Abstract
Chronic infection with the gastric pathogen Helicobacter pylori significantly increases the risk of developing atrophic gastritis, peptic ulcer disease, and gastric adenocarcinoma. H. pylori strains that possess the cag pathogenicity island, which translocates CagA into the host cells, augment these risks. The aim of this study was to determine the molecular mechanisms through which H. pylori upregulates the expression of plasminogen activator inhibitor 1 (PAI-1), a member of the urokinase activator system that is involved in tumor metastasis and angiogenesis. Levels of PAI-1 mRNA and protein were examined in tissues from H. pylori-infected patients and in vitro using AGS gastric epithelial cells. In vitro, cells were infected with toxigenic cag-positive or nontoxigenic cag-negative strains of H. pylori or isogenic mutants. The amount of PAI-1 secretion was measured by enzyme-linked immunosorbent assay, and mRNA levels were determined using real-time PCR. The regulation of PAI-1 was examined using the extracellular signal-regulated kinase 1/2 (ERK1/2) inhibitor and small interfering RNA. Analysis of human biopsy samples revealed an increase in both PAI-1 mRNA and protein levels in patients with H. pylori gastritis compared to those of uninfected controls. Infection of AGS cells with H. pylori significantly increased PAI-1 mRNA expression and the secretion of PAI-1 protein. Moreover, PAI-1 mRNA and protein production was more pronounced when AGS cells were infected by H. pylori strains carrying a functional cag secretion system than when cells were infected by strains lacking this system. PAI-1 secretion was also reduced when cells were infected with either cagE-negative or cagA-negative mutants. The ectopic overexpression of CagA significantly increased the levels of PAI-1 mRNA and protein, whereas blockade of the ERK1/2 pathway inhibited H. pylori-mediated PAI-1 upregulation. These findings suggest that the upregulation of PAI-1 in H. pylori-infected gastric epithelial cells may contribute to the carcinogenic process.
Helicobacter pylori is a type I carcinogen, and infection with this pathogenic bacterium is the leading cause of gastric cancer worldwide (28). It has been postulated that infection by H. pylori may contribute to this disease in two distinct ways. One way is by activating a chronic inflammatory response which causes a cascade of molecular and morphological changes in the inflamed epithelium, leading to mucosal atrophy, metaplasia, dysplasia, and eventually gastric cancer. The other is that this bacterium may directly modify epithelial-cell function and promote carcinogenesis by interfering with genes such as those regulating apoptosis, cell cycle control, tumor suppression, and cell-to-cell contacts (25).
Plasminogen activator inhibitor 1 (PAI-1) is a 50-kDa protein belonging to a family of serine protease inhibitors known as serpins. PAI-1 is synthesized by a variety of cells and is induced by growth factors, cytokines, hormones, and other stimuli, and high PAI-1 levels are present in plasma from patients with acute or chronic inflammatory conditions. The main function of PAI-1 in tissue is to inhibit the action of the urokinase-type plasminogen activator (uPA), a serine protease involved in tissue remodeling and cell migration (6).
Recent studies have shown that PAI-1 levels are elevated in tumors, and the extent of the increase correlates with an increased potential for the spread of the malignancy (15). It has also been noted that cancer patients with high PAI-1 levels have a poor prognosis for survival (3, 11, 15). High PAI-1 levels may promote the degradation of the extracellular matrix by decreasing the ability of cells to adhere to their substratum and detaching cells from the extracellular matrices by inactivating integrins (8).
Previous reports have shown that PAI-1 levels are increased in H. pylori-associated gastric carcinoma and intestinal metaplasia (4, 33). The aims of this study were (i) to determine whether PAI-1 is upregulated in patients with H pylori gastritis, (ii) to determine whether the infection of AGS gastric epithelial cells with H. pylori can directly result in increased levels of PAI-1 mRNA and protein, (iii) to then identify the signal transduction pathways that are required to regulate this process, and (iv) to examine which pathogenic bacterial factors influence the upregulation of PAI-1.
MATERIALS AND METHODS
Cell culture and reagents.
AGS gastric epithelial cells (American Type Culture Collection, Rockville, MD) were maintained in RPMI medium with 10% fetal calf serum. Cell culture experiments were carried out using 6-, 12-, and 24-well polypropylene tissue culture plates (Corning Costar, Cambridge, MA). All experiments were carried out using 90%-confluent monolayers unless otherwise stated. The extracellular signal-regulated kinase 1/2 (ERK1/2) inhibitor PD98059 was obtained from Calbiochem (La Jolla, CA).
H. pylori strains, clinical isolates, and isogenic mutants.
H. pylori was cultured and prepared as previously described (19). Unless otherwise stated, experiments were performed using the cag-positive H. pylori strain 43504 (American Type Culture Collection, Rockville, MD). Isogenic H. pylori mutants lacking the cagE or cagA gene were studied together with the parental, toxigenic, cag-positive wild-type strains 60190 and J166. Mutants and clinical isolates were obtained from the Vanderbilt University Campylobacter and Helicobacter Laboratory (Nashville, TN). RNA isolated from isogenic H. pylori mutants was analyzed by reverse transcriptase PCR (RT-PCR) to confirm that the transcription of the adjacent upstream and downstream cag genes (cagD, cagF, and cagL) was intact. The bacterial strains used in this study are outlined in Table 1. H. pylori was heat killed by boiling it for 10 min, pelleted by centrifugation, and resuspended in fresh medium before being added to cells.
TABLE 1.
H. pylori strains and mutants used in the study
| Strain of H. pylori | Virulence factor | Mutants |
|---|---|---|
| ATCC 43504 | cag positive | |
| J68 | cag negative | |
| J166 | cag positive | cagE-negative and cagA-negative mutants |
| 60190 | cag positive | cagE-negative and cagA-negative mutants |
Patients and biopsy samples.
Biopsy samples were obtained from patients who were recruited from a group receiving routine endoscopies performed at Beth Israel Deaconess Medical Center, Boston, MA. Full ethical approval for the study was granted by the Beth Israel Deaconess Medical Center Committee for Clinical Investigations. The eight patients used for the study comprised five males and three females, with an average age of 50 years, and comprised five Caucasians and three persons of Asian descent. Gastric biopsy specimens for PAI-1 protein analysis were taken from the antrum, snap-frozen in liquid nitrogen, and stored at −80°C until needed for further processing. Biopsy specimens were also collected for H. pylori culture. For the analysis of H. pylori infection, adjacent biopsy specimens were placed in formalin and stained with hematoxylin and eosin. Biopsy samples with equivocal stains were analyzed further by Warthin-Starry silver staining. The slides were examined microscopically for H. pylori gastritis using the modified Sydney System (31). H. pylori infection was confirmed by culturing on a selection medium (Skirrow agar) that tests for urease and identified by colony morphology (pinhead-sized translucent colonies) and microscopy (gram-negative curved organisms).
