Abstract
Measles is an acute febrile infectious disease with high morbidity and mortality. The genome of measles virus (MV), the causative agent, encodes two accessory products, V and C proteins, that play important roles in MV virulence. The V but not the C protein of the IC-B strain (a well-characterized virulent strain of MV) has been shown to block the Jak/Stat signaling pathway and counteract the cellular interferon (IFN) response. We have recently shown that a recombinant IC-B strain that lacks C protein expression replicates poorly in certain cell lines, and its growth defect is related to translational inhibition and strong IFN induction. Here, we show that the V protein of the MV IC-B strain also blocks the IFN induction pathway mediated by the melanoma differentiation-associated gene 5 product, thus actively interfering with the host IFN response at two different steps. On the other hand, the C protein per se possesses no activity to block the IFN induction pathway. Our data indicate that the C protein acts as a regulator of viral RNA synthesis, thereby acting indirectly to suppress IFN induction. Since recombinant MVs with C protein defective in modulating viral RNA synthesis or lacking C protein expression strongly stimulate IFN production, in spite of V protein production, both the C and V proteins must be required for MV to fully circumvent the host IFN response.
Measles is an acute febrile infectious disease with high morbidity and mortality and accounts for ∼4% of deaths in children aged <5 years worldwide (5). Measles virus (MV), the causative agent, belongs to the genus Morbillivirus of the family Paramyxoviridae and has a nonsegmented negative-strand RNA genome of ∼16 kb in length. The genome has six genes that encode the phosphoprotein (P) and the nucleocapsid (N), matrix (M), fusion (F), hemagglutinin (H), and large (L) proteins (24). The P gene encodes two additional proteins, V and C, by a process of RNA editing and an alternative translational initiation in a different reading frame, respectively (4, 9). The V and C proteins are nonessential products (58, 62) but play important roles in MV virulence (12, 54, 70, 74, 75). Although the V protein has been shown to counteract the cellular interferon (IFN) response (7, 13, 50, 52, 69), molecular mechanisms by which the C protein contributes to virus virulence are poorly understood. We have recently shown that a recombinant MV that lacks C protein expression (MVΔC) replicates poorly in certain cell lines, and its growth defect is related to translational inhibition and strong IFN induction (47).
Host antiviral responses are initiated by detecting pathogen-associated molecular patterns, such as cytoplasmic single-stranded RNA (ssRNA) bearing a 5′ triphosphate and double-stranded RNA (dsRNA) (19, 71). The retinoic acid inducible gene I (RIG-I) and melanoma differentiation-associated gene 5 (mda-5) products are known to be intracellular receptors (sensors) for these virus-derived RNA molecules (32). Recently, some RNAs processed by RNase L have also been shown to be recognized by these sensor molecules (40). RIG-I and mda-5 transmit signals to a downstream adapter molecule, which in turn activates specific kinases (71). The activated kinases phosphorylate IFN-regulatory factors (IRFs), which leads to the production of IFN-α/β. Secreted IFNs bind to the IFN-α/β receptor on the surface of adjacent cells and activate the Jak/Stat signaling pathway, which stimulates transcription of a variety of antiviral genes (59). Much evidence has indicated that many viruses encode specific proteins to inhibit IFN induction or the Jak/Stat signaling pathway (21, 25, 33). The IC-B strain (also referred to as the 84-01 B95a isolate) is a well-characterized virulent strain of MV and is believed to retain original phenotypes of MV circulating in patients (34, 35). The V but not the C protein of the MV IC-B strain blocks the Jak/Stat signaling pathway (7, 47, 50, 69, 70).
In this study, we showed that the V protein of the MV IC-B strain blocks the mda-5-mediated IFN induction pathway, as previously shown for the V protein of the neurotropic MV strain, HNT-PI (10). Thus, the V protein of the MV IC-B strain actively interferes with the host IFN response at two different steps: IFN induction and Jak/Stat signaling. On the other hand, the C protein per se possesses no activity to block the IFN induction pathway. However, our data indicate that the C protein is a regulator of viral RNA synthesis, which acts indirectly to suppress the IFN induction pathway. Since MVΔC cannot block IFN induction, V protein expression alone is insufficient, and both the C and V proteins must be required for MV to fully circumvent the host IFN response.
MATERIALS AND METHODS
Cells and viruses.
Vero cells constitutively expressing the human signaling lymphocyte activation molecule (SLAM) (Vero/hSLAM) (51) were maintained in Dulbecco's modified Eagle's medium (DMEM; ICN Biomedicals, Aurora, OH) supplemented with 7.5% fetal bovine serum (FBS) and 500 μg/ml geneticin (G418; Nacalai Tesque, Tokyo, Japan). A549 cells constitutively expressing human SLAM (A549/hSLAM) (67) were maintained in RPMI 1640 medium (ICN Biomedicals) supplemented with 10% FBS and 500 μg/ml G418. VV5-4 (2), B95a (34), and CHO cells constitutively expressing hSLAM (CHO/hSLAM) (73) were maintained in RPMI 1640 medium supplemented with 7.5% FBS. Vero and HEK293 cells were maintained in DMEM supplemented with 7.5% FBS. Recombinant MVs were generated as described previously, with minor modifications (46, 66). Briefly, VV5-4 cells were infected with vTF7-3 (18), a vaccinia virus encoding the T7 RNA polymerase (a gift from B. Moss), and transfected with a full-length genome plasmid that encodes the antigenome of MV under the control of the T7 promoter and three support plasmids, pCAG-T7-IC-N, pCAG-T7-IC-PΔC, and pGEMCR-9301B-L. On the next day, the transfected VV5-4 cells were cocultured with B95a and Vero/hSLAM cells to amplify recombinant MV rescued from the full-length genome plasmid. Some of the recombinant MVs used in the present study had a transcriptional unit of enhanced green fluorescent protein (EGFP) at the first 3′ locus of the virus genome (27). A stock of the Edmonston strain that contains defective interfering (DI) particles (our unpublished observation) was used as a control.
Reagents and antibodies.
A fusion-blocking peptide, Z-D-Phe-Phe-Gly (Peptide Institute, Osaka, Japan) (61), was used at 100 μg/ml in appropriate experiments. A mouse monoclonal antibody (MAb) against the MV N protein (E137; a gift from T. A. Sato) and a rabbit polyclonal antibody raised against the common amino terminus of the P and V proteins (47) were used to detect the N, P, and V proteins. A hybridoma cell line producing an antibody against the MV C protein, clone 2D10, was generated as follows. A C57BL/6 mouse received two footpad injections of peptide (from the C protein) WPSRKPWQHGQKYQTTQDRTE. The peptide corresponded to 21 amino acids at position 20 to 40 of the C protein. Spleen cells were fused with X63Ag8.653 myeloma cells using polyethylene glycol 4000 (Merck, Darmstadt, Germany). After cultivation for 2 weeks, culture supernatants of fused cells were screened for antibody by Western blotting. A horseradish peroxidase-conjugated sheep anti-mouse immunoglobulin (Ig) antibody (Amersham Biosciences, Piscataway, NJ) was used as a secondary antibody for immunoblotting. Conjugation of Alexa Fluor 555 to 2D10 MAb was performed using an Alexa Fluor 555 MAb labeling kit (Molecular Probes, Eugene, OR), according to the manufacturer's protocol. A mouse MAb against human IRF3 (SL-12.1; BD Pharmingen, Heidelberg, Germany) was used for indirect immunofluorescence assays. Alexa Fluor 488-conjugated donkey anti-mouse and anti-rabbit IgG(H+L) antibodies and Alexa Fluor 594-conjugated donkey anti-mouse IgG(H+L) antibody were purchased from Molecular Probes and used as secondary antibodies for indirect immunofluorescence assays. Poly(I:C) and cytosine 1-β-d-arabinofuranoside (AraC) were purchased from Amersham Biosciences and Sigma (St. Louis, MO), respectively.
Construction of plasmids.
All full-length genome plasmids were derived from p(+)MV323, which encodes the full-length antigenomic cDNA of the virulent IC-B strain of MV (68). The recombinant MV obtained from p(+)MV323 is called IC323 (68). The plasmid p(+)MV323-EGFP, which contained an additional transcriptional unit of EGFP, and p(+)MVΔC-EGFP were described previously (27, 47). p(+)MV-C174-EGFP and p(+)MV-C157-EGFP were generated by introducing a nonsense mutation at amino acid positions 175 and 158, respectively, in the reading frame of the C protein of p(+)MV323-EGFP (Table 1). These mutations were synonymous in the reading frame of the P and V proteins (Table 1). Recombinant MVs generated from p(+)MV323-EGFP, p(+)MVΔC-EGFP, p(+)MV-C174-EGFP, and p(+)MV-C157-EGFP were referred to as wild-type (wt) MV (also called IC323-EGFP [27]), MVΔC, MV-C174, and MV-C157, respectively, in this study. DNA fragments encoding the intact C protein and mutant C proteins (C174, C157, C145, and C85, which corresponded to amino acids 174, 157, 145, and 85, respectively, of the amino terminus of the C protein) were cloned into the eukaryotic expression plasmid pCA7 (67), a derivative of pCAGGS (49), generating pCA7-IC-C(wt), pCA7-IC-C174, pCA7-IC-C157, pCA7-IC-C145, and pCA7-IC-C85, respectively. In a reporter assay for IFN-β, we used pIFΔ(−125)lucter (a gift from S. Goodbourn) and pRL-TK (Promega, Madison, WI). pEF-FLAG-human-mda-5 was generated by inserting a DNA fragment encoding FLAG-tagged human mda-5 into the eukaryotic expression plasmid pEFneo, a derivative of pEF-BOS (44). DNA fragments coding for the C and V proteins of the IC-B strain and FLAG-tagged NS1 protein of the influenza A virus PR8 strain (47) were also cloned into pEFneo, generating pEF-IC-C, pEF-IC-V, and pEF-FLAG-PR8-NS1, respectively. For in vitro synthesis of MV leader RNA, pUC18-T7-leader-55 was generated as follows. A DNA fragment containing MV leader sequence of 55 nucleotides in length, ending in a restriction enzyme recognition site for AleI, was inserted into the pUC18 vector just downstream of the T7 promoter. For a minigenome assay, p18MGFLuc01 (36) (a gift from K. Komase) was used. pCA7-PKIα-EGFP was generated by inserting the EGFP-tagged human protein kinase inhibitor α (PKIα) cDNA into pCA7.
TABLE 1.
Substitutions in C174 and C157
| Protein | Nucleotide substitution at the indicated position of:a
|
Amino acid substitution at the indicated position of:b
|
||||||
|---|---|---|---|---|---|---|---|---|
| P/V reading frame
|
C reading frame +1
|
P/V
|
C
|
|||||
| nt 2350-2352 | nt 2299-2301 | nt 2351-2353 | nt 2300-2302 | 182 | 165 | 175 | 158 | |
| wt | GTT | ATC | TTG | TCA | Val | Ile | Leu | Ser |
| C174 | GTA | * | TAG | * | * | * | Stop | * |
| C157 | * | ATA | * | TAA | * | * | * | Stop |
Substituted nucleotides are underlined. An asterisk indicates that the trinucleotide is the same as that of the wt. nt, nucleotide.
An asterisk indicates that the amino acid is identical to that of the wt. Stop, introduced stop codon.
Virus titration.
Monolayers of Vero/hSLAM cells on six-well plates were incubated with serially diluted virus samples for 1 h at 37°C, washed with phosphate-buffered saline (PBS), and overlaid with DMEM containing 5% FBS and 0.75% agarose. At 3 days postinfection (p.i.), the number of plaque-forming units was counted.
Minigenome assay.
A minigenome assay was performed as described previously, with minor modifications (46). Monolayers of CHO/hSLAM or VV5-4 cells cultured in Opti-MEM (Gibco-BRL, Grand Island, NY) on 24-well plates were infected with vTF7-3 at a multiplicity of infection (MOI) of 0.5 and then transfected with 0.2 μg of p18MGFLuc01 (36), 0.2 μg of pCAG-T7-IC-N, 0.3 μg of pCAG-T7-IC-PΔC, 0.2 μg of pGEMCR-9301B-L, and 0.2 μg of pCA7 encoding the viral proteins to be analyzed, by using Lipofectamine 2000 reagent (Invitrogen Life Technologies, Carlsbad, CA). At 4 h p.i., culture medium was replaced with RPMI 1640 medium supplemented with 7.5% FBS and 40 μg/ml AraC or 7.5% FBS alone. At 48 h p.i., enzymatic activity of firefly luciferase was measured by a Dual Glo luciferase assay system (Promega) and luminometer (Mithras LB 940; Berthold Technologies, Bad Wildbad, Germany).
Immunoblotting.
Monolayers of VV5-4 cells were infected with vTF7-3 at an MOI of 0.5 and transfected with viral protein-expressing plasmids, according to the protocol used for the minigenome assay. At 48 h p.i., cells were lysed in a radioimmunoprecipitation assay buffer (150 mM NaCl, 10 mM Tris-HCl, pH 7.4, 1% Triton X-100, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate), and polypeptides in cells were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and blotted onto polyvinylidene difluoride membranes (Hybond-P; Amersham Biosciences). Polyvinylidene difluoride membranes were then incubated for 16 h with MAb 2D10, which is specific for the C protein, washed five times with Tris-buffered saline containing 0.05% Tween 20, and incubated with peroxidase-conjugated sheep anti-mouse IgG antibody for 1 h at room temperature. After membranes were washed with Tris-buffered saline-0.05% Tween 20 five times, they were treated with ECL Plus reagent (Amersham Biosciences), and chemiluminescent signals were detected and visualized using a VersaDoc 3000 imager (Bio-Rad, Hercules, CA).
In vitro synthesis of MV leader RNA.
MV leader RNA was synthesized in vitro using MEGAshortscript kit (Ambion, Austin, TX) according to the manufacturer's instructions. A reaction mixture containing T7 RNA polymerase, nucleoside triphosphates, and pUC18-T7-leader-55 linearized with the restriction enzyme AleI was incubated at 37°C for 4 h. In vitro transcription products were then purified using TRIzol reagent (Invitrogen Life Technologies) after treatment with DNase.
Extraction of total RNA from MV-infected or mock-infected cells.
Monolayers of Vero/hSLAM cells were infected with wt MV at an MOI of 0.5 or were not infected. At 36 h p.i., total RNA was extracted from cells and purified using TRIzol reagent.
Reporter assay.
Monolayers of Vero cells seeded on 24-well plates were transfected with 0.2 μg of pIFΔ(−125)lucter, 0.016 μg of pRL-TK, 0.1 μg of pEFneo or pEF-FLAG-human-mda-5, and 0.6 μg of pEF encoding the viral proteins to be analyzed. At 24 h posttransfection, MV leader RNA, poly(I:C), or total RNAs extracted from MV-infected or mock-infected cells were transfected into cells using Lipofectamine 2000. After 6 h, enzymatic activities of firefly luciferase and Renilla luciferase were measured using a Dual Glo luciferase assay system and luminometer. Data were normalized with Renilla luciferase activity. Monolayers of HEK293 cells were transfected with 0.1 μg of pIFΔ (−125) lucter, 0.1 μg of pRL-TK, and 0.8 μg of pEF encoding viral proteins to be analyzed. After 24 h posttransfection, cells were infected with a vesicular stomatitis virus (VSV) construct, VSVΔG*-G, and enzymatic activities of firefly and Renilla luciferase were measured using a Dual Glo luciferase assay system and luminometer at 6 h p.i. VSVΔG*-G is a recombinant VSV, in which the coding region of the G protein is replaced with that of GFP (65).
Immunofluorescence staining.
Cells were seeded on coverslips in six-well plates and infected with recombinant MV in the presence of the fusion-blocking peptide or transfected with protein expression plasmids. For the inhibition of the chromosomal region maintenance protein 1 (CRM1)-dependent nuclear export pathway, leptomycin B (LMB) was added at 20 nM. At appropriate time points p.i. or posttransfection, cells were fixed and permeabilized with PBS containing 2.5% formaldehyde and 0.5% Triton X-100. Fixed cells were washed with PBS and incubated with a primary antibody, followed by incubation with a secondary antibody. For the counterstaining of the nucleus, cells were treated with 10 μg/ml RNase A (Sigma) and incubated with 10 μg/ml propidium iodide (Sigma) for 20 min. Cells were then observed using a confocal microscope (Radiance 2100; Bio-Rad).
Reverse transcription-quantitative PCR (RT-QPCR).
Total RNA was extracted from virus-infected cells with TRIzol reagent, treated with RQ1 DNase (Promega), and reverse transcribed into cDNAs using SuperScript III reverse transcriptase (Invitrogen Life Technologies). The amounts of cDNA for viral mRNAs and the genome were quantified using Sybr Premix Ex Taq (TaKaRa Bio, Shiga, Japan) and a LightCycler (Roche Diagnostics, Indianapolis, IN), as described previously (47, 67). Levels of β-actin mRNA were also quantified as an internal control using the primer pair CTGGAACGGTGAAGGTGACA and AAGGGACTTCCTGTAACAATGCA. Levels of IFN-β mRNA were also quantified using the primer pair CTCCTGGCTAATGTCTATCA and GCAGAATCCTCCCATAATAT.
Northern blotting.
Total RNA was extracted from virus-infected cells with the TRIzol reagent (Life Technologies, Gaithersburg, MD). Thirty micrograms of the total RNA was electrophoresed, transferred to positively charged Nylon membranes (Hybond-N+; Amersham Biosciences, Piscataway, NJ), and hybridized with 32P-labeled cDNA probes synthesized with a Prime-it II random primer labeling kit (Stratagene, La Jolla, CA) and MV gene-specific cDNA fragments. The gene-specific cDNA fragments used were reported previously (67). The 5′ genome end-specific cDNA probe at nucleotide positions 15591 to 15894 was also used to detect the DI particle genome. The membranes were stripped and rehybridized with a glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA probe used as an internal control. Radioactivity was analyzed and quantified with a Fuji BioImager 1000 and Mac Bas software (Fuji Medical Systems, Stamford, CT).
RESULTS
The MV C protein per se does not possess activity to block the IFN induction pathway.
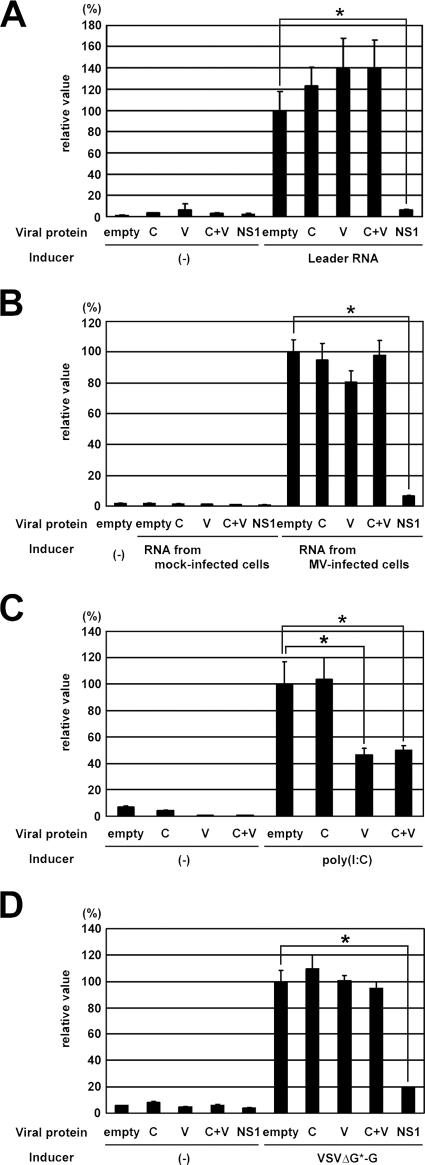
Many viruses encode specific viral proteins to block the IFN induction pathway (21, 25, 33). As our previous study has shown that infection with MVΔC strongly stimulates IFN synthesis in cells, the ability of the C protein to block the IFN induction pathway was analyzed using reporter assays. Various stimulators were used to induce IFN synthesis, and the C and V proteins of the MV IC-B strain were examined. RIG-I is a sensor molecule to detect ssRNA bearing a 5′ triphosphate (29, 55), and MV leader RNA synthesized in vitro has been shown to stimulate RIG-I-mediated IFN induction (57). When MV leader RNA synthesized in vitro was used as an inducer, the C and V proteins did not block the IFN induction pathway whether they were expressed individually or together (Fig. 1A). By contrast, the NS1 protein of influenza A virus efficiently inhibited the pathway, as reported previously (43, 72) (Fig. 1A). We also used RNAs extracted from MV-infected cells as inducers (Fig. 1B). These RNAs stimulated IFN induction, but those from mock-infected cells did not (Fig. 1B). The influenza virus NS1 protein again inhibited the IFN induction pathway, whereas the C and V proteins, whether expressed individually or together, did not block it (Fig. 1B). mda-5 is a sensor molecule to detect dsRNAs, and the V proteins of many paramyxoviruses are shown to interact with mda-5, thereby blocking mda-5-mediated IFN-induction (1, 10). mda-5 was overexpressed in cells using the expression vector pEF-FLAG-mda-5, and the mda-5-mediated IFN-induction signal was stimulated by poly(I:C) (Fig. 1C). The V protein of the IC-B strain inhibited the mda-5-mediated IFN induction signal, whereas the C protein failed to so do (Fig. 1C). Coexpression of the C protein did not affect the inhibitory effect of the V protein (Fig. 1C). Finally, recombinant VSV (VSVΔG*-G) (65) was used to induce IFN (Fig. 1D). Whether expressed individually or together, neither the C nor V protein blocked the IFN induction pathway after infection with VSVΔG*-G, whereas the influenza virus NS1 protein could so do (Fig. 1D). These data indicate that the C protein per se does not have the ability to block the IFN induction pathway, unlike the influenza virus NS1 and MV V proteins. Thus, the C protein appears to suppress IFN induction via an indirect mechanism in infected cells. Since the C protein has been shown to suppress MV RNA synthesis, we hypothesized that it inhibits the IFN induction pathway by reducing the production of inducer RNAs in infected cells.
FIG. 1.
Effect of the MV C and V proteins on IFN induction. (A) Vero cells were transfected with pIFΔ(−125)lucter and pRL-TK, together with pEFneo vector expressing the MV C, MV V, or influenza virus NS1 protein or empty pEFneo vector. The MV C and V protein-expressing plasmids were transfected either individually (C or V) or together (C+V). At 24 h posttransfection, cells were mock-transfected (−) or transfected with MV leader RNA synthesized in vitro. After incubation for 6 h, activities of intracellular firefly and Renilla luciferase were measured. The ratio of firefly luciferase activity to Renilla luciferase activity was calculated, and relative values are indicated. The average value for cells transfected with empty pEFneo vector and MV leader RNA was set to 100%. Data represent means ± standard deviations of triplicate samples. The asterisk indicates a significant difference based on a t test (P < 0.01). (B) Vero cells were transfected with expression plasmids as described in panel A. At 24 h posttransfection, cells were transfected with total RNA extracted from mock- or wt MV-infected cells. The average value for cells transfected with empty pEFneo vector and total RNAs from wt MV-infected cells was set to 100%. (C) Vero cells were transfected with pIFΔ(−125)lucter, pRL-TK, and pEF-FLAG-mda-5, together with pEFneo vector expressing MV C or MV V protein or empty pEFneo vector. The MV C and V protein-expressing plasmids were also transfected together (C+V). At 24 h posttransfection, cells were mock transfected (−) or transfected with poly(I:C). The average value for cells transfected with empty pEFneo vector and poly(I:C) was set to 100%. (D) HEK293 cells were transfected with pIFΔ(−125)lucter and pRL-TK together with pEFneo vector expressing the MV C, MV V, or influenza NS1 protein or with empty pEFneo vector. The MV C and V protein-expressing plasmids were also transfected together (C+V). At 24 h posttransfection, cells were mock infected (−) or infected with VSVΔG*-G at an MOI of 10. The average value for cells transfected with empty pEFneo vector and infected with VSVΔG*-G was set to 100%.
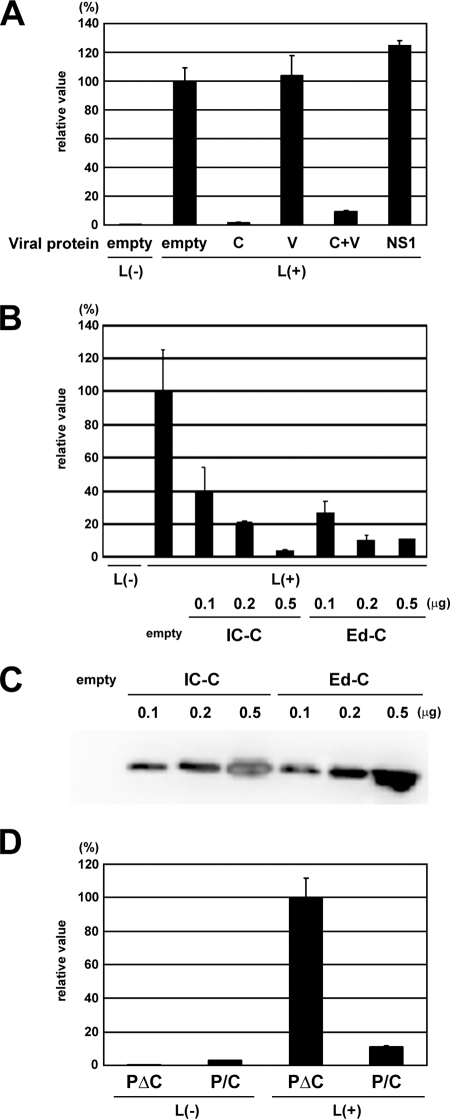
MV C protein but not V protein suppresses viral gene expression.
Previous studies have reported that both the C and V proteins of MV are capable of inhibiting viral RNA synthesis and that their abilities are different among different MV strains (3, 60, 77). We analyzed the abilities of the C and V proteins of the MV IC-B strain to suppress viral gene expression by using an MV minigenome system. As shown in Fig. 2A, the C protein but not the V protein of the IC-B strain suppressed gene expression from the MV minigenome. The C protein also inhibited the minigenome gene expression when the V protein was coexpressed (Fig. 2A). In these experiments, we confirmed the expression of the V proteins by Western blotting and their ability to inhibit Jak-STAT signaling by using methods reported previously (data not shown) (47, 50). The C protein of the Edmonston strain (the C proteins of the Edmonston ATCC and Edmonston tag strains have the same amino acid sequences) has been shown to strongly suppress MV minigenome RNA synthesis (3, 60). The C protein of the IC-B strain (IC-C) exhibited activity similar to that of the Edmonston strain (Fig. 2B, Ed-C). The comparable expression levels of the proteins were ascertained by Western blotting (Fig. 2C). The ability of the C protein to suppress MV minigenome gene expression was also analyzed in another way. The P protein-expressing plasmid, pCAG-T7-IC-PΔC (66), used as a support plasmid in the MV minigenome assay, has two nucleotide substitutions to knock out the expression of the C protein. These substitutions cause nonsense mutations in the C protein reading frame but are synonymous in the P protein reading frame (66). In contrast, the pCAG-T7-IC-P/C plasmid does not have these substitutions and expresses both the P and C proteins from two overlapping reading frames. When pCAG-T7-IC-P/C was used in place of pCAG-T7-IC-PΔC, reporter gene expression was severely restricted (Fig. 2D). All these results indicate that the C protein of the IC-B strain has the ability to suppress MV gene expression, whereas the V protein possesses little such activity.
FIG. 2.
Effect of the MV C and V proteins on MV minigenome expression. (A) CHO/hSLAM cells infected with vTF7-3 at an MOI of 0.5 were transfected with p18MGFLuc01, pCAG-T7-IC-N, pCAG-T7-IC-PΔC, and pGEMCR-9301B-L, together with the pCA7 vector expressing the C or V protein of the IC-B strain (C or V), influenza NS1 protein, or empty pCA7 vector. The MV IC-C and IC-V protein-expressing plasmids were also transfected together (C+V). pGEMCR-9301B-L was omitted from a transfection mixture for some cells [L(−)]. At 48 h posttransfection, intracellular luciferase activity was measured, and relative values are indicated. The average value for cells transfected with empty pCA7 vector was set to 100%. The data represent means ± standard deviations of triplicate samples. (B) VV5-4 cells infected with vTF7-3 at an MOI of 0.5 were transfected with p18MGFLuc01, pCAG-T7-IC-N, pCAG-T7-IC-PΔC, and pGEMCR-9301B-L together with various amounts (0.1, 0.2, and 0.5 μg) of pCA7 vector expressing the C protein of the IC-B (IC-C) or Edmonston strain (Ed-C) or empty pCA7 vector. pGEMCR-9301B-L was omitted from a transfection mixture for some cells [L(−)]. At 48 h posttransfection, intracellular luciferase activity was measured, and relative values are indicated. The average value for cells transfected with all three support plasmids and empty pCA7 vector was set to 100%. (C) VV5-4 cells were treated as described in panel B, lysed, and subjected to Western blotting. The C protein of MV was detected using MAb 2D10. (D) CHO/hSLAM cells infected with vTF7-3 at an MOI of 0.5 were transfected with p18MGFLuc01, pCAG-T7-IC-N, and pGEMCR-9301B-L together with pCAG-T7-IC-PΔC or pCAG-T7-IC-P/C. At 48 h posttransfection, intracellular luciferase activity was measured, and relative values are indicated. The average value for cells transfected with pCAG-T7-IC-PΔC was set to 100%. pGEMCR-9301B-L was omitted from a transfection mixture for some cells [L(−)].
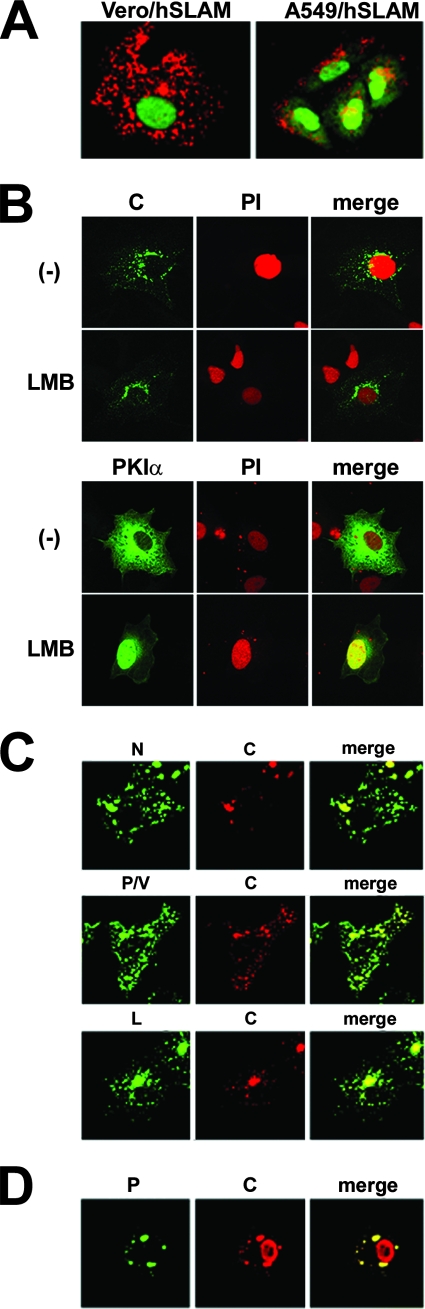
MV C protein colocalizes with proteins forming the ribonucleoprotein (RNP) complex in MV-infected cells.
Bellini et al. have shown that the C protein of MV is colocalized with the N protein in both the nucleus and cytoplasm of acutely or persistently infected cells, and they have suggested that the C protein may play a role in nucleocapsid assembly or viral RNA synthesis (4). Nishie et al. have reported that the C protein of MV shuttles between the nucleus and cytoplasm using a CRM1-dependent nuclear export pathway (48). These results were obtained using the C proteins from the attenuated Edmonston or brain-adapted MV strains (4) or the epitope-tagged C protein (48). Furthermore, the C protein of the Edmonston strain has been shown to have an amino acid substitution in its nuclear localization signal (48). We therefore reexamined the intracellular distribution of the C protein in cells infected with the virulent IC-B strain of MV. At 12 h p.i., expression of the C protein was below a detectable level (data not shown). When the C protein became detectable (at 16 h p.i.), it was localized exclusively in the cytoplasm of infected cells, forming small dots (data not shown). Figure 3A shows the distribution of the C protein in MV-infected Vero/hSLAM and A549/hSLAM cells at 36 h p.i., which was essentially the same as that at 16 h p.i. Although the C protein was hardly detected in the nucleus using MAb 2D10, its possible intranuclear localization and altered structure cannot be excluded, as suggested by Bellini et al. (4). However, when the CRM1-dependent nuclear export pathway was blocked by LMB, the distribution pattern of the C protein was not altered (Fig. 3B). By contrast, PKIα-EGFP, which is known to use the CRM1-dependent nuclear export pathway (17), accumulated in the nucleus (Fig. 3B). These data strongly suggest that the C protein of the virulent MV strain is mostly localized in the cytoplasm, not the nucleus, when it is expressed in the context of viral infection.
FIG. 3.
Intracellular localization of C protein. (A) Vero/hSLAM and A549/hSLAM cells were infected with IC323-EGFP (wt MV) at an MOI of 0.5 in the presence of the fusion-blocking peptide. At 36 h p.i., intracellular distribution of C protein was analyzed by indirect immunofluorescence assay using MAb 2D10 against MV C protein. Green fluorescence indicates EGFP, and red fluorescence indicates the MV C protein. (B) Vero/hSLAM cells were infected with IC323 (wt MV) or transfected with EGFP-tagged PKIα-expressing plasmids. Some cells were treated with 20 nM LMB, and others remained untreated (−). For counterstaining of the nucleus, cells were treated with 10 μg/ml RNase A and incubated with 10 μg/ml propidium iodide (PI). At 24 h p.i. the C protein was detected by indirect immunofluorescence assay using MAb 2D10. (C) Vero/hSLAM cells were infected with IC323 wt MV (upper and middle panels) or IC323-EGFPtag-L (lower panels) at an MOI of 0.5 in the presence of the fusion-blocking peptide. At 36 h p.i., intracellular distribution of N and C proteins (upper panel), P/V and C proteins (middle panel), or C protein (lower panel) was analyzed by indirect immunofluorescence assay using specific antibodies against the C, N, or P/V protein. Red fluorescence indicates the C protein, and green fluorescence indicates the N (upper panel), P/V (middle panel), or EGFP-tagged L protein (lower panel). (D) Vero/hSLAM cells were transfected with MV N, P, and C protein-expressing plasmids (pCAG-T7-IC-N, pCAG-T7-IC-PΔC, and pCA7-IC-C). At 48 h posttransfection, intracellular distribution of P and C proteins was analyzed by indirect immunofluorescence assay using specific antibodies raised against the P/V or C protein. Green fluorescence indicates the P protein, and red fluorescence indicates the C protein.
Next, we examined by immunofluorescence staining whether the C protein was colocalized with proteins forming the RNP complex (N, P, and L proteins) in MV-infected cells. Since no antibody was available to detect the MV L protein, a recombinant MV (IC323-EGFPtagL) harboring the EGFP-tagged L protein was generated using a reverse genetics technique, as reported previously (14, 46, 66, 68). The C protein was shown to be colocalized in the cytoplasm with both the N and L proteins (Fig. 3C). To detect the P protein, only the rabbit serum raised against the amino-terminal half of the P protein, which is shared by the V protein, was available. Both viral proteins recognized by the serum (the P and V proteins) were mostly colocalized with the C protein (Fig. 3C). Colocalization of the C protein with the P protein was further verified by using protein-expressing plasmids. When the C protein was coexpressed with the N and P proteins (the plasmid did not direct the synthesis of the V protein), it showed a colocalization pattern similar to that in MV-infected cells, and dual staining indicated that the C protein was colocalized with the P protein in the cytoplasm (Fig. 3D). These results indicated that the C protein of the MV IC-B strain was colocalized with the proteins that formed the RNP complex in MV-infected cells, consistent with its ability to modulate viral RNA synthesis.
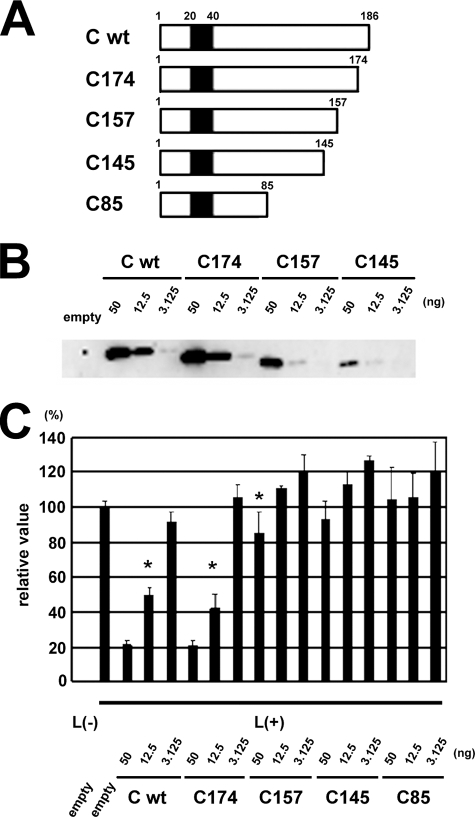
Truncation of the carboxyl terminus affects the ability of the C protein to inhibit MV gene expression.
To determine the region on the C protein required to suppress MV RNA synthesis, four plasmids encoding mutant C proteins, with stepwise truncations at the carboxyl terminus, were generated (Fig. 4A). The intact C protein (C wt) is composed of 186 amino acid residues, and stop codons were introduced at positions 175, 158, 146, and 86, as these substitutions can be introduced into the virus genome without altering the amino acid sequence of the P and V proteins (Table 1). These mutant C proteins with the carboxyl terminus truncations were designated C174, C157, C145, and C85, respectively. Expression levels of these mutant C proteins were analyzed by Western blotting using the C protein-specific MAb 2D10 that recognizes the amino terminus of the C protein (Fig. 4A). C174 was expressed at a level similar to that of C wt, whereas the longer truncations decreased the expression levels of the protein (Fig. 4B). C85 was barely detectable (data not shown). Using these plasmids, we analyzed the activity of mutant C proteins to suppress MV gene expression (Fig. 4C). Expression of the reporter gene was severely suppressed by C wt and C174. C157 suppressed the minigenome expression weakly, even when the protein was expressed at a level similar to that of C wt and C174 (Fig. 4C). C145 and C85 hardly suppressed MV gene expression, partly because they were expressed at levels much lower than those of C wt and C174 (Fig. 4B and data not shown). These data indicate that the carboxyl-terminal 12 amino acid residues at positions 175 to 186 were dispensable for the C protein to suppress MV minigenome expression, whereas the next 17 residues at positions 158 to 174 were important for this function.
FIG. 4.
Effect of carboxyl terminus truncation of C protein on its ability to inhibit MV minigenome expression. (A) Diagram of wt and carboxyl terminus-truncated C proteins. Filled box indicates the region used as an antigen to generate MAb 2D10. (B) Expression levels of mutant C proteins used in the minigenome assay. VV5-4 cells infected with vTF7-3 at an MOI of 0.5 were transfected with p18MGFLuc01, pCAG-T7-IC-N, pCAG-T7-IC-PΔC, and pGEMCR-9301B-L together with empty pCA7 vector or various amounts (50, 12.5, and 3.125 ng) of pCA7-IC-C(wt), pCA7-IC-C174, pCA7-IC-C157, or pCA7-IC-C145. At 48 h posttransfection, C proteins were detected by Western blotting using MAb 2D10. (C) Inhibitory activity of C protein against MV minigenome expression. VV5-4 cells infected with vTF7-3 and transfected with plasmids as indicated in panel B were used for the minigenome analysis. At 48 h posttransfection, intracellular luciferase activity was measured, and relative values are indicated. The average value for cells transfected with all three support plasmids and empty pCA7 vector was set to 100%. pGEMCR-9301B-L was omitted from a transfection mixture for some cells [L(−)]. The data represent means ± standard deviations of triplicate samples. Asterisks indicate the samples in which similar amounts of C protein were detected by the method described in panel B.
C protein of MV modulates viral RNA synthesis, thereby contributing to circumvention of the host IFN response.
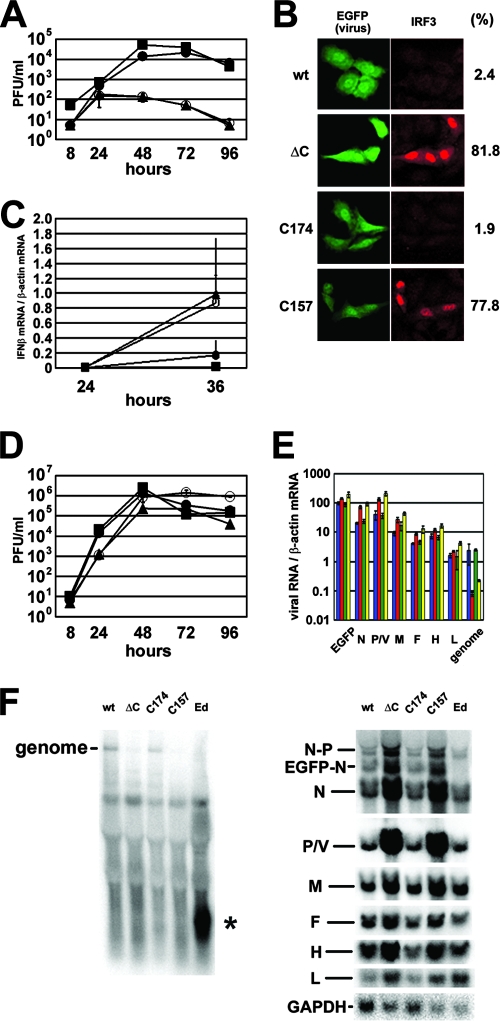
The above data indicate that C174 and C157 differ in their ability to inhibit viral RNA synthesis. Since nonsense mutations at positions 175 and 158 in the C protein reading frame are synonymous in the overlapping P/V protein reading frame, they could be introduced into infectious recombinant virus genomes without altering the P and V protein sequences (Table 1). Infectious MVs bearing C174 or C157 (designated MV-C174 and MV-C157, respectively) were generated by reverse genetics (46, 66, 68) and examined for their ability to grow and modulate IFN induction in infected cells. MV-C174 replicated in A549/hSLAM cells as efficiently as wt MV, whereas MV-C157 replicated very poorly, like MVΔC (Fig. 5A) (47). Viral protein levels were also much higher in wt MV- and MV-C174-infected cells than levels in MV-C157- and MVΔC-infected cells (data not shown). The nuclear translocation of IRF3 was often observed in MV-C157- and MVΔC-infected cells, whereas it was rarely seen in MV-C174- and wt MV-infected cells (Fig. 5B). Accordingly, higher levels of IFN-β mRNA were observed in MV-C157- and MVΔC-infected cells than in MV-C174- and wt MV-infected cells (Fig. 5C). All these data are consistent with those reported previously for wt MV and MVΔC (47). We interpreted these results as follows. wt MV and MV-C174 with functional C proteins are capable of controlling viral RNA synthesis at optimal levels such that the amount of IFN induced by inducer RNAs is minimized, and yet progeny virions are efficiently produced from available viral RNAs. In contrast, MVΔC and MV-C157 bearing defective C proteins produce excessive RNAs in infected cells, which may induce a larger amount of IFN, thus resulting in poor virus growth. However, we could not find much difference in viral RNA levels between wt MV- or MV-C174-infected cells and MVΔC- or MV-C157-infected cells (data not shown). Since IFN produced upon viral infection may affect the subsequent viral RNA production, we repeated the experiments using Vero/hSLAM cells. Vero cells are known to be defective in IFN synthesis (16, 45), whereas A549 cells are competent to produce IFN (64, 76). Indeed, IFN-β mRNA was below the detectable level in Vero/hSLAM cells infected with any of the recombinant viruses (data not shown). In Vero/hSLAM cells, MVΔC and MV-C157 grew almost as efficiently as wt MV and MV-C174 (Fig. 5D), although their viral protein synthesis was still partially restricted (data not shown). On the other hand, RT-QPCR (Fig. 5E) and Northern blotting analyses (Fig. 5F) indicated that levels of viral mRNAs in MVΔC- or MV-C157-infected Vero/hSLAM cells were much greater than those in wt MV- or MV-C174-infected cells. These results indicate that viral transcription is suppressed by the C protein and that the lack of C protein leads to high levels of viral transcripts in the absence of IFN production. Thus, the C protein of MV likely contributes to the circumvention of the host IFN response by modulating the level of viral RNA synthesis.
FIG. 5.
Viral growth, IFN induction, and viral RNA synthesis in cells infected with recombinant MVs. (A) Growth kinetics of wt MV, MVΔC, MV-C174, and MV-C157 in A549/hSLAM cells. A549/hSLAM cells were infected with recombinant MVs at an MOI of 0.01. At various time intervals, cells were scraped into culture medium, and virus titers were determined on Vero/hSLAM cells. (B) Indirect immunofluorescence assay for IRF3 in virus-infected cells. A549/hSLAM cells were infected with wt MV, MV-C174, MV-C157, or MVΔC at an MOI of 0.5 and incubated in the presence of the fusion-blocking peptide. At 36 h p.i., intracellular distribution of IRF3 was analyzed by indirect immunofluorescence assay. Green fluorescence indicates EGFP, and red fluorescence indicates IRF3. The percentage of cells showing nuclear translocation of IRF3 is indicated. (C) Quantification of IFN-β mRNA. A549/hSLAM cells were infected with wt MV, MV-C174, MV-C157, or MVΔC at an MOI of 0.5. At 24 and 36 h p.i., total RNA was extracted from cells, and levels of IFN-β mRNA were analyzed by RT-QPCR. Data were normalized by the levels of β-actin mRNA. (D) Growth kinetics of wt MV, MVΔC, MV-C174, and MV-C157 in Vero/hSLAM cells. Experiments were performed as described in panel A, using Vero/hSLAM cells instead of A549/hSLAM cells. (A, C, and D) Filled circles, wt MV; filled squares, MV-C174; filled triangles, MV-C157; open circles, MVΔC. Data represent the means ± standard deviations of triplicate samples. (E) Quantification of viral mRNAs and genome by RT-QPCR. Vero/hSLAM cells were infected with wt MV (blue), MVΔC (red), MV-C174 (green), or MV-C157 (yellow) at an MOI of 0.5. At 36 h p.i., levels of viral mRNAs and viral genomes in infected cells were analyzed by RT-QPCR. Data were normalized by the levels of β-actin mRNA. (F) Northern blot analyses. Vero/hSLAM cells were infected with wt MV (wt), MVΔC (ΔC), MV-C174 (C174), MV-C157 (C157), or a stock of the MV Edmonston strain containing DI particles (Ed) at an MOI of 0.1. At 48 h p.i., viral mRNAs and viral genomes in infected cells were detected by Northern blot analyses using 32P-labeled DNA probes specific for the viral N, P/V, M, F, H, L, cellular GAPDH mRNAs (right panels) or the 5′ genome terminus (left panel). The asterisk in the left panel indicates the DI genome detected by the 5′ genome terminus probe. The right top panel shows mono- (N) and bicistronic (N-P and EGFP-N) messages detected by the N gene probe.
Interestingly, viral genome levels were decreased in MVΔC- or MV-C157-infected cells (Fig. 5E and F), which may be attributed to the limited protein synthesis in those cells. No significant difference was found in the 3′ to 5′ gradient of MV transcripts (Fig. 5E) and the ratio of bicistronic mRNAs (Fig. 5F) among cells infected with the different recombinant viruses. Furthermore, no aberrant transcripts including the DI genomes were detected in cells infected with any of the recombinant viruses (a strong signal for the DI genome was detected in cells infected with a stock of the Edmonston strain known to contain DI particles) (Fig. 5F). The presence of the DI genomes was also examined in viral particles. When many (more than 25) cycles of PCR were performed, the DI genomes were detected in the MV-C174 and MV-C157 particles but not in the wt MV and MVΔC particles (data not shown). However, their levels were much less than those of the authentic virus genomes. Thus, although the lack or functional defect of the C protein enhances the viral RNA synthesis, there is no evidence that it affects the quality or ratios of the viral RNA produced.
DISCUSSION
In this paper, we showed that the MV C protein plays an important role in the circumvention of IFN induction in the host by acting as a regulator of viral RNA synthesis. Unlike the V protein, the C protein is produced only by members of three genera (Respirovirus, Henipavirus, and Morbillivirus) in the subfamily Paramyxovirinae, using an overlapping reading frame of the P and V proteins (37). The C proteins are small basic polypeptides and have little homology in the amino acid sequence among the genera (37). There is substantial evidence that the C protein of members of the Respirovirus genus (Sendai virus and human parainfluenza virus type 3) inhibits the Jak/Stat signaling pathway and is critical for evasion of host innate immune responses (15, 22, 23, 30, 31, 41). The C protein of Sendai virus is also required for efficient virus assembly (26). The C protein of Nipah virus, a member of the Henipavirus genus, counteracts the cellular IFN response (53). Takeuchi et al. have shown that the C protein of MV is essential for virus growth in macaques (70). Although one study has reported that the C protein of MV inhibits the IFN response (63), other studies have indicated that it does not possess the ability to block the IFN-stimulated Jak/Stat signaling cascade (50, 69, 70). Our previous study has shown that expression of the C protein is required for the MV IC-B strain to grow well in IFN-producing cell lines but not in cells having a defect in IFN production (47). All these observations may suggest that the C protein of the IC-B strain supports MV growth by inhibiting IFN induction. However, the present study indicates that the C protein per se does not have the ability to modulate the IFN induction pathway.
ssRNA bearing a 5′ triphosphate and dsRNA are known to be recognized as pathogen-associated molecular patterns by the intracellular sensor molecules RIG-I and mda-5 and are believed to stimulate IFN induction in RNA virus-infected cells (19, 71). Previous studies have shown that infection with negative-strand RNA viruses is recognized by RIG-I, a sensor molecule for ssRNA bearing a 5′ triphosphate, but not by mda-5, a sensor molecule for dsRNA (32). Recently, Plumet et al. (57) have suggested that MV leader RNA, which is predicted to be ssRNA bearing a 5′ triphosphate, is a stimulant for IFN induction. Our results confirmed that such MV leader RNA synthesized in vitro does indeed stimulate the IFN induction pathway in cells. However, the relevance of these data in vivo remains to be determined, as MV leader RNA is barely detected in MV-infected cells (8). Also importantly, the V protein of MV interferes with the mda-5-mediated IFN induction pathway rather than the RIG-I-mediated pathway (10). Thus, the mechanism of IFN induction in MV-infected cells still needs further investigation. Gainey et al. (20) have recently proposed that paramyxovirus P/V gene products limit host innate immune responses by inhibiting synthesis of aberrant viral mRNAs and dsRNAs. The V protein of parainfluenza virus type 5 and the C proteins of Sendai virus and human parainfluenza virus type 3 inhibit their RNA synthesis (6, 11, 28, 31, 38, 42). Both the C and V proteins of MV have also been shown to inhibit viral RNA synthesis (3, 60, 77). However, our data with the MV IC-B strain showed that only the C protein but not the V protein inhibits MV gene expression efficiently. MV strain variations may explain the discrepancy from previous studies, but we found that the V protein of the Edmonston ATCC and Edmonston tag strains also exhibited little inhibitory function (data not shown).
Using recombinant viruses, we demonstrated a complete correlation between the ability of the C protein to inhibit viral RNA synthesis and its ability to suppress the IFN induction pathway. Recombinant MVs carrying a C protein defective in suppressing viral RNA synthesis (MV-C157) or lacking C protein expression (MVΔC) strongly stimulated IFN synthesis and failed to replicate well in A549/hSLAM cells. Nonetheless, the amount of viral RNA in A549/hSLAM cells infected with MVΔC or MV-C157 was maintained at a level similar to that in cells infected with wt MV or MV-C174. A plausible explanation for these results is that RNA synthesis by MVΔC or MV-C157 is much more accelerated at the single-cell level than synthesis by wt MV or MV-C174, leading to a robust IFN response, which controls subsequent viral replication. This explanation is supported by the large amount of viral RNA in Vero/hSLAM cells infected with MVΔC or MV-C157 compared with that in wt MV- or MV-C174-infected cells. The lower levels of viral genomes observed in Vero/hSLAM cells infected with MVΔC or MV-C157 may be due to the IFN-independent inhibition of protein synthesis in those cells, as also observed in HeLa/NS1 cells infected with MVΔC (47). Since the N protein must be accumulated at a high level in the cytoplasm to allow efficient genome replication (56), the inhibition of protein synthesis will lead to decreased genome replication. Although the exact mechanism is currently unknown, we suspect that the activation of the dsRNA-dependent protein kinase R by increased viral RNAs may be involved in the impaired protein synthesis in cells infected with MVΔC.
Indirect immunofluorescence staining and confocal microscopy showed that the C protein was colocalized with all three proteins (N, P, and L) that comprise the RNP complex. These data suggest that the C protein plays a regulatory role in viral RNA synthesis by interacting with the RNP complex, as suggested by Bellini et al. (4). However, previous studies using a yeast two-hybrid system and a glutathione S-transferase pull-down assay have shown that the C protein of MV does not interact with the N, P, or L protein (39). We also failed to detect interaction of the C protein with N, P, or L proteins using a yeast two-hybrid system and coimmunoprecipitation (M. Iwasaki et al., unpublished observations). Thus, interaction of the C protein with the RNP complex may be indirect or very weak. We are searching for cellular factors that interact with the C protein to elucidate molecular mechanisms by which the C protein exhibits its functions.
In conclusion, our data indicate that the C protein of MV acts as a regulator of viral RNA synthesis and thus contributes to evasion of the host IFN response. Since MVΔC and MV-C157 strongly stimulate IFN production and replicate poorly in IFN-producing cells, in spite of the production of the V protein, both the C and V proteins must be required for MV to fully circumvent the host IFN response.
Acknowledgments
We thank B. Moss, W. Chang, T. A. Sato, K. Komase, and S. Goodbourn for providing vTF7-3, VV5-4 cells, MAb E137, p18MGFLuc01, and pIFΔ(−125)lucter, respectively.
This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology and the Ministry of Health, Labor and Welfare of Japan.
Footnotes
Published ahead of print on 18 June 2008.
REFERENCES
- 1.Andrejeva, J., K. S. Childs, D. F. Young, T. S. Carlos, N. Stock, S. Goodbourn, and R. E. Randall. 2004. The V proteins of paramyxoviruses bind the IFN-inducible RNA helicase, mda-5, and inhibit its activation of the IFN-beta promoter. Proc. Natl. Acad. Sci. USA 10117264-17269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bair, C. H., C. S. Chung, I. A. Vasilevskaya, and W. Chang. 1996. Isolation and characterization of a Chinese hamster ovary mutant cell line with altered sensitivity to vaccinia virus killing. J. Virol. 704655-4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bankamp, B., J. Wilson, W. J. Bellini, and P. A. Rota. 2005. Identification of naturally occurring amino acid variations that affect the ability of the measles virus C protein to regulate genome replication and transcription. Virology 336120-129. [DOI] [PubMed] [Google Scholar]
- 4.Bellini, W. J., G. Englund, S. Rozenblatt, H. Arnheiter, and C. D. Richardson. 1985. Measles virus P gene codes for two proteins. J. Virol. 53908-919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bryce, J., C. Boschi-Pinto, K. Shibuya, and R. E. Black. 2005. WHO estimates of the causes of death in children. Lancet 3651147-1152. [DOI] [PubMed] [Google Scholar]
- 6.Cadd, T., D. Garcin, C. Tapparel, M. Itoh, M. Homma, L. Roux, J. Curran, and D. Kolakofsky. 1996. The Sendai paramyxovirus accessory C proteins inhibit viral genome amplification in a promoter-specific fashion. J. Virol. 705067-5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caignard, G., M. Guerbois, J. L. Labernardiere, Y. Jacob, L. M. Jones, F. Wild, F. Tangy, and P. O. Vidalain. 2007. Measles virus V protein blocks Jak1-mediated phosphorylation of STAT1 to escape IFN-alpha/beta signaling. Virology 368351-362. [DOI] [PubMed] [Google Scholar]
- 8.Castaneda, S. J., and T. C. Wong. 1989. Measles virus synthesizes both leaderless and leader-containing polyadenylated RNAs in vivo. J. Virol. 632977-2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cattaneo, R., K. Kaelin, K. Baczko, and M. A. Billeter. 1989. Measles virus editing provides an additional cysteine-rich protein. Cell 56759-764. [DOI] [PubMed] [Google Scholar]
- 10.Childs, K., N. Stock, C. Ross, J. Andrejeva, L. Hilton, M. Skinner, R. Randall, and S. Goodbourn. 2007. mda-5, but not RIG-I, is a common target for paramyxovirus V proteins. Virology 359190-200. [DOI] [PubMed] [Google Scholar]
- 11.Curran, J., J. B. Marq, and D. Kolakofsky. 1992. The Sendai virus nonstructural C proteins specifically inhibit viral mRNA synthesis. Virology 189647-656. [DOI] [PubMed] [Google Scholar]
- 12.Devaux, P., and R. Cattaneo. 2004. Measles virus phosphoprotein gene products: conformational flexibility of the P/V protein amino-terminal domain and C protein infectivity factor function. J. Virol. 7811632-11640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Devaux, P., V. von Messling, W. Songsungthong, C. Springfeld, and R. Cattaneo. 2007. Tyrosine 110 in the measles virus phosphoprotein is required to block STAT1 phosphorylation. Virology 36072-83. [DOI] [PubMed] [Google Scholar]
- 14.Duprex, W. P., F. M. Collins, and B. K. Rima. 2002. Modulating the function of the measles virus RNA-dependent RNA polymerase by insertion of green fluorescent protein into the open reading frame. J. Virol. 767322-7328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Durbin, A. P., J. M. McAuliffe, P. L. Collins, and B. R. Murphy. 1999. Mutations in the C, D, and V open reading frames of human parainfluenza virus type 3 attenuate replication in rodents and primates. Virology 261319-330. [DOI] [PubMed] [Google Scholar]
- 16.Emeny, J. M., and M. J. Morgan. 1979. Regulation of the interferon system: evidence that Vero cells have a genetic defect in interferon production. J. Gen. Virol. 43247-252. [DOI] [PubMed] [Google Scholar]
- 17.Fornerod, M., M. Ohno, M. Yoshida, and I. W. Mattaj. 1997. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell 901051-1060. [DOI] [PubMed] [Google Scholar]
- 18.Fuerst, T. R., E. G. Niles, F. W. Studier, and B. Moss. 1986. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc. Natl. Acad. Sci. USA 838122-8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujita, T., K. Onoguchi, K. Onomoto, R. Hirai, and M. Yoneyama. 2007. Triggering antiviral response by RIG-I-related RNA helicases. Biochimie 89754-760. [DOI] [PubMed] [Google Scholar]
- 20.Gainey, M. D., P. J. Dillon, K. M. Clark, M. J. Manuse, and G. D. Parks. 2008. Paramyxovirus-induced shutoff of host and viral protein synthesis: role of the P and V proteins in limiting PKR activation. J. Virol. 82828-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia-Sastre, A. 2002. Mechanisms of inhibition of the host interferon alpha/beta-mediated antiviral responses by viruses. Microbes Infect. 4647-655. [DOI] [PubMed] [Google Scholar]
- 22.Garcin, D., P. Latorre, and D. Kolakofsky. 1999. Sendai virus C proteins counteract the interferon-mediated induction of an antiviral state. J. Virol. 736559-6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcin, D., J. B. Marq, L. Strahle, P. le Mercier, and D. Kolakofsky. 2002. All four Sendai virus C proteins bind Stat1, but only the larger forms also induce its mono-ubiquitination and degradation. Virology 295256-265. [DOI] [PubMed] [Google Scholar]
- 24.Griffin, D. E. 2007. Measles virus, p. 1551-1585. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 5th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 25.Haller, O., G. Kochs, and F. Weber. 2006. The interferon response circuit: induction and suppression by pathogenic viruses. Virology 344119-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hasan, M. K., A. Kato, M. Muranaka, R. Yamaguchi, Y. Sakai, I. Hatano, M. Tashiro, and Y. Nagai. 2000. Versatility of the accessory C proteins of Sendai virus: contribution to virus assembly as an additional role. J. Virol. 745619-5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hashimoto, K., N. Ono, H. Tatsuo, H. Minagawa, M. Takeda, K. Takeuchi, and Y. Yanagi. 2002. SLAM (CD150)-independent measles virus entry as revealed by recombinant virus expressing green fluorescent protein. J. Virol. 766743-6749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horikami, S. M., R. E. Hector, S. Smallwood, and S. A. Moyer. 1997. The Sendai virus C protein binds the L polymerase protein to inhibit viral RNA synthesis. Virology 235261-270. [DOI] [PubMed] [Google Scholar]
- 29.Hornung, V., J. Ellegast, S. Kim, K. Brzozka, A. Jung, H. Kato, H. Poeck, S. Akira, K. K. Conzelmann, M. Schlee, S. Endres, and G. Hartmann. 2006. 5′-Triphosphate RNA is the ligand for RIG-I. Science 314994-997. [DOI] [PubMed] [Google Scholar]
- 30.Kato, A., K. Kiyotani, T. Kubota, T. Yoshida, M. Tashiro, and Y. Nagai. 2007. Importance of the anti-interferon capacity of Sendai virus C protein for pathogenicity in mice. J. Virol. 813264-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kato, A., Y. Ohnishi, M. Kohase, S. Saito, M. Tashiro, and Y. Nagai. 2001. Y2, the smallest of the Sendai virus C proteins, is fully capable of both counteracting the antiviral action of interferons and inhibiting viral RNA synthesis. J. Virol. 753802-3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kato, H., O. Takeuchi, S. Sato, M. Yoneyama, M. Yamamoto, K. Matsui, S. Uematsu, A. Jung, T. Kawai, K. J. Ishii, O. Yamaguchi, K. Otsu, T. Tsujimura, C. S. Koh, C. Reis e Sousa, Y. Matsuura, T. Fujita, and S. Akira. 2006. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441101-105. [DOI] [PubMed] [Google Scholar]
- 33.Katze, M. G., Y. He, and M. Gale, Jr. 2002. Viruses and interferon: a fight for supremacy. Nat. Rev. Immunol. 2675-687. [DOI] [PubMed] [Google Scholar]
- 34.Kobune, F., H. Sakata, and A. Sugiura. 1990. Marmoset lymphoblastoid cells as a sensitive host for isolation of measles virus. J. Virol. 64700-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kobune, F., H. Takahashi, K. Terao, T. Ohkawa, Y. Ami, Y. Suzaki, N. Nagata, H. Sakata, K. Yamanouchi, and C. Kai. 1996. Nonhuman primate models of measles. Lab. Anim. Sci. 46315-320. [PubMed] [Google Scholar]
- 36.Komase, K., T. Nakayama, M. Iijima, K. Miki, R. Kawanishi, and H. Uejima. 2006. The phosphoprotein of attenuated measles AIK-C vaccine strain contributes to its temperature-sensitive phenotype. Vaccine 24826-834. [DOI] [PubMed] [Google Scholar]
- 37.Lamb, R. A., and G. D. Parks. 2007. Paramyxoviridae: the viruses and their replication, p. 1449-1496. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 5th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 38.Lin, Y., F. Horvath, J. A. Aligo, R. Wilson, and B. He. 2005. The role of simian virus 5 V protein on viral RNA synthesis. Virology 338270-280. [DOI] [PubMed] [Google Scholar]
- 39.Liston, P., C. DiFlumeri, and D. J. Briedis. 1995. Protein interactions entered into by the measles virus P, V, and C proteins. Virus Res. 38241-259. [DOI] [PubMed] [Google Scholar]
- 40.Malathi, K., B. Dong, M. Gale, Jr., and R. H. Silverman. 2007. Small self-RNA generated by RNase L amplifies antiviral innate immunity. Nature 448816-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malur, A. G., S. Chattopadhyay, R. K. Maitra, and A. K. Banerjee. 2005. Inhibition of STAT 1 phosphorylation by human parainfluenza virus type 3 C protein. J. Virol. 797877-7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malur, A. G., M. A. Hoffman, and A. K. Banerjee. 2004. The human parainfluenza virus type 3 (HPIV 3) C protein inhibits viral transcription. Virus Res. 99199-204. [DOI] [PubMed] [Google Scholar]
- 43.Mibayashi, M., L. Martinez-Sobrido, Y. M. Loo, W. B. Cardenas, M. Gale, Jr., and A. Garcia-Sastre. 2007. Inhibition of retinoic acid-inducible gene I-mediated induction of beta interferon by the NS1 protein of influenza A virus. J. Virol. 81514-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mizushima, S., and S. Nagata. 1990. pEF-BOS, a powerful mammalian expression vector. Nucleic Acids Res. 185322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mosca, J. D., and P. M. Pitha. 1986. Transcriptional and posttranscriptional regulation of exogenous human beta interferon gene in simian cells defective in interferon synthesis. Mol. Cell. Biol. 62279-2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakatsu, Y., M. Takeda, M. Kidokoro, M. Kohara, and Y. Yanagi. 2006. Rescue system for measles virus from cloned cDNA driven by vaccinia virus Lister vaccine strain. J. Virol. Methods 137152-155. [DOI] [PubMed] [Google Scholar]
- 47.Nakatsu, Y., M. Takeda, S. Ohno, R. Koga, and Y. Yanagi. 2006. Translational inhibition and increased interferon induction in cells infected with C protein-deficient measles virus. J. Virol. 8011861-11867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nishie, T., K. Nagata, and K. Takeuchi. 2007. The C protein of wild-type measles virus has the ability to shuttle between the nucleus and the cytoplasm. Microbes Infect. 9344-354. [DOI] [PubMed] [Google Scholar]
- 49.Niwa, H., K. Yamamura, and J. Miyazaki. 1991. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108193-199. [DOI] [PubMed] [Google Scholar]
- 50.Ohno, S., N. Ono, M. Takeda, K. Takeuchi, and Y. Yanagi. 2004. Dissection of measles virus V protein in relation to its ability to block alpha/beta interferon signal transduction. J. Gen. Virol. 852991-2999. [DOI] [PubMed] [Google Scholar]
- 51.Ono, N., H. Tatsuo, Y. Hidaka, T. Aoki, H. Minagawa, and Y. Yanagi. 2001. Measles viruses on throat swabs from measles patients use signaling lymphocytic activation molecule (CDw150) but not CD46 as a cellular receptor. J. Virol. 754399-4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Palosaari, H., J. P. Parisien, J. J. Rodriguez, C. M. Ulane, and C. M. Horvath. 2003. STAT protein interference and suppression of cytokine signal transduction by measles virus V protein. J. Virol. 777635-7644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park, M. S., M. L. Shaw, J. Munoz-Jordan, J. F. Cros, T. Nakaya, N. Bouvier, P. Palese, A. Garcia-Sastre, and C. F. Basler. 2003. Newcastle disease virus (NDV)-based assay demonstrates interferon-antagonist activity for the NDV V protein and the Nipah virus V, W, and C proteins. J. Virol. 771501-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Patterson, J. B., D. Thomas, H. Lewicki, M. A. Billeter, and M. B. Oldstone. 2000. V and C proteins of measles virus function as virulence factors in vivo. Virology 26780-89. [DOI] [PubMed] [Google Scholar]
- 55.Pichlmair, A., O. Schulz, C. P. Tan, T. I. Naslund, P. Liljestrom, F. Weber, and C. Reis e Sousa. 2006. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science 314997-1001. [DOI] [PubMed] [Google Scholar]
- 56.Plumet, S., W. P. Duprex, and D. Gerlier. 2005. Dynamics of viral RNA synthesis during measles virus infection. J. Virol. 796900-6908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Plumet, S., F. Herschke, J. M. Bourhis, H. Valentin, S. Longhi, and D. Gerlier. 2007. Cytosolic 5′-triphosphate ended viral leader transcript of measles virus as activator of the RIG I-mediated interferon response. PLoS ONE 2e279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Radecke, F., and M. A. Billeter. 1996. The nonstructural C protein is not essential for multiplication of Edmonston B strain measles virus in cultured cells. Virology 217418-421. [DOI] [PubMed] [Google Scholar]
- 59.Randall, R. E., and S. Goodbourn. 2008. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J. Gen. Virol. 891-47. [DOI] [PubMed] [Google Scholar]
- 60.Reutter, G. L., C. Cortese-Grogan, J. Wilson, and S. A. Moyer. 2001. Mutations in the measles virus C protein that up regulate viral RNA synthesis. Virology 285100-109. [DOI] [PubMed] [Google Scholar]
- 61.Richardson, C. D., A. Scheid, and P. W. Choppin. 1980. Specific inhibition of paramyxovirus and myxovirus replication by oligopeptides with amino acid sequences similar to those at the N-termini of the F1 or HA2 viral polypeptides. Virology 105205-222. [DOI] [PubMed] [Google Scholar]
- 62.Schneider, H., K. Kaelin, and M. A. Billeter. 1997. Recombinant measles viruses defective for RNA editing and V protein synthesis are viable in cultured cells. Virology 227314-322. [DOI] [PubMed] [Google Scholar]
- 63.Shaffer, J. A., W. J. Bellini, and P. A. Rota. 2003. The C protein of measles virus inhibits the type I interferon response. Virology 315389-397. [DOI] [PubMed] [Google Scholar]
- 64.Spann, K. M., K. C. Tran, B. Chi, R. L. Rabin, and P. L. Collins. 2004. Suppression of the induction of alpha, beta, and lambda interferons by the NS1 and NS2 proteins of human respiratory syncytial virus in human epithelial cells and macrophages [corrected]. J. Virol. 784363-4369. (Erratum, 78:6705.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Takada, A., C. Robinson, H. Goto, A. Sanchez, K. G. Murti, M. A. Whitt, and Y. Kawaoka. 1997. A system for functional analysis of Ebola virus glycoprotein. Proc. Natl. Acad. Sci. USA 9414764-14769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Takeda, M., S. Ohno, F. Seki, K. Hashimoto, N. Miyajima, K. Takeuchi, and Y. Yanagi. 2005. Efficient rescue of measles virus from cloned cDNA using SLAM-expressing Chinese hamster ovary cells. Virus Res. 108161-165. [DOI] [PubMed] [Google Scholar]
- 67.Takeda, M., S. Ohno, F. Seki, Y. Nakatsu, M. Tahara, and Y. Yanagi. 2005. Long untranslated regions of the measles virus M and F genes control virus replication and cytopathogenicity. J. Virol. 7914346-14354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Takeda, M., K. Takeuchi, N. Miyajima, F. Kobune, Y. Ami, N. Nagata, Y. Suzaki, Y. Nagai, and M