LN ablation protocol (27).
Gestational treatment of mice with LTβR-IgG and anti-TNF antibody.
Female C57BL/6 wt mice were screened daily for the presence of a vaginal plug as a sign of conception. Pregnant mice were intravenously injected with 100 μg LTβR-IgG and anti-TNF antibody TN3-19.12 (Abcam, Cambridge, United Kingdom) on days 11, 13, 15, and 17 following conception. Progeny (male and female) of gestationally treated mice were used at the age of 10 to 12 weeks for L. major infection. At the end of infection experiments, mice were euthanized and the presence of LNs was individually investigated. The progeny of all fusion protein-treated mothers lacked popliteal, inguinal, and cervical LNs. Mesenteric LNs could be detected in all of these mice.
Generation of chimeric mice.
wt C57BL/6 and LTβR−/− mice received a lethal dose of radiation (5.0 Gy on 2 days, resulting in a cumulative dose of 10 Gy) and were reconstituted with bone marrow cells (107 cells/mouse) obtained from wt C57BL/6 mice or from LTβR−/− mice. For experimental leishmaniasis, chimeric mice were used 5 weeks after bone marrow reconstitution.
Cytokine and proliferation assay.
For cytokine assay, mice were euthanized and spleens were aseptically removed. A single-cell suspension was prepared, and CD4+ T cells were collected using biomagnetic enrichment procedures (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's recommendations.
Bone marrow-derived DC were generated as previously described (35). Shortly thereafter, the femur bone was aseptically removed from euthanized C57BL/6 mice and the bone marrow was flushed out. Bone marrow DC were expanded with interleukin 4 (IL-4) and granulocyte-macrophage colony-stimulating factor for 6 days. DC (1 × 106 cells/ml) were incubated with soluble leishmania antigen equivalent to 5 × 106 L. major organisms for 48 h. For assessment of cytokine secretion, DC and CD4+ T cells (5 × 104/100 μl) were mixed in a 1:5 ratio and cultured in RPMI 1640 plus 2 mM glutamine, 50 μM mercaptoethanol, and 10% fetal calf serum for 48 h. Culture supernatants were assayed by enzyme-linked immunosorbent assay (ELISA) (Pharmingen) for IL-4 (detection limit, <14 pg/ml) and gamma interferon (IFN-γ) (detection limit, < 2.5 pg/ml) according to the manufacturer's instructions.
Parasites and experimental infection.
L. major (WHO nomenclature MHOM/IL/81/FE/BNI) was cultivated in Schneider's drosophila medium supplemented with 10% fetal calf serum, 2% human urine, 2% glutamine, and 1% penicillin-streptomycin (14). Soluble leishmania antigen was prepared by five repeated freeze and thaw cycles in phosphate-buffered saline.
Experimental leishmaniasis.
Cutaneous leishmaniasis was initiated by subcutaneous application of 2 × 107 promastigotes (stationary phase) of L. major in 50 μl phosphate-buffered saline into the left hind footpad of 4 to 14 mice per experimental group. In additional experiments, smaller parasite inocula (105 and 106 stationary-phase promastigotes) and 2 × 107 nonviable L. major organisms were used. The latter were obtained by three repeated freeze-thaw cycles with living parasites. Footpad thickness was assessed weekly using a metric caliper. Specific swelling of the infected footpad was assessed by subtracting the diameter of the infected footpad from that of the noninfected footpad. The course of infection was monitored for 8 to 12 weeks. When mice developed significant foot ulcers, the experiment was terminated instantly and the mice were euthanized. Footpads, bone marrow, liver, and spleen from each mice were harvested for limiting dilution assay (LDA) and determination of the cytokine profile. The experiments were repeated two to three times.
LDA.
Parasite numbers in bone marrow and liver as a parameter for systemic spread were determined 8 to 12 weeks after infection by an LDA (37) modified by using leishmania medium as specified above instead of slant blood agar (8).
Statistical analysis.
Means and standard errors (SE) of the means of all numerical data were calculated. Statistical significance between groups was judged by either the Mann-Whitney U test or Student's t test. A comparison of data sets yielding a P value of >0.05 was considered to not be statistically significant.
RESULTS
Mice lacking pLNs are susceptible to L. major infection and show an L. major-specific Th2 response.
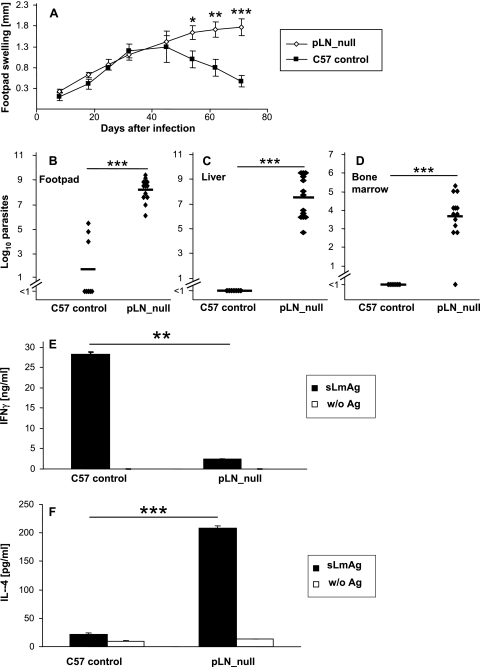
wt mice lacking pLNs were generated by gestational blockade of membrane-bound lymphotoxin by injection of LTβR-IgG and anti-TNF antibody on days 11, 13, 15, and 17 following conception. Their offspring were infected with stationary-phase L. major promastigotes, and the lack of pLNs was confirmed in each mouse after sacrifice. In these mice, lesions progressed further than in control mice (Fig. 1A). Ten weeks after infection, lesions had nearly completely resolved in control mice, as expected, while in wt mice lacking pLNs, chronic lesions with occasional small ulcers had developed. Correspondingly, wt mice lacking pLNs harbored markedly more parasites in cutaneous lesions (Fig. 1B).