PAI-1 ELISA.
PAI-1 protein levels were determined using a commercially available human-PAI-1 enzyme-linked immunosorbent assay (ELISA) kit that detects the latent and active forms of PAI-1 as well as uPA-PAI-1 complexes (Oncogene Science). This was used in accordance with the manufacturer's instructions.
Immunohistochemical staining for PAI-1.
Immunohistochemical studies were performed on archival, formalin-fixed, paraffin-embedded gastric biopsy samples obtained from Vanderbilt University Medical Center, Nashville, TN, and the Department of Pathology, Technische Universität München, Munich, Germany. Sections were stained using PAI-1 monoclonal antibody (MAb) TJA6 (Abcam) according to the manufacturer's instructions. In brief, 4-μm sections were deparaffinized, subjected to antigen retrieval by boiling them in 10 mM sodium citrate buffer, pH 6.0, for 10 min, placed on ice for 20 min, and blocked in 3% H2O2 for 10 min. The slides were washed twice in water, twice in Tris-buffered saline (TBS) with 0.1% Tween 20, and twice in TBS.
Sections were blocked with the avidin/biotin blocking kit (Vector Laboratories) and then treated with 5% normal donkey serum (Jackson Immunolab) in TBS for an hour at room temperature. Sections were then incubated with anti-PAI-1 mouse MAb (1:20) at 4°C overnight. After slides were washed twice in TBS with 0.1% Tween 20 and once in TBS, biotin-conjugated donkey anti-mouse secondary antibody (1:200) was added to the slides, which were then incubated for an hour at room temperature. Signal was enhanced with a Vectastatin ABC kit (Vector Laboratories). Peroxidase substrate was added for 4 min with a 3,3′-diaminobenzidine kit (Vector Laboratories). Slides were counterstained for 3 seconds in hematoxylin (Fisher Scientific) and then dehydrated and mounted using Permount (Fisher Scientific).
Human gastric biopsy samples.
Gastric biopsy samples were homogenized on ice in 0.1 M Tris, pH 7.6, containing 0.1% Tween 80. Homogenates were centrifuged at 14,000 × g for 15 min at 4°C. Protein concentrations of the supernatant were then determined using the bicinchoninic acid protein assay (Pierce).
Western blotting.
Cell lysates were loaded onto a 7.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel and transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA). Western blotting analysis was carried out using CagA antibodies (Austral Biologicals, San Ramon, CA), ERK1/2 antibodies (Cell Signaling Technology Inc., Danvers, MA), PAI-1 (Abcam), and an actin antibody (Sigma-Aldrich, St. Louis, MO). Secondary antibodies were obtained from Santa Cruz Biotechnology.
Analysis of PAI-1 and GAPDH mRNA levels using real-time RT-PCR (TaqMan assay).
Total RNA and cDNA were prepared as described previously (16). PAI-1 and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) mRNA levels were determined by real-time PCR using an ABI PRISM 7700 sequence detection system (ABI/Perkin-Elmer). cDNA was incubated for 2 min at 50°C, denatured for 10 min at 95°C, and then subjected to 40 cycles of annealing at 55°C for 20 seconds and extension at 60°C for 1 min, followed by denaturation at 95°C for 15 seconds. The gene-specific primers used were PAI-1 sense primer, 5′ AAGGGTCTGCTGTGCACCAT 3′; PAI-1 antisense primer, 5′ AAACACCCTCACCCCAAGT 3′; GAPDH sense primer, 5′ GACCACAGTCCATGCCATCA 3′; and GAPDH antisense primer, 5′ CATCACGCCACAGTTTCCC 3′. To detect amplicons generated using gene-specific primers, dually labeled fluorogenic (real-time) probes containing 6-carboxyfluorescein (at the 5′ end) and 6-carboxytetramethylrhodamine (at the 3′ end) were synthesized (Sigma-Genosys, The Woodlands, TX). The real-time probes used were PAI-1, 5′ CCCCATCCTACGTGGC 3′, and GAPDH, 5′ ACCCAGAAGACTGTGGATGGCCCC 3′. PAI-1 levels in each sample were normalized to GAPDH expression, and the change in the mRNA level was expressed relative to the level of induction in untreated cells by using the ΔΔCT method, where CT is the threshold cycle (22).
Plasmids and transfection.
The transfection of AGS cells was carried out by using JetPEI (Polyplus Transfection, NY) according to the manufacturer's recommendations: 1 μg DNA for 24-well plates and 3 μg DNA for 6-well plates. Cells were transfected with the green fluorescent protein-CagA expression vector containing the full-length CagA sequence from H. pylori strain G27 (2), which was kindly provided by Manuel Amieva, Stanford University.
siRNA transfection.
Validated small interference RNA (siRNA) targeting mitogen-activated protein kinase 1 (MAPK1) mRNA was obtained from Qiagen (Valencia, CA) and prepared according to manufacturer's instructions. Nonsilencing siRNA duplexes (Qiagen) were used as negative controls. On the day prior to transfection, AGS cells were seeded at a density of 8 × 104 cells/ml in 24-well plates. Cells were transiently transfected with 100 nM MAPK siRNA or with the negative control using HiPerfect transfection reagent (Qiagen) according to the manufacturer's instructions. Cells were used 48 hours posttransfection, and lysates were analyzed by Western blotting to assess gene knockdown.
Statistical analyses.
Statistical analyses were performed using SigmaStat for Windows version 2.0 (Jandel Scientific Software, San Rafael, CA). Analysis of variance followed by protected t tests were used for intergroup comparisons, except where otherwise stated.
RESULTS
PAI-1 protein and mRNA levels are increased in patients with H. pylori gastritis.
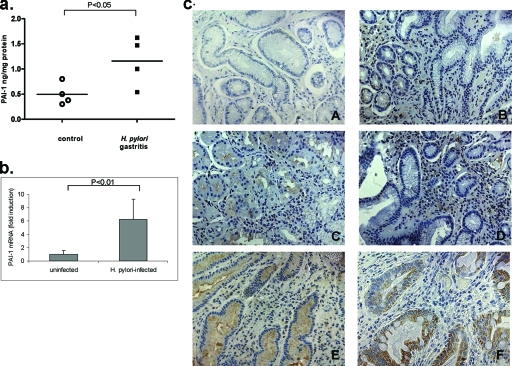
Gastric biopsy specimens were taken from uninfected patients with normal pathology and from patients with H. pylori gastritis. In order to determine PAI-1 protein levels, antral biopsy samples were homogenized and then lysates were assayed by PAI-1 ELISA. The ELISA used measures free, total, active, or complexed PAI-1 and is therefore able to measure total PAI-1 rather than just enzymatically active forms, which was the case with previously available kits. The data presented in Fig. 1a show the total concentration of PAI-1 (ng/mg protein) in each biopsy sample. Patients with H. pylori gastritis were found to have a mean twofold-higher level of PAI-1 than normal uninfected controls (four patients for each group). PAI-1 mRNA levels were also analyzed using cDNA from control and H. pylori-infected subjects. Figure 1b shows the results of the real-time PCR analysis in which PAI-1 mRNA levels were found to be increased 5.6-fold (P < 0.01) in gastric biopsy specimens from H. pylori-infected patients compared to those in biopsy specimens from uninfected controls (four patients for each group).
FIG. 1.
(a) PAI-1-protein levels are elevated in gastric biopsy samples from patients with H. pylori gastritis compared with those of controls. PAI-1 levels in gastric biopsy samples were measured by ELISA and are expressed as mean concentrations (ng) of PAI-1/mg protein. The graph shows mean PAI-1 concentrations ± standard deviations (SD) for each group. P was <0.01 for H. pylori-infected patients compared to values for biopsy specimens from uninfected controls (n = 4). (b) PAI-1 mRNA levels are elevated in gastric biopsy samples obtained from patients infected with H. pylori compared with those of healthy controls. Data are normalized to GAPDH data (four patients for each group). (c) Histochemical staining of formalin-fixed, paraffin-embedded gastric biopsy specimens with PAI-1 MAb. (A) Normal uninfected control tissue, (B) uninfected NAG patient tissue, (C) tissue from a NAG patient infected with cag-positive H. pylori, (D) tissue from a NAG patient infected with cag-negative H. pylori, (E) tissue from an intestinal metaplasia patient, and (F) tissue from a patient with gastric cancer. Magnification, ×40.
To ascertain the cellular source of PAI-1 production in gastric tissue, we performed an immunohistochemical analysis of PAI-1 in gastric biopsy specimens from patients with different disease conditions (Fig. 1c). These specimens represented tissues from an uninfected control (Fig. 1c, panel A), an uninfected patient with nonatrophic gastritis (NAG) (panel B), a cag-positive H. pylori-infected NAG patient (panel C), a cag-negative H. pylori-infected NAG patient (panel D), an intestinal metaplasia patient (panel E), and a gastric cancer patient (panel F). Normal antral biopsy specimens showed little or no immunoreactivity for PAI-1; however, biopsy specimens from patients with gastritis (Fig. 1c, panels B, C, and D) showed a marked increase in PAI-1 levels. Increased staining was observed in both epithelial and lamina propria inflammatory cells in all NAG patient samples; however, increased epithelial PAI-1 expression was most pronounced in the NAG patient specimens of those infected with cag-positive H. pylori (panel C). Intense PAI-1 staining of epithelial cells was evident in both intestinal metaplasia (panel E) and gastric cancer (panel F) patients, which confirms previous findings (4, 33).
cag-positive H. pylori selectively upregulates PAI-1 mRNA and protein secretions in AGS gastric epithelial cells.
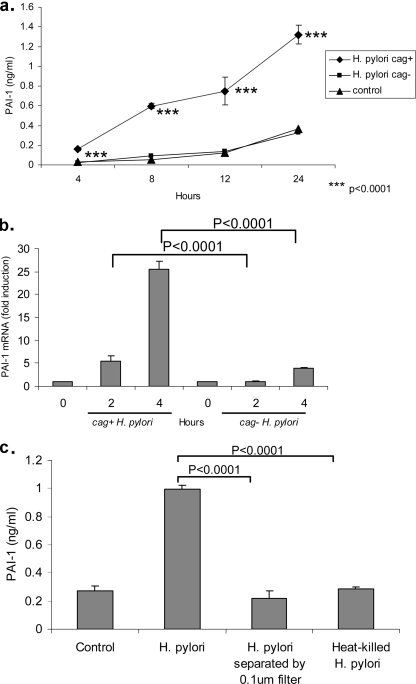
To confirm that gastric epithelial cells could be a source of the increased level of PAI-1 during infection with H. pylori, we next investigated whether this bacterium could directly induce PAI-1 production by AGS cells. One aspect that may affect disease outcome is H. pylori strain variation. Studies have demonstrated that strains harboring a 40-kb region of the H. pylori genome known as the cag pathogenicity island, which encodes a type IV secretion system, are more likely to induce symptomatic disease than those that do not carry this particular genetic element (29). Therefore, to address this issue, AGS gastric epithelial cells were infected with either a cag-positive strain (J166) or a cag-negative strain (J68) of H. pylori at a multiplicity of infection (MOI) of 20 bacteria to 1 cell. Uninfected cells were used as a control. Medium was harvested over a 24-hour time course, and the level of PAI-1 in the conditioned medium at 4, 8, 12, and 24 h was determined by ELISA. As shown in Fig. 2a, infection with the cag-positive strain resulted in a significant increase in secreted PAI-1 levels compared to those of uninfected control AGS cells or AGS cells infected with the cag-negative strain. PAI-1 secretion was also found to be dependent upon H. pylori concentrations; H. pylori at an MOI of 100:1 increased the production of PAI-1 protein by approximately 28% compared to that produced by H. pylori at an MOI of 20:1. In contrast, PAI-1 protein production was increased by only 7% when AGS cells were treated with H. pylori at an MOI of 200:1; however, this treatment was also associated with a marked increase in cell death (data not shown). To determine whether PAI-1 mRNA expression was increased in H. pylori-infected cells, AGS cells were infected with cag-positive (J166) or cag-negative (J68) strains (MOI of 20:1). RNA was isolated and reverse transcribed, and the resulting cDNA was subjected to real-time PCR to quantify PAI-1 mRNA levels. In Fig. 2b, infection of AGS cells with the cag-positive strain resulted in an increase in PAI-1 mRNA levels: 5-fold at 2 h and 25-fold at 4 h. In contrast, infection with the cag-negative strain produced only a threefold increase in PAI-1 mRNA levels at 4 h. Data are expressed as levels of induction above those in uninfected control cells as normalized to GAPDH mRNA levels.
FIG. 2.
(a) Time course of PAI-1 secretion by cag-positive-H. pylori-infected AGS cells. The graph shows the secretion of PAI-1 by AGS cells over 4, 8, 12, and 24 h in response to no infection (control; medium alone) and infection with cag-positive strain J166 (cag+) and cag-negative strain J68 (cag−). At each time point, medium was collected and PAI-1 levels were measured by ELISA. Data are presented as means ± SD of results from triplicates. ***, P was <0.0001 for control levels of PAI-1 versus the value with cag-positive H. pylori. Results are representative of three independent experiments. (b) The infection of AGS cells with cag-positive H. pylori upregulates PAI-1 mRNA expression. AGS cells were infected with either cag-positive H. pylori strain J166 or cag-negative H. pylori strain J68. RNA was isolated from infected cells at 2 and 4 h, reverse transcribed, and then analyzed by real-time PCR. Data are presented as levels of induction above the level in the control and normalized to GAPDH data. (c) PAI-1 secretion by H. pylori-infected AGS cells is not mediated by soluble factors. AGS cells were infected with live bacteria of cag-positive H. pylori strain ATCC 43504, live bacteria separated by a 0.1-μm filter, or heat-killed bacteria. Medium was harvested at 24 h, and PAI-1 protein levels were determined by ELISA. Data are presented as means ± SD from triplicates. P was <0.0001 for PAI-1 levels in cells infected with live H. pylori versus levels in cells infected with filtered bacteria, and P was <0.0001 for cells infected with live H. pylori versus cells infected with heat-killed H. pylori. Results are representative of three independent experiments.
Contact with live H. pylori is required to induce PAI-1 production by AGS cells.
Our next experiment was carried out to determine whether PAI-1 upregulation was mediated by soluble secreted factors, such as urease or vacuolating toxin, or through direct contact with epithelial cells. AGS cells were either directly infected with live H. pylori (cag-positive strain ATCC 43504) or cocultured with H. pylori using 0.1-μm filters to separate the bacteria from the cells. Heat-killed bacteria were also added to the cells. Only contact with live H. pylori was able to cause an increase in AGS cell PAI-1 secretion (Fig. 2c).
PAI-1 upregulation is dependent on CagA and the type IV secretion system.
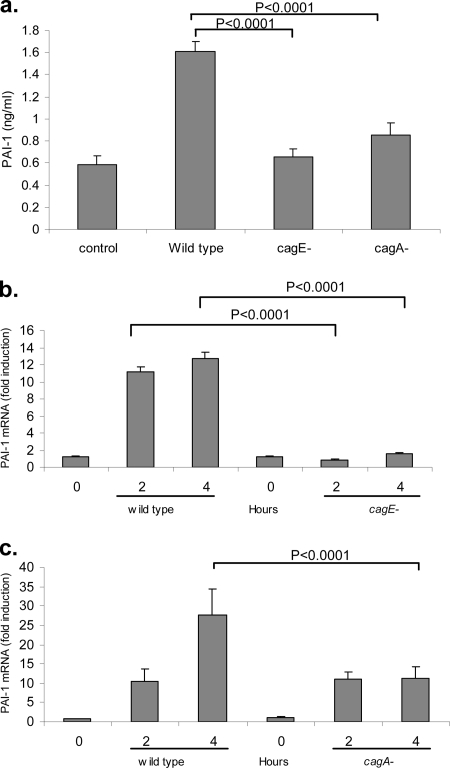
H. pylori has a number of virulence factors aside from soluble factors. The cag pathogenicity island encodes a type IV secretion system that has been demonstrated to activate an inflammatory response in gastric epithelial cells (5). In addition to its role in generating an inflammatory response, the type IV secretion system functions as a molecular syringe to deliver the immunodominant 120-kDa CagA protein into the cytosol of the cell (1, 26, 37). CagA interacts with several host cell proteins, including Grb2, SHP-2, and src kinases, and is thought to be important in tumorigenesis (13, 23, 36). We set out to determine whether the cag pathogenicity island genes and/or the CagA protein regulates PAI-1 production by H. pylori-infected AGS cells. In order to do this, we used isogenic H. pylori mutants. The mutants selected were the cagE-negative mutant, which contains a dysfunctional secretion system and thereby prevents translocation of the CagA protein into eukaryotic cells, and the cagA-negative mutant, which although it has a functional type IV secretion system is not capable of expressing the CagA protein (1, 26). AGS cells were infected with the parent strain 60190 or cagE-negative or cagA-negative isogenic mutants (MOI of 20:1) for a 24-hour period, and conditioned medium was harvested and assayed for PAI-1 by ELISA. As shown in Fig. 3a, wild-type H. pylori stimulated PAI-1 protein production ∼2.5-fold. This induction was significantly decreased following coculture with either cagE-negative or cagA-negative mutants.
FIG. 3.
(a) Infection of AGS cells with cagE-negative and cagA-negative H. pylori mutants generated a weaker PAI-1 secretory response than infection with their wild-type counterparts. AGS cells were infected with wild-type strain 60190 or its isogenic cagE-negative (cagE−) or cagA-negative (cagA−) mutant for 24 h. Medium was collected and assayed for PAI-1 protein by ELISA. Data are presented as means ± SD from triplicates. P was <0.0001 for PAI-1 levels in cells infected with wild-type H. pylori versus levels in cagE-negative cells, and P was <0.0001 for cells infected with wild-type H. pylori versus cagA-negative cells. Results are representative of three independent experiments. (b) Infection of AGS cells with the cagE-negative mutant resulted in an attenuation in the PAI-1 mRNA response compared with that after infection with its wild-type counterpart. AGS cells were infected with wild-type strain 60190 or its isogenic cagE-negative mutant for 2 and 4 h. RNA was isolated and reverse transcribed, and PAI-1 mRNA was analyzed by real-time PCR. Data are presented as levels of induction above the control level and corrected by normalization to GAPDH data. (c) Infection of AGS cells with the cagA-negative mutant resulted in a decrease in the PAI-1 mRNA response compared with that after infection with its wild-type counterpart. AGS cells were infected with wild-type strain 60190 or its isogenic cagA-negative mutant for 2 and 4 h. RNA was isolated and reverse transcribed, and PAI-1 mRNA was analyzed by real-time PCR. Data are presented as levels of induction above the control level and corrected by normalization to GAPDH data.
There was no statistical difference between the results obtained from the cagE-negative and cagA-negative mutants in terms of PAI-1 protein secretion, suggesting that the CagA protein is required for PAI-1 protein production by H. pylori-infected gastric epithelial cells. We also examined whether PAI-1 mRNA levels were reduced when cells were infected with cagE-negative or cagA-negative mutants, compared to the level in the parental wild-type strain. As shown in Fig. 3b, infection with the H. pylori cagE-negative mutant caused a significant reduction in PAI-1 mRNA levels at 2 and 4 h compared to the level in the wild-type strain; however, with the cagA-negative mutant, decreased PAI-1 mRNA levels were observed only at the 4-h time point (Fig. 3C). Additional experiments using an independent H. pylori cag-positive strain (J166) and its isogenic cagE-negative and cagA-negative mutants produced results similar to those obtained with strain 60190. The effects of the mutants on PAI-1 regulation were not a result of polar effects induced by gene inactivation, since RT-PCR analysis of the isogenic mutants revealed no decrease in the expression of other cag genes adjacent to or downstream of cagA and cagE (data not shown).
Overexpression of CagA results in increases in PAI-1 mRNA levels.
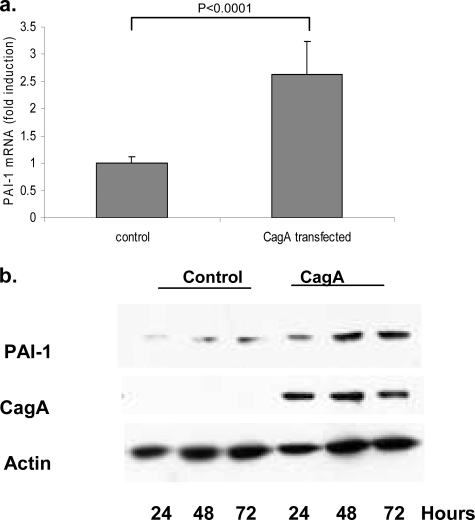
To investigate whether CagA per se was sufficient to induce the upregulation of PAI-1, we next overexpressed CagA in AGS cells. Forty-eight hours after transfection, PAI-1 mRNA levels were determined using real-time PCR. As shown in Fig. 4a, the overexpression of CagA was able to induce a two- to threefold increase in PAI-1 mRNA levels. To determine whether CagA overexpression could result in increased levels of PAI-1 protein, AGS cells were transfected for up to 72 h, and lysates were collected at 24, 48, and 72 h. CagA expression and PAI-1 levels were then determined by Western blotting. As shown in Fig. 4b, CagA was detected at all three time points. In cells expressing CagA, PAI-1 protein levels increased approximately 2.5-fold (by densitometry) at 24, 48, and 72 h after transfection compared with levels in cells transfected with the control plasmid.
FIG. 4.
(a) Transfection of CagA into AGS cells results in increased PAI-1 mRNA levels. The graph shows PAI-1 mRNA levels in AGS cells 48 h after they were transfected with either a CagA or a control plasmid. Samples were analyzed by real-time PCR, and data are presented as levels of induction above the control level and corrected by normalization to GAPDH data. P was <0.0001 for the PAI-1 mRNA control value versus the CagA transfection value (n = 3). Results are representative of four independent experiments. (b) Western blot demonstrating the time course of CagA and PAI-1 expression in AGS cells following transfection over a 72-h period. Blots were stripped and reprobed with actin, which acts as a loading control. Results are representative of two independent experiments.
PAI-1 production is dependent on the activation of ERK1/2.
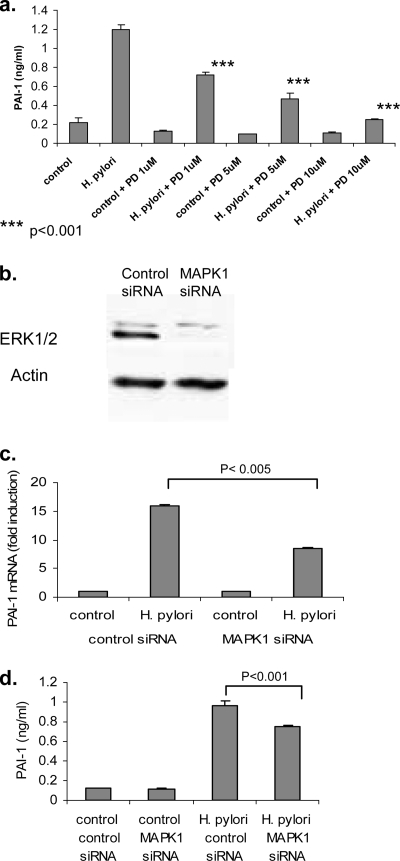
We next explored the signal transduction pathways involved in H. pylori-mediated PAI-1 upregulation. The involvement of the MAPK signaling pathway in PAI-1 upregulation in epithelial cells and other cell types has been well documented (9, 32). In previous studies, we have found that certain strains of H. pylori are able to induce epidermal growth factor receptor transactivation and, through this, increased ERK1/2 signaling (17, 19). In our next series of experiments, we examined the contribution of the MAPK signaling pathway to the regulation of PAI-1 in response to H. pylori infection. AGS cells were pretreated for 1 hour with different concentrations of the ERK1/2 inhibitor PD98059 (1 μM, 5 μM, and 10 μM). Cells were then infected with the cag-positive H. pylori strain ATCC 43504 for 24 h. Conditioned medium was collected and assayed for PAI-1 protein by ELISA. The results of this experiment, shown in Fig. 5a, demonstrate that H. pylori-mediated PAI-1 production was inhibited in a dose-dependent manner by the ERK1/2 inhibitor PD98059. Real-time PCR analysis also revealed that PD98059 at 10 μM reduced H. pylori-induced PAI-1 mRNA levels to baseline (data not shown).
FIG. 5.
(a) The ERK1/2 inhibitor PD98059 inhibits H. pylori-mediated PAI-1 protein production. AGS cells were pretreated for 1 hour with 1 μM, 5 μM, or 10 μM PD98059 (PD) and then infected with the cag-positive H. pylori strain ATCC 43504. Medium was harvested at 24 h, and PAI-1 protein levels were determined by ELISA. Data are presented as means ± SD from triplicates. Results are representative of three independent experiments. (b) Representative Western blot showing ERK1/2 expression in AGS cells following transfection with control siRNA and MAPK1 siRNA. Blots were stripped and reprobed with actin, which acts as a loading control. (c) Transfection of MAPK1 siRNA into AGS cells results in decreased PAI-1 mRNA levels. The graph shows PAI-1 mRNA levels in AGS cells 48 h after they were transfected with either control or MAPK siRNA. Samples were analyzed by real-time PCR, and data are presented as levels of induction above the control level and corrected by normalization to GAPDH data. P was <0.005 for PAI-1 mRNA control siRNA values versus MAPK1 siRNA values (n = 3). Results are representative of three independent experiments. (d) MAPK1 siRNA inhibits H. pylori-mediated PAI-1 protein production. The graph shows the levels of PAI-1 secreted by AGS cells transfected with control or MAPK siRNA. Data are presented as means ± SD (n = 3). Results are representative of three independent experiments.
To confirm that ERK proteins participate in the signal transduction process, we also used siRNA directed toward MAPK1 (ERK2). Figure 5b shows a reduction in the MAPK1 protein as a result of treatment of the cells with siRNA. Compared to cells transfected with the control siRNA, we found that PAI-1 mRNA levels were reduced by approximately 50% in AGS cells treated with MAPK1 siRNA (Fig. 5c). A significant decrease in the level of PAI-1 protein was also observed in H. pylori-infected AGS cells transfected with the MAPK1 siRNA (Fig. 5d).
DISCUSSION
PAI-1 plays a key role in tumor progression and is believed to an important regulator of invasion, metastasis, and angiogenesis (6). In this report, we have shown that the infection of gastric epithelial cells by the gastric pathogen H. pylori upregulates PAI-1, which if dysregulated may contribute to the process of oncogenic transformation.
A number of studies have shown that PAI-1 is upregulated in patients with gastric cancer (15, 33). In a study by Sakakibara et al., PAI-1 expression scores markedly increased with tumor stage, and there was a significant increase in PAI-1 expression scores in metastasis-positive gastric cancers compared to those of metastasis-negative cancers. It was also determined in this study that the overexpression of PAI-1 is a strong and independent prognostic factor for gastric cancer (33).
In the current study, we demonstrated that patients with H. pylori gastritis have elevated PAI-1 mRNA and protein levels, compared with those in patients who are uninfected. We have also shown that AGS gastric epithelial cells infected with H. pylori respond by upregulating PAI-1 mRNA and protein production. While other investigators have examined the upregulation of different members of the urokinase plasminogen system and found increased levels of both the uPA and the uPA receptor (14), this is, to our knowledge, the first report of PAI-1 upregulation by H. pylori-infected gastric epithelial cells. Iwamoto et al. found a significant upregulation of both the uPA and uPA receptor in MKN45 and KATO-III cells and also reported that this effect was limited to H. pylori carrying the cag pathogenicity island genes, which parallels our findings with PAI-1 (14).
Another study by Varro et al. (37a) examined the upregulation of PAI-2 by H. pylori in AGS cells. PAI-2 is another serpin closely related to PAI-1, and in that study, the authors were able to demonstrate that the induction of PAI-2 by H. pylori was mediated by the release of interleukin-8 and the activation of cyclooxygenase-2.
In our study, we determined that one of the bacterial factors required for generating the PAI-1 response was the type IV cag secretion system. Metalloproteinases (MMPs) are functionally similar to PAI-1 in that they play a role in the processes of tumor growth and metastasis (35). Interestingly, the type IV secretion system also appears to be required for the upregulation of a number of epithelial-cell MMPs by H. pylori. Oliveira et al. have reported that H. pylori-induced increases in MMP-2 and MMP-9 activity are dependent on the presence of a functional bacterial type IV secretion system (27). Krueger et al. have shown that the type IV secretion system is also required for MMP-1 upregulation (21). Similar findings have been reported for MMP-7 by Crawford et al. (7).
Once CagA is translocated into host cells, it activates various signal transduction pathways, resulting in pathological cellular responses such as increased cell proliferation, motility, and apoptosis, all of which can potentially contribute to the carcinogenic process (12). Recently, investigators have shown that CagA can upregulate MMP-1 (30), activate β-catenin (10), and also cause the disruption of tight junctions and the loss of apical-basolateral polarity (2). In this study, we found that infection of AGS cells with cagA-negative mutants resulted in a decreased PAI-1 response compared with that of the wild-type parental strains, at the level of both protein and RNA. However, the reduction in PAI-1 mRNA data with the cagA-negative mutants was not as low as that observed with the cagE-negative mutants. One possible explanation for this apparent discrepancy is that the bacteria may regulate PAI-1 production at both the transcriptional and posttranscriptional levels, and therefore, mRNA data and protein production may not necessarily correlate (24). However, the overexpression of CagA in the absence of infection produced a modest but statistically significant increase in PAI-1 mRNA and, over a 72-h period, resulted in increased PAI-1 protein levels, demonstrating that CagA is required for the maximal upregulation of PAI-1 by H. pylori.
We and others have previously reported that the MAPKs are differentially regulated by cag-positive and cag-negative strains of H. pylori, with cag-negative bacteria inducing weak or no activation of the various MAKs in infected epithelial cells (18). In the present study, we found that ERK1/2 activation is required in part for H. pylori-mediated PAI-1 upregulation. This may offer one potential explanation as to why there is a decreased PAI-1 response to infection with both cag-negative H. pylori strains and mutants. We also observed that some cag-positive H. pylori strains were able to induce a more robust upregulation of PAI-1 than others; this is currently being investigated in greater detail by our laboratory.
In summary, we have shown that PAI-1 is elevated both in patients with H. pylori gastritis and in AGS cells infected with H. pylori. PAI-1 is upregulated by a number of known carcinogenic agents (20, 34, 38) and plays a pivotal role in invasion, metastasis, and angiogenesis. Our findings suggest that increased PAI-1 production during H. pylori infection may contribute to H. pylori-associated carcinogenesis.
Acknowledgments
This work was supported by National Institutes of Health grant KO1-DK075942 to S. Keates; NIH grants DK58587, CA77955, and DK73902 to R. M. Peek, Jr.; and NIH grant CA-028842 to P. Correa. S. C. Robson acknowledges support from NIH grants HL57307, HL63972, and HL076540. In addition, the American Gastroenterological Association, Foundation for Digestive Health and Nutrition, presented a TAP Endowed Designated Research Award in Acid Related Diseases to S. Keates.
Editor: S. R. Blanke
Footnotes
Published ahead of print on 2 June 2008.
REFERENCES
- 1.Backert, S., E. Ziska, V. Brinkmann, U. Zimny-Arndt, A. Fauconnier, P. R. Jungblut, M. Naumann, and T. F. Meyer. 2000. Translocation of the Helicobacter pylori CagA protein in gastric epithelial cells by a type IV secretion apparatus. Cell. Microbiol. 2155-164. [DOI] [PubMed] [Google Scholar]
- 2.Bagnoli, F., L. Buti, L. Tompkins, A. Covacci, and M. R. Amieva. 2005. Helicobacter pylori CagA induces a transition from polarized to invasive phenotypes in MDCK cells. Proc. Natl. Acad. Sci. USA 10216339-16344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berger, D. H. 2002. Plasmin/plasminogen system in colorectal cancer. World J. Surg. 26767-771. [DOI] [PubMed] [Google Scholar]
- 4.Beyer, B. C., M. M. Heiss, E. H. Simon, K. U. Gruetzner, R. Babic, K. W. Jauch, F. W. Schildberg, and H. Allgayer. 2006. Urokinase system expression in gastric carcinoma: prognostic impact in an independent patient series and first evidence of predictive value in preoperative biopsy and intestinal metaplasia specimens. Cancer 1061026-1035. [DOI] [PubMed] [Google Scholar]
- 5.Censini, S., C. Lange, Z. Xiang, J. E. Crabtree, P. Ghiara, M. Borodovsky, R. Rappuoli, and A. Covacci. 1996. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc. Natl. Acad. Sci. USA 9314648-14653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chorostowska-Wynimko, J., E. Skrzypczak-Jankun, and J. Jankun. 2004. Plasminogen activator inhibitor type-1: its structure, biological activity and role in tumorigenesis. Int. J. Mol. Med. 13759-766. [PubMed] [Google Scholar]
- 7.Crawford, H. C., U. S. Krishna, D. A. Israel, L. M. Matrisian, M. K. Washington, and R. M. Peek, Jr. 2003. Helicobacter pylori strain-selective induction of matrix metalloproteinase-7 in vitro and within gastric mucosa. Gastroenterology 1251125-1136. [DOI] [PubMed] [Google Scholar]
- 8.Czekay, R. P., and D. J. Loskutoff. 2004. Unexpected role of plasminogen activator inhibitor 1 in cell adhesion and detachment. Exp. Biol. Med. (Maywood) 2291090-1096. [DOI] [PubMed] [Google Scholar]
- 9.Demyanets, S., C. Kaun, K. Rychli, G. Rega, S. Pfaffenberger, T. Afonyushkin, V. N. Bochkov, G. Maurer, K. Huber, and J. Wojta. 2007. The inflammatory cytokine oncostatin M induces PAI-1 in human vascular smooth muscle cells in vitro via PI 3-kinase and ERK1/2-dependent pathways. Am. J. Physiol. Heart Circ. Physiol. 293H1962-H1968. [DOI] [PubMed] [Google Scholar]
- 10.Franco, A. T., D. A. Israel, M. K. Washington, U. Krishna, J. G. Fox, A. B. Rogers, A. S. Neish, L. Collier-Hyams, G. I. Perez-Perez, M. Hatakeyama, R. Whitehead, K. Gaus, D. P. O'Brien, J. Romero-Gallo, and R. M. Peek, Jr. 2005. Activation of beta-catenin by carcinogenic Helicobacter pylori. Proc. Natl. Acad. Sci. USA 10210646-10651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujii, T., T. Obara, S. Tanno, H. Ura, and Y. Kohgo. 1999. Urokinase-type plasminogen activator and plasminogen activator inhibitor-1 as a prognostic factor in human colorectal carcinomas. Hepatogastroenterology 462299-2308. [PubMed] [Google Scholar]
- 12.Handa, O., Y. Naito, and T. Yoshikawa. 2007. CagA protein of Helicobacter pylori: a hijacker of gastric epithelial cell signaling. Biochem. Pharmacol. 731697-1702. [DOI] [PubMed] [Google Scholar]
- 13.Higashi, H., R. Tsutsumi, S. Muto, T. Sugiyama, T. Azuma, M. Asaka, and M. Hatakeyama. 2002. SHP-2 tyrosine phosphatase as an intracellular target of Helicobacter pylori CagA protein. Science 295683-686. [DOI] [PubMed] [Google Scholar]
- 14.Iwamoto, J., Y. Mizokami, K. Takahashi, K. Nakajima, T. Ohtsubo, S. Miura, T. Narasaka, H. Takeyama, T. Omata, K. Shimokobe, M. Ito, H. Takehara, and T. Matsuoka. 2005. Expressions of urokinase-type plasminogen activator, its receptor and plasminogen activator inhibitor-1 in gastric cancer cells and effects of Helicobacter pylori. Scand. J. Gastroenterol. 40783-793. [DOI] [PubMed] [Google Scholar]
- 15.Kaneko, T., H. Konno, M. Baba, T. Tanaka, and S. Nakamura. 2003. Urokinase-type plasminogen activator expression correlates with tumor angiogenesis and poor outcome in gastric cancer. Cancer Sci. 9443-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keates, S., A. C. Keates, E. Mizoguchi, A. Bhan, and C. P. Kelly. 1997. Enterocytes are the primary source of the chemokine ENA-78 in normal colon and ulcerative colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 273G75-G82. [DOI] [PubMed] [Google Scholar]
- 17.Keates, S., A. C. Keates, S. Nath, R. M. Peek, Jr., and C. P. Kelly. 2005. Transactivation of the epidermal growth factor receptor by cag+ Helicobacter pylori induces upregulation of the early growth response gene Egr-1 in gastric epithelial cells. Gut 541363-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keates, S., A. C. Keates, M. Warny, R. M. Peek, Jr., P. G. Murray, and C. P. Kelly. 1999. Differential activation of mitogen-activated protein kinases in AGS gastric epithelial cells by cag+ and cag− Helicobacter pylori. J. Immunol. 1635552-5559. [PubMed] [Google Scholar]
- 19.Keates, S., S. Sougioultzis, A. C. Keates, D. Zhao, R. M. Peek, Jr., L. M. Shaw, and C. P. Kelly. 2001. cag+ Helicobacter pylori induce transactivation of the epidermal growth factor receptor in AGS gastric epithelial cells. J. Biol. Chem. 27648127-48134. [DOI] [PubMed] [Google Scholar]
- 20.Kim, K. R., T. Yoshizaki, H. Miyamori, K. Hasegawa, T. Horikawa, M. Furukawa, S. Harada, M. Seiki, and H. Sato. 2000. Transformation of Madin-Darby canine kidney (MDCK) epithelial cells by Epstein-Barr virus latent membrane protein 1 (LMP1) induces expression of Ets1 and invasive growth. Oncogene 191764-1771. [DOI] [PubMed] [Google Scholar]
- 21.Krueger, S., T. Hundertmark, T. Kalinski, U. Peitz, T. Wex, P. Malfertheiner, M. Naumann, and A. Roessner. 2006. Helicobacter pylori encoding the pathogenicity island activates matrix metalloproteinase 1 in gastric epithelial cells via JNK and ERK. J. Biol. Chem. 2812868-2875. [DOI] [PubMed] [Google Scholar]
- 22.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25402-408. [DOI] [PubMed] [Google Scholar]
- 23.Mimuro, H., T. Suzuki, J. Tanaka, M. Asahi, R. Haas, and C. Sasakawa. 2002. Grb2 is a key mediator of Helicobacter pylori CagA protein activities. Mol. Cell 10745-755. [DOI] [PubMed] [Google Scholar]
- 24.Montuori, N., G. Rossi, and P. Ragno. 2002. Post-transcriptional regulation of gene expression in the plasminogen activation system. Biol. Chem. 38347-53. [DOI] [PubMed] [Google Scholar]
- 25.Mueller, A., S. Falkow, and M. R. Amieva. 2005. Helicobacter pylori and gastric cancer: what can be learned by studying the response of gastric epithelial cells to the infection? Cancer Epidemiol. Biomarkers Prev. 141859-1864. [DOI] [PubMed] [Google Scholar]
- 26.Odenbreit, S., J. Puls, B. Sedlmaier, E. Gerland, W. Fischer, and R. Haas. 2000. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science 2871497-1500. [DOI] [PubMed] [Google Scholar]
- 27.Oliveira, M. J., A. C. Costa, A. M. Costa, L. Henriques, G. Suriano, J. C. Atherton, J. C. Machado, F. Carneiro, R. Seruca, M. Mareel, A. Leroy, and C. Figueiredo. 2006. Helicobacter pylori induces gastric epithelial cell invasion in a c-Met and type IV secretion system-dependent manner. J. Biol. Chem. 28134888-34896. [DOI] [PubMed] [Google Scholar]
- 28.Parsonnet, J. 1993. Helicobacter pylori and gastric cancer. Gastroenterol. Clin. North Am. 2289-104. [PubMed] [Google Scholar]
- 29.Peek, R. M., Jr., G. G. Miller, K. T. Tham, G. I. Perez-Perez, X. Zhao, J. C. Atherton, and M. J. Blaser. 1995. Heightened inflammatory response and cytokine expression in vivo to cagA+ Helicobacter pylori strains. Lab. Investig. 73760-770. [PubMed] [Google Scholar]
- 30.Pillinger, M. H., N. Marjanovic, S. Y. Kim, Y. C. Lee, J. U. Scher, J. Roper, A. M. Abeles, P. I. Izmirly, M. Axelrod, M. Y. Pillinger, S. Tolani, V. Dinsell, S. B. Abramson, and M. J. Blaser. 2007. Helicobacter pylori stimulates gastric epithelial cell MMP-1 secretion via CagA-dependent and -independent ERK activation. J. Biol. Chem. 28218722-18731. [DOI] [PubMed] [Google Scholar]
- 31.Price, A. B. 1991. The Sydney System: histological division. J. Gastroenterol. Hepatol. 6209-222. [DOI] [PubMed] [Google Scholar]
- 32.Providence, K. M., and P. J. Higgins. 2004. PAI-1 expression is required for epithelial cell migration in two distinct phases of in vitro wound repair. J. Cell Physiol. 200297-308. [DOI] [PubMed] [Google Scholar]
- 33.Sakakibara, T., K. Hibi, M. Koike, M. Fujiwara, Y. Kodera, K. Ito, and A. Nakao. 2006. Plasminogen activator inhibitor-1 as a potential marker for the malignancy of gastric cancer. Cancer Sci. 97395-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Son, D. S., and K. K. Rozman. 2002. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) induces plasminogen activator inhibitor-1 through an aryl hydrocarbon receptor-mediated pathway in mouse hepatoma cell lines. Arch. Toxicol. 76404-413. [DOI] [PubMed] [Google Scholar]
- 35.Stamenkovic, I. 2000. Matrix metalloproteinases in tumor invasion and metastasis. Semin. Cancer Biol. 10415-433. [DOI] [PubMed] [Google Scholar]
- 36.Stein, M., F. Bagnoli, R. Halenbeck, R. Rappuoli, W. J. Fantl, and A. Covacci. 2002. c-Src/Lyn kinases activate Helicobacter pylori CagA through tyrosine phosphorylation of the EPIYA motifs. Mol. Microbiol. 43971-980. [DOI] [PubMed] [Google Scholar]
- 37.Stein, M., R. Rappuoli, and A. Covacci. 2000. Tyrosine phosphorylation of the Helicobacter pylori CagA antigen after cag-driven host cell translocation. Proc. Natl. Acad. Sci. USA 971263-1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37a.Varro, A., P. J. Noble, D. M. Pritchard, S. Kennedy, C. A. Hart, R. Dimaline, and G. J. Dockray. 2004. Helicobacter pylori induces plasminogen activator inhibition in gastric epithelial cells through nuclear factor-κB and Rho: implications for invasion and apoptosis. Cancer Res. 641695-1702. [DOI] [PubMed] [Google Scholar]
- 38.Vidal, B., M. Parra, M. Jardi, S. Saito, E. Appella, and P. Munoz-Canoves. 2005. The alkylating carcinogen N-methyl-N′-nitro-N-nitrosoguanidine activates the plasminogen activator inhibitor-1 gene through sequential phosphorylation of p53 by ATM and ATR kinases. Thromb. Haemost. 93584-591. [DOI] [PubMed] [Google Scholar]