FIG. 1.
(A) Footpad swelling (compared to the uninfected contralateral footpad) (mm) of wt mice lacking pLNs (pLN_null) generated by gestational treatment with anti-TNF and LTβR-IgG fusion protein (see Materials and Methods) and control C57BL/6 (C57 control) mice (mean ± SE; n = 13 for wt mice lacking pLNs; n = 7 for C57BL/6). (B to D) LDA of footpads (B), liver (C), or bone marrow (D) of wt mice lacking pLNs or control C57BL/6 mice (C57 control) 10 weeks after infection. The number of living parasites for single mice (diamonds) and the means for wt mice lacking pLN and C57BL/6 mice (horizontal bar) are indicated. (E and F) Cytokine secretion of CD4+ cells isolated from spleens of wt mice lacking pLNs or control C57BL/6 mice 10 weeks after infection. Cells were incubated for 48 h with syngeneic DC stimulated for 48 h with soluble Leishmania antigen (sLmAg) (filled bars) or with unstimulated syngeneic dendritic cells (w/o Ag) (open bars). IFN-γ (E) or IL-4 (F) secretion was measured by ELISA (mean ± SE; n = 5). For all panels: *, P < 0.05; **, P < 0.01; ***, P < 0.001 (for differences between wt mice lacking pLNs and control C57BL/6 mice).
To analyze whether in wt mice lacking pLNs the course of disease was also qualitatively altered with regard to visceralization, we determined parasite numbers in liver and bone marrow to detect systemic infection. As depicted in Fig. 1C and D, wt mice lacking pLNs harbored high numbers of parasites in liver and bone marrow, while, as expected, there was no systemic dissemination in control mice. Since sufficient differentiation and propagation (9, 33) of Th1 cells are indispensable for resistance to L. major infection, we analyzed cytokine production by CD4+ T cells. As shown in Fig. 1E and F, wt mice lacking pLNs secreted larger amounts IL-4 while the production of IFN-γ was markedly reduced compared to results with control mice. In conclusion, wt mice lacking pLNs present an L. major-specific immune response despite the lack of pLNs; however, the absence of pLNs was associated with a switch from Th1 toward Th2 differentiation.
Mice lacking LNs due to genetic depletion of lymphotoxin ligands or receptors are susceptible to L. major infection and show Th2-cell differentiation.
In wt mice lacking pLNs, non-skin-draining mesenteric LNs are still present. To clarify whether the presence of these LNs is responsible for the observed Th2 differentiation, we analyzed the course of L. major infection in LTβR−/− mice, which completely lack LNs, including mesenteric LNs.
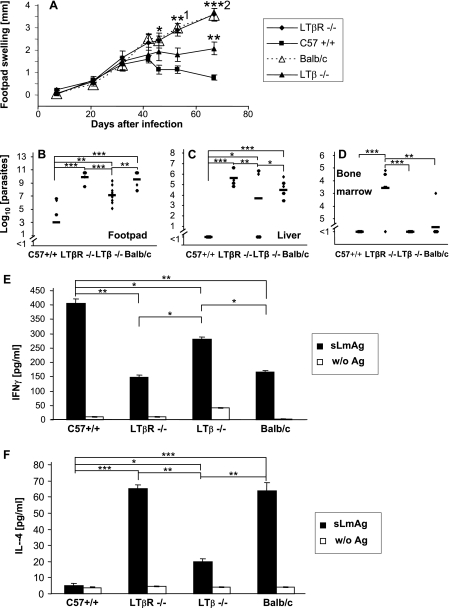
As shown in Fig. 2A, lesions were markedly progressive in LTβR−/− mice. The progression of lesions was similar to that observed in the prototypically susceptible BALB/c mice. Conversely, there was regression of lesions in control C57BL/6 wt mice 6 weeks after infection, as expected.
FIG. 2.
(A) Footpad swelling (compared to the uninfected contralateral footpad) (mm) of infected LTβR−/−, LTβ−/−, wt C57BL/6 (C57 +/+), and BALB/c mice (mean ± SE; n = 9 for C57BL/6 wt and LTβ−/− mice; n = 8 for LTβR−/− and BALB/c mice). 1, P < 0.05; 2, P < 0.01 (for differences between LTβR−/− and LTβ−/− mice). *, P < 0.05; **, P < 0.01; ***, P < 0.001 (compared to results for wt C57BL/6 mice). (B to D) LDA of footpads (B), liver (C), or bone marrow (D) of LTβR−/−, LTβ−/−, wt C57BL/6 (C57+/+), or BALB/c mice 10 weeks after infection. The number of living parasites for single mice (diamonds) and the means for LTβR−/−, LTβ−/−, wt C57BL/6, and BALB/c mice (horizontal bars) are indicated. (E and F) Cytokine secretion of CD4+ cells isolated from spleens of LTβR−/−, LTβ−/−, wt C57BL/6 (C57+/+), or BALB/c mice 10 weeks after infection. Cells were incubated for 48 h with syngeneic DC stimulated for 48 h with soluble Leishmania antigen (sLmAg) (filled bars) or with unstimulated syngeneic DC (open bars). IFN-γ (E) or IL-4 (F) secretion was measured by ELISA (mean ± SE; n = 5). For panels B to F: *, P < 0.05; **, P < 0.01; ***, P < 0.001 (for differences between indicated mice).
Accordingly, footpads of LTβR−/− mice contained as many parasites as those of susceptible BALB/c mice (up to 1 × 105-fold more parasites than in wt C57BL/6 mice) (Fig. 2B). Moreover, high numbers of parasites were detected in livers and bone marrow of LTβR−/− mice, as was found for BALB/c mice (Fig. 2C and D).
To clarify the contribution of T-helper-cell differentiation in the deficient parasite containment, we analyzed the presence and extent of an L. major-specific T-cell response 10 weeks after infection (Fig. 2E and F).
For LTβR−/− mice, we found an L. major-specific IL-4 secretion which was as high as that for BALB/c mice.
Since even in susceptible BALB/c mice there is a higher absolute concentration of released IFN-γ than of IL-4, the degree of Th1 or Th2 response can best be expressed as the ratio of released antigen-specific IFN-γ to IL-4.
This ratio revealed that the Th2 response was indeed as strong in LTβR−/− mice as in BALB/c mice due to equal IFN-γ secretion.
Thus, LTβR−/− mice, which revealed the same degree of parasite dissemination as BALB/c mice, also presented the same strong Th2 switch.
Experimental leishmaniasis has previously also been analyzed with LTβ−/− mice. These mice resemble wt mice lacking pLNs, since they lack pLNs and develop mesenteric LNs. Mucosa-draining cervical and sacral LNs are present in LTβ−/− mice. Like wt mice lacking pLNs, LTβ−/− mice were shown to develop a spread of parasites to visceral organs, but they were not reported to develop a Th2 switch.
In comparison with our results, this could mean that the Th2 switch is prevented by the presence of mucosa-draining cervical and sacral LNs, the only lymphoid organs present in LTβ−/− mice but not in wt mice lacking pLNs and also not in LTβR−/− mice.
When we analyzed infection in these mice, we found that in similarity to wt mice lacking pLNs, they developed stable but nonhealing lesions with occasional small ulcers (Fig. 2A) and also visceralization (Fig. 2B). In agreement with Xu et al. (42), we found lower antigen-specific IFN-γ secretion in LTβ−/− mice, but in contrast to previously published data, we detected a marked, antigen-specific secretion of IL-4 by CD4+ T cells derived from LTβ−/− mice (Fig. 2E and F). IL-4 levels were significantly higher than those in wt C57BL/6 mice, where antigen-induced IL-4 secretion was barely detectable (the IFNγ/IL-4 ratio in C57BL/6 wt mice is approximately 250, versus 2.7 in BALB/c mice, while the values are 15 for LTβ−/− mice and 2.3 for LTβR−/− mice). This clearly indicates that a switch toward a Th2 response pattern had occurred in LTβ−/− mice, associated with systemic disease in these mice.
The Th2 response in LN-deficient mice occurs early after infection and also after injection of nonviable parasites.
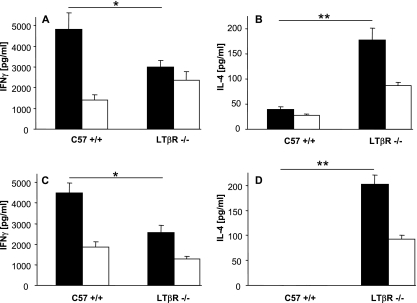
It could be argued that the Th2 switch observed in LN-deficient mice is a consequence rather than the cause for susceptibility, since it could be due to constant and prolonged L. major stimulation. To address this issue, we also analyzed cytokine production in LTβR−/− and C57BL/6 wt mice very early after infection, when there was no apparent difference in the clinical course of disease. As such, we already observed a clear L. major-specific Th2 response pattern, with lower IFN-γ secretion and higher IL-4 levels than were found for C57BL/6 wt mice (Fig. 3A and B), 2 weeks after infection. To further exclude an influence of uncontrolled parasite growth on Th2 differentiation, we injected parasites killed by repeated freeze-thaw cycles into LTβR−/− and C57BL/6 wt mice and measured L. major-specific cytokine production 2 weeks later. We detected L. major-specific cytokine secretion in LTβR−/− and C57BL/6 wt mice, which was clearly shifted toward a Th2 response pattern in LTβR −/− mice (Fig. 3C and D). These experiments indicate that Th2 differentiation is not secondary to susceptibility in LN-deficient mice but is likely to be the cause of the observed susceptibility in these mice.
FIG. 3.
(A and B) Cytokine secretion of CD4+ cells isolated from spleens of LTβR−/− or wt C57BL/6 (C57 +/+) mice 2 weeks after L. major infection. Cells were incubated for 48 h with syngeneic DC stimulated for 48 h with soluble Leishmania antigen (filled bars) or with unstimulated syngeneic DC (open bars). IFN-γ (A) or IL-4 (B) secretion was measured by ELISA (mean ± SE; n = 3). (C and D) Cytokine secretion of CD4+ cells isolated from spleens of LTβR−/− or wt C57BL/6 (C57 +/+) mice 2 weeks after injection of 2 × 107 nonviable L. major parasites. Cells were incubated for 48 h with syngeneic DC stimulated for 48 h with soluble Leishmania antigen (filled bars) or with unstimulated syngeneic DC (open bars). IFN-γ (C) or IL-4 (D) secretion was measured by ELISA (mean ± SE; n = 3). For all panels: *, P < 0.05; **, P < 0.01; ***, P < 0.001 (for differences between indicated mice).
Infection of LTβR−/− mice with smaller L. major inocula also induces a Th2 response.
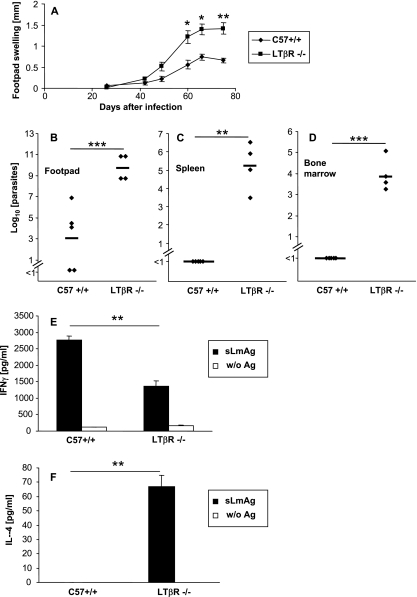
Different numbers of parasites used to initiate an infection can have a distinct impact on the course of infection (3, 4, 38). Moreover, with use of large parasite inocula, an early dissemination of parasites beyond the local lymph nodes has been linked to susceptibility in BALB/c mice (20). A DC-independent transport of L. major antigen to skin-draining LNs resulting in initiation of an adaptive immune response by LN-resident DC populations has been reported (15, 22). To test whether the observed Th2 switch in LN-deficient mice is related to a DC-independent parasite dissemination as a result of the high-dose infection, we also infected LTβR−/− and C57BL/6 wt mice with 105 (data not shown) and 104 (Fig. 4) promastigote parasites. While the course of infection was prolonged, as expected, due to the smaller parasite inocula, we did not observe any differences in Th2 differentiation (Fig. 4E and F), local parasite containment (Fig. 4B), and visceralization (Fig. 4C and D) compared to results with the high-dose infection model. Thus, Th2 differentiation was not restricted to large parasite inocula in LN-deficient mice.
FIG. 4.
(A) Footpad swelling (compared to the uninfected contralateral footpad) (mm) of LTβR−/− and wt C57BL/6 (C57+/+) mice infected with a small parasite inoculum (104 parasites) (mean ± SE; n = 4 for LTβR−/− mice and n = 5 for C57+/+ mice). *, P < 0.05; **, P < 0.01; ***, P < 0.001 (compared to wt C57BL/6 mice). (B to D) LDA of footpads (B), spleen (C), or bone marrow (D) of LTβR−/− or control C57BL/6 wt (C57 +/+) mice 11 weeks after infection. The number of living parasites for single mice (diamonds) and the means for LTβR−/− and wt C57BL/6 mice (horizontal bars) are indicated. (E and F) Cytokine secretion of CD4+ cells isolated from spleens of LTβR−/− and wt C57BL/6 (C57+/+) mice 11 weeks after infection. Cells were incubated for 48 h with syngeneic DC stimulated for 48 h with soluble Leishmania antigen (sLmAg) (black bars) or with unstimulated syngeneic DC (open bars). IFN-γ (E) or IL-4 (F) secretion was measured by ELISA (mean ± SE; n = 3). For all panels: *, P < 0.05; **, P < 0.01; ***, P < 0.001 (for differences between LTβR−/− and wt C57BL/6 mice).
LTβR-mediated signaling has no effect on L. major infections.
In contrast to results with wt mice lacking pLNs, the genetic models present with alterations of LTβR-mediated signaling during infection. In LTβR−/− mice, LTβR-mediated signaling is completely abolished, while in LTβ−/− mice, LIGHT, a second ligand of the LTβR, can still bind to the receptor (40). LTβR is expressed on monocytic cells, and the relevance of LTβR-mediated signaling for the course of adaptive and innate immune responses has previously been reported (7, 34).
Therefore, we cannot exclude an influence of LTβR-mediated signaling on L. major infection in these genetically depleted mice.
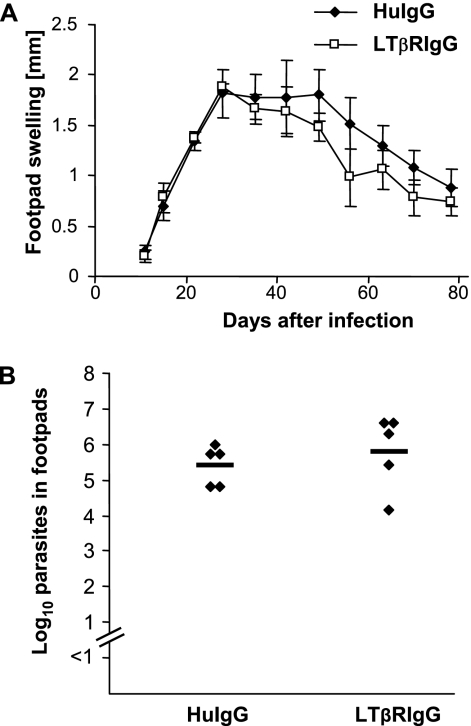
In order to exclude an influence of LIGHT-LTβR signaling on the course of L. major infection which is not secondary to the lack of pLNs, we treated control C57BL/6 wt mice repeatedly with LTβR-IgG prior to and during L. major infection. As shown in Fig. 5A and B, we detected no influence of LTβR-IgG treatment on footpad swelling, parasite load in feet (Fig. 5B), and prevention of parasite dissemination (data not shown).
FIG. 5.
(A) Footpad swelling (compared to the uninfected contralateral footpad) (mm) of LTβR-IgG fusion protein-treated (LTβRlgG) or human IgG-treated (HuIgG) C57BL/6 mice (mean ± SE; n = 5). (B) LDA of footpads of LTβR-IgG fusion protein-treated or huIgG-treated C57BL/6 mice 12 weeks after infection. The number of living parasites for single mice (diamonds) and the means (horizontal bars) are indicated.
Thus, in the presence of pLNs, we could not detect an influence of LIGHT-LTβR signaling on L. major infection.
In agreement with these data, we further showed that the presence of LTβR on monocytes and other hematopoietic cells has no decisive influence on the high susceptibility of LTβR−/− mice: in wt-to-LTβR bone marrow chimeric (wt→LTβR) mice, we detected systemic disease similar to that in LTβR mice (log parasites in footpads, 6.7 for wt→LTβR mice versus 7.8 for LTβR−/− mice; in liver, 6.4 versus 6.5; in bone marrow, 4.0 versus 4.5). wt→LTβR mice developed a Th2 response with high levels of antigen-specific IL-4 (IFNγ/IL-4 ratio, 12.6, versus 5.3 in LTβR−/− mice and 381 in control C57BL/6 wt mice). Correspondingly, an absence of LTβR on hematopoietic cells had no decisive impact on the course of infection in LTβR−/−-to-wt bone marrow chimeric (LTβR→wt) mice. LTβR→wt mice developed a Th1 response like that of wt C57BL/6 mice (IFNγ/IL-4 ratio of 376, versus 389 in control C57BL/6 wt mice). In agreement with this, there was no dissemination to the spleen, liver, or bone-marrow in LTβR→wt mice as in control mice and we found no significant differences in the local parasite burden (log parasites in footpads, 5.2 in LTβR→wt mice versus 4.5 in control wt C57BL/6 mice).
The spleen is dispensable for resistance to cutaneous L. major infections.
An antigen-specific immune response after subcutaneous infection with L. major is also generated in mice lacking all LNs (LTβR−/− mice). This suggests that in the absence of LNs, T-cell priming must then take place in the spleen, resulting in Th2 cells. Therefore, in C57BL/6 mice with draining LNs but without a spleen, skin-draining LNs should suffice to induce a protective Th1 response. To test this hypothesis, we infected splenectomized and sham-operated C57BL/6 mice with L. major.
We detected no influence of splenectomy on footpad swelling (data not shown) and parasite clearance in L. major infection (log parasites in footpads of splenectomized mice, 6.2, versus 5.9 in sham-operated mice). There was no evidence for L. major dissemination in splenectomized mice.
DISCUSSION
In the absence of skin-draining LNs, infection of C57BL/6 mice with L. major results in an L. major-specific T-cell response. However, despite the genetically resistant background, the infection is associated with a Th2 response and systemic dissemination of parasites. The presence of non-skin-draining LNs in these mice was not sufficient to induce resistance against L. major. Therefore, our results clearly indicate that for the generation of an L. major-specific Th1 response, the presence of skin-draining LNs appears to be indispensable.
The nature of an immune response has been thought to classically depend on two signals: (i) the antigen and (ii) the costimulatory signals. More recently a third signal has been proposed, consisting of additional signals by DC triggered via the local cytokine milieu (16). Our results strongly suggest that the secondary lymphatic organs utilized for antigen presentation provide a fourth signal which critically influences the type of T-cell response.
These results were obtained by using mice with a resistant C57BL/6 background, which were made deficient in skin-draining peripheral LNs by gestational treatment with LTβR-IgG and anti-TNF. After such treatment, mice with the BALB/c background had already been demonstrated to be able to generate normal antigen-specific T-cell responses against small antigens in the contact hypersensitivity model. The morphological and functional composition of the remaining lymphoid organs (mucosal and mesenteric lymph nodes and spleen) was not influenced, nor were the prerequisites for direct interactions between T cells and APCs decisively impaired (28).
In our study, we also used mice with genetic depletions of LTβ signaling components (LTβ−/− and LTβR−/− mice). This allowed us to compare the immune response of wt mice lacking pLNs with those of mice that completely lack LNs and mice that lack pLNs but develop mucosa-draining cervical and sacral LNs (LTβ−/− mice).
The effect of draining LNs on the course of Leishmania infection has been analyzed in one previous study, but there, LNs were surgically removed from BALB/c mice. Infection with L. major in these mice resulted in more-severe disease (24), but these results are difficult to interpret because surgical removal of LNs also entails disruption of lymph vessels. In contrast, our procedure allowed us to analyze the effect of the absence of secondary lymphoid organs on the T-cell-dependent immune response in the presence of intact lymphatic vessels.
The importance of different routes of infection has been analyzed in two previous studies. Intranasal infection of resistant C57BL/6 mice was associated with a Th2 immune response (5). A shift toward a Th2 response was also observed in (C57BL/6 × BALB/c) F1 mice when mice were infected in the dorsal skin instead of the footpad (23).
Mesenteric LNs are known to favor a Th2 response. As such, ovalbumin-pulsed DC derived from pLNs induce a Th1 response, while DC derived from mesenteric LNs induce a Th2 response (2).
Therefore, the type of T-cell response and the ensuing course of L. major infection may depend on the site of antigen delivery, partly because of the type of APCs utilized for antigen presentation (2, 5, 23).
A recent study demonstrates that in subcutaneous infection, as applied in our model, the predominant uptake and transport of L. major to draining LNs is mainly executed by dermal DC and not by epidermal Langerhans cells (31). This is important, since dermal DC, in contrast to Langerhans cells, have been demonstrated to enter the spleen for antigen presentation (28). Recently, a rapid, DC-independent transport of parasite antigen and living parasites to skin-draining LNs using high-dose infection has been reported (22). Moreover, LN-resident cells rather than skin-derived DC have been demonstrated to induce a T-cell response directed against the L. major antigen LACK (Leishmania homolog for receptors of activated C kinase) in the high-dose infection model (15). Early parasite dissemination beyond the local LNs has previously been linked to susceptibility in BALB/c mice. Therefore, we also analyzed L. major infection in LTβR−/− mice infected with small parasite inocula, having thus a much lesser probability of DC-independent parasite dissemination. Using small parasite inocula, we observed no differences from high-dose infection, indicating that the observed Th2 switch is not restricted to large parasite inocula. Thus, an effect of the absence of T-cell priming by LN-resident DC in LN-deficient mice on the observed Th2 switch cannot be excluded but seems unlikely. Additionally, since T-cell priming by LN-resident DC resulted in a Th2 response in susceptible and resistant mice (22), the absence of this mechanism in LN-deficient mice cannot easily explain the observed Th2 switch.
Since an L. major-specific immune response was induced in mice deficient in peripheral lymph nodes, it appears likely that T-cell priming has taken place in the spleen or in the remaining nondraining mesenteric LNs.
Recent studies demonstrate that in the absence of certain secondary lymphoid organs, a specific immune response can indeed be induced in the remaining lymphoid organs, including the spleen. As such, in the absence of draining LNs, dermal DC were shown to still be able to induce a contact hypersensitivity response after subcutaneous administration of hapten. When antigen was applied epicutaneously, a single mesenteric LN was sufficient to induce contact hypersensitivity (28). In transplant rejection there was an overlapping requirement for the spleen or LNs (19).
To clarify how far the presence of different non-skin-draining LNs is responsible for Th2 differentiation in wt mice lacking pLNs, we had additionally analyzed experimental leishmaniasis in LTβR−/− mice, which completely lack LNs, and in LTβ−/− mice, which closely resemble wt mice lacking pLNs but which still develop mucosa-draining cervical and sacral LNs.
Experimental leishmaniasis has previously been analyzed with the latter and shown to result in systemic infection. Previous studies showed either no differences in Th differentiation or a reduction in IFN-γ secretion without increased IL-4 release (41, 42).
In contrast to findings of Xu et al. (42) and Wilhelm et al. (41), however, we detected a clear Th2 response in LTβ−/− mice.
The different results regarding IL-4 are most likely due to the methods used for analyzing the T-cell response. Wilhelm et al. (41) were analyzing cytokine mRNA expression in splenic cells when they found no alterations in Th differentiation, while Xu et al. (42) were stimulating whole-spleen-cell populations with L. major antigen when they reported no IL-4 secretion. We analyzed the cytokine response of isolated CD4+ T cells and were able to detect a marked IL-4 secretion after stimulation with Leishmania antigen-primed DC.
Thus, we conclude that in LTβ−/− mice, just as in wt mice lacking pLNs, a Th2 response is induced in the remaining non-skin-draining LNs or the spleen.
It could be argued that the Th2 switch observed in all LN-deficient mice analyzed in this study is not the cause for susceptibility but may be a consequence of constant and prolonged L. major stimulation. However, a Th2 cytokine response pattern was detected very early in L. major-infected LTβR−/− mice and also after inoculation with nonviable parasites. This clearly supports our conclusion that susceptibility in LN-deficient mice is a consequence of early Th2 differentiation due to the absence of pLNs.
While our results suggest that LNs are indispensable for an L. major-specific Th1 response, the spleen appears to be dispensable for a protective Th1-type immunity against L. major in the presence of pLNs. We could show that splenectomized wt mice cleared L. major similarly to sham-operated mice.
The question remained of whether in LTβR- or LTβ-deficient mice it was the absence of pLNs which led to a Th2 switch or rather the alterations in LTβR-mediated signaling.
In LTβR-deficient mice, LTβR-mediated signaling is completely abolished, while in LTβ−/− mice, LIGHT, a second ligand of the LTβR, can still bind to the receptor (40). LTβR is expressed on monocytic cells, and LTβR-mediated signaling influences the course of adaptive and innate immune responses (7, 34). Recently LIGHT has been reported to be important for IL-12 production of DC and resistance in experimental leishmaniasis (43). However, the authors argue that these effects are primarily mediated by binding of LIGHT to the HVEM receptor.
This raises the question of impaired DC or macrophage function as a cause distinct from the absence of secondary lymphoid tissues. There are several observations which argue against this hypothesis: no alterations in DC and macrophage functions were described for mice deficient in lymphotoxin ligands and receptors (1, 30, 42). In similarity to previously published data regarding LTβ−/− mice (41), we observed no decisive effect on infection following transfer either of LTβR−/− bone marrow to wt mice or wt bone marrow to LTβR−/− mice. Therefore, the lack of LTβR expression on bone marrow-derived APC does not interfere with immunity against L. major infection, and the presence of the LTβR on bone marrow-derived cells was not sufficient to induce resistance. Furthermore, in contrast to the findings of Xu et al. (43), we could not demonstrate that interruption of LTβR-mediated signaling by LTβR-IgG treatment prior to and during infection affected resistance to L. major in control (C57BL/6 wt) mice.
All of these results provide no evidence for a dominant role of LTβR-mediated signaling in experimental leishmaniasis.
In conclusion, we have demonstrated for the first time that L. major infection in the absence of pLNs in mice of resistant background (C57BL/6) results in a Th2 immune response and susceptibility. Thus, in contrast to pLNs, the lymphoid microenvironment of mucosa-draining LNs and/or the spleen results in the formation of Th2 effector cells, which fail to control leishmaniasis.
The Th2 bias of these lymphoid organs may comprise a physiological mechanism for antigens which enter the organism via the intestine or the systemic circulation. In both cases, induction of Th1-type cell-mediated immunity would result in severe tissue damage. Thus, our data indicate that the initiation of a tissue-adapted immune response is controlled at two sites: at the tissue itself by the local microenvironment and/or different populations of DC (13) and at the draining lymphatic organs. The mechanism by which lymphoid organs regulate T-cell differentiation might be signals either from LN stroma cells or from the T-cell and DC populations present in the organ.
Acknowledgments
We thank Sonja Jansen, Sandra Doths, and Eva Nattkemper for outstanding technical support.
This work was supported by grants from the Innovative Medizinische Forschung (SP 1 2 03 18, to T.W.S.) and the Interdisciplinary Clinical Research Center, Münster (Sun 2/019/07, to C.S. and J.M.E.), of the University of Münster.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 14 July 2008.
REFERENCES
- 1.Abe, K., F. O. Yarovinsky, T. Murakami, A. N. Shakhov, A. V. Tumanov, D. Ito, L. N. Drutskaya, K. Pfeffer, D. V. Kuprash, K. L. Komschlies, and S. A. Nedospasov. 2003. Distinct contributions of TNF and LT cytokines to the development of dendritic cells in vitro and their recruitment in vivo. Blood 1011477-1483. [DOI] [PubMed] [Google Scholar]
- 2.Alpan, O., G. Rudomen, and P. Matzinger. 2001. The role of dendritic cells, B cells, and M cells in gut-oriented immune responses. J. Immunol. 1664843-4852. [DOI] [PubMed] [Google Scholar]
- 3.Belkaid, Y., S. Mendez, R. Lira, N. Kadambi, G. Milon, and D. Sacks. 2000. A natural model of Leishmania major infection reveals a prolonged “silent” phase of parasite amplification in the skin before the onset of lesion formation and immunity. J. Immunol. 165969-977. [DOI] [PubMed] [Google Scholar]
- 4.Belkaid, Y., E. von Stebut, S. Mendez, R. Lira, E. Caler, S. Bertholet, M. C. Udey, and D. Sacks. 2002. CD8+ T cells are required for primary immunity in C57BL/6 mice following low-dose, intradermal challenge with Leishmania major. J. Immunol. 1683992-4000. [DOI] [PubMed] [Google Scholar]
- 5.Constant, S. L., J. L. Brogdon, D. A. Piggott, C. A. Herrick, I. Visintin, N. H. Ruddle, and K. Bottomly. 2002. Resident lung antigen-presenting cells have the capacity to promote Th2 T cell differentiation in situ. J. Clin. Investig. 1101441-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diefenbach, A., H. Schindler, N. Donhauser, E. Lorenz, T. Laskay, J. MacMicking, M. Rollinghoff, I. Gresser, and C. Bogdan. 1998. Type 1 interferon (IFNα/β) and type 2 nitric oxide synthase regulate the innate immune response to a protozoan parasite. Immunity 877-87. [DOI] [PubMed] [Google Scholar]
- 7.Ehlers, S., C. Holscher, S. Scheu, C. Tertilt, T. Hehlgans, J. Suwinski, R. Endres, and K. Pfeffer. 2003. The lymphotoxin beta receptor is critically involved in controlling infections with the intracellular pathogens Mycobacterium tuberculosis and Listeria monocytogenes. J. Immunol. 1705210-5218. [DOI] [PubMed] [Google Scholar]
- 8.Ehrchen, J., L. Helming, G. Varga, B. Pasche, K. Loser, M. Gunzer, C. Sunderkotter, C. Sorg, J. Roth, and A. Lengeling. 2007. Vitamin D receptor signaling contributes to susceptibility to infection with Leishmania major. FASEB J. 213208-3218. [DOI] [PubMed] [Google Scholar]
- 9.Ehrchen, J., A. Sindrilaru, S. Grabbe, F. Schonlau, C. Schlesiger, C. Sorg, K. Scharffetter-Kochanek, and C. Sunderkotter. 2004. Senescent BALB/c mice are able to develop resistance to Leishmania major infection. Infect. Immun. 725106-5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Endres, R., M. B. Alimzhanov, T. Plitz, A. Futterer, M. H. Kosco-Vilbois, S. A. Nedospasov, K. Rajewsky, and K. Pfeffer. 1999. Mature follicular dendritic cell networks depend on expression of lymphotoxin beta receptor by radioresistant stromal cells and of lymphotoxin beta and tumor necrosis factor by B cells. J. Exp. Med. 189159-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu, Y. X., and D. D. Chaplin. 1999. Development and maturation of secondary lymphoid tissues. Annu. Rev. Immunol. 17399-433. [DOI] [PubMed] [Google Scholar]
- 12.Futterer, A., K. Mink, A. Luz, M. H. Kosco-Vilbois, and K. Pfeffer. 1998. The lymphotoxin beta receptor controls organogenesis and affinity maturation in peripheral lymphoid tissues. Immunity 959-70. [DOI] [PubMed] [Google Scholar]
- 13.Gallucci, S., and P. Matzinger. 2001. Danger signals: SOS to the immune system. Curr. Opin. Immunol. 13114-119. [DOI] [PubMed] [Google Scholar]
- 14.Howard, M. K., M. M. Pharoah, F. Ashall, and M. A. Miles. 1991. Human urine stimulates growth of Leishmania in vitro. Trans. R. Soc. Trop. Med. Hyg. 85477-479. [DOI] [PubMed] [Google Scholar]
- 15.Iezzi, G., A. Frohlich, B. Ernst, F. Ampenberger, S. Saeland, N. Glaichenhaus, and M. Kopf. 2006. Lymph node resident rather than skin-derived dendritic cells initiate specific T cell responses after Leishmania major infection. J. Immunol. 1771250-1256. [DOI] [PubMed] [Google Scholar]
- 16.Kalinski, P., C. M. Hilkens, E. A. Wierenga, and M. L. Kapsenberg. 1999. T-cell priming by type-1 and type-2 polarized dendritic cells: the concept of a third signal. Immunol. Today 20561-567. [DOI] [PubMed] [Google Scholar]
- 17.Koni, P. A., and R. A. Flavell. 1999. Lymph node germinal centers form in the absence of follicular dendritic cell networks. J. Exp. Med. 189855-864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuprash, D. V., M. B. Alimzhanov, A. V. Tumanov, A. O. Anderson, K. Pfeffer, and S. A. Nedospasov. 1999. TNF and lymphotoxin beta cooperate in the maintenance of secondary lymphoid tissue microarchitecture but not in the development of lymph nodes. J. Immunol. 1636575-6580. [PubMed] [Google Scholar]
- 19.Lakkis, F. G., A. Arakelov, B. T. Konieczny, and Y. Inoue. 2000. Immunologic ‘ignorance’ of vascularized organ transplants in the absence of secondary lymphoid tissue. Nat. Med. 6686-688. [DOI] [PubMed] [Google Scholar]
- 20.Laskay, T., A. Diefenbach, M. Rollinghoff, and W. Solbach. 1995. Early parasite containment is decisive for resistance to Leishmania major infection. Eur. J. Immunol. 252220-2227. [DOI] [PubMed] [Google Scholar]
- 21.Mackay, F., and J. L. Browning. 1998. Turning off follicular dendritic cells. Nature 39526-27. [DOI] [PubMed] [Google Scholar]
- 22.Misslitz, A. C., K. Bonhagen, D. Harbecke, C. Lippuner, T. Kamradt, and T. Aebischer. 2004. Two waves of antigen-containing dendritic cells in vivo in experimental Leishmania major infection. Eur. J. Immunol. 34715-725. [DOI] [PubMed] [Google Scholar]
- 23.Nabors, G. S., T. Nolan, W. Croop, J. Li, and J. P. Farrell. 1995. The influence of the site of parasite inoculation on the development of Th1 and Th2 type immune responses in (BALB/c x C57BL/6) F1 mice infected with Leishmania major. Parasite Immunol. 17569-579. [DOI] [PubMed] [Google Scholar]
- 24.Poulter, L. W., and C. R. Pandolph. 1982. Mechanisms of immunity to leishmaniasis. IV. Significance of lymphatic drainage from the site of infection. Clin. Exp. Immunol. 48396-402. [PMC free article] [PubMed] [Google Scholar]
- 25.Reiner, S. L., and R. M. Locksley. 1995. The regulation of immunity to Leishmania major. Annu. Rev. Immunol. 13151-177. [DOI] [PubMed] [Google Scholar]
- 26.Rennert, P. D., J. L. Browning, and P. S. Hochman. 1997. Selective disruption of lymphotoxin ligands reveals a novel set of mucosal lymph nodes and unique effects on lymph node cellular organization. Int. Immunol. 91627-1639. [DOI] [PubMed] [Google Scholar]
- 27.Rennert, P. D., J. L. Browning, R. Mebius, F. Mackay, and P. S. Hochman. 1996. Surface lymphotoxin alpha/beta complex is required for the development of peripheral lymphoid organs. J. Exp. Med. 1841999-2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rennert, P. D., P. S. Hochman, R. A. Flavell, D. D. Chaplin, S. Jayaraman, J. L. Browning, and Y. X. Fu. 2001. Essential role of lymph nodes in contact hypersensitivity revealed in lymphotoxin-alpha-deficient mice. J. Exp. Med. 1931227-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rennert, P. D., D. James, F. Mackay, J. L. Browning, and P. S. Hochman. 1998. Lymph node genesis is induced by signaling through the lymphotoxin beta receptor. Immunity 971-79. [DOI] [PubMed] [Google Scholar]
- 30.Ritter, U., A. Meissner, J. Ott, and H. Korner. 2003. Analysis of the maturation process of dendritic cells deficient for TNF and lymphotoxin-alpha reveals an essential role for TNF. J. Leukoc. Biol. 74216-222. [DOI] [PubMed] [Google Scholar]
- 31.Ritter, U., A. Meissner, C. Scheidig, and H. Korner. 2004. CD8 alpha- and Langerin-negative dendritic cells, but not Langerhans cells, act as principal antigen-presenting cells in leishmaniasis. Eur. J. Immunol. 341542-1550. [DOI] [PubMed] [Google Scholar]
- 32.Sacks, D., and N. Noben-Trauth. 2002. The immunology of susceptibility and resistance to Leishmania major in mice. Nat. Rev. Immunol. 2845-858. [DOI] [PubMed] [Google Scholar]
- 33.Schonlau, F., K. Scharffetter-Kochanek, S. Grabbe, B. Pietz, C. Sorg, and C. Sunderkotter. 2000. In experimental leishmaniasis deficiency of CD18 results in parasite dissemination associated with altered macrophage functions and incomplete Th1 cell response. Eur. J. Immunol. 302729-2740. [DOI] [PubMed] [Google Scholar]
- 34.Spahn, T. W., H. P. Eugster, A. Fontana, W. Domschke, and T. Kucharzik. 2005. Role of lymphotoxin in experimental models of infectious diseases: potential benefits and risks of a therapeutic inhibition of the lymphotoxin-β receptor pathway. Infect. Immun. 737077-7088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spahn, T. W., S. Issazadah, A. J. Salvin, and H. L. Weiner. 1999. Decreased severity of myelin oligodendrocyte glycoprotein peptide 33-35-induced experimental autoimmune encephalomyelitis in mice with a disrupted TCR delta chain gene. Eur. J. Immunol. 294060-4071. [DOI] [PubMed] [Google Scholar]
- 36.Spahn, T. W., C. Maaser, L. Eckmann, J. Heidemann, A. Lugering, R. Newberry, W. Domschke, H. Herbst, and T. Kucharzik. 2004. The lymphotoxin-beta receptor is critical for control of murine Citrobacter rodentium-induced colitis. Gastroenterology 1271463-1473. [DOI] [PubMed] [Google Scholar]
- 37.Titus, R. G., M. Marchand, T. Boon, and J. A. Louis. 1985. A limiting dilution assay for quantifying Leishmania major in tissues of infected mice. Parasite Immunol. 7545-555. [DOI] [PubMed] [Google Scholar]
- 38.von Stebut, E., J. M. Ehrchen, Y. Belkaid, S. L. Kostka, K. Molle, J. Knop, C. Sunderkotter, and M. C. Udey. 2003. Interleukin 1α promotes Th1 differentiation and inhibits disease progression in Leishmania major-susceptible BALB/c mice. J. Exp. Med. 198191-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.von Stebut, E. 2007. Cutaneous Leishmania infection: progress in pathogenesis research and experimental therapy. Exp. Dermatol. 16340-346. [DOI] [PubMed] [Google Scholar]
- 40.Ware, C. F. 2005. Network communications: lymphotoxins, LIGHT, and TNF. Annu. Rev. Immunol. 23787-819. [DOI] [PubMed] [Google Scholar]
- 41.Wilhelm, P., D. S. Riminton, U. Ritter, F. A. Lemckert, C. Scheidig, R. Hoek, J. D. Sedgwick, and H. Korner. 2002. Membrane lymphotoxin contributes to anti-leishmanial immunity by controlling structural integrity of lymphoid organs. Eur. J. Immunol. 321993-2003. [DOI] [PubMed] [Google Scholar]
- 42.Xu, G., D. Liu, Y. Fan, X. Yang, H. Korner, Y. X. Fu, and J. E. Uzonna. 2007. Lymphotoxin alpha beta 2 (membrane lymphotoxin) is critically important for resistance to Leishmania major infection in mice. J. Immunol. 1795358-5366. [DOI] [PubMed] [Google Scholar]
- 43.Xu, G., D. Liu, I. Okwor, Y. Wang, H. Korner, S. K. Kung, Y. X. Fu, and J. E. Uzonna. 2007. LIGHT Is critical for IL-12 production by dendritic cells, optimal CD4+ Th1 cell response, and resistance to Leishmania major. J. Immunol. 1796901-6909. [DOI] [PubMed] [Google Scholar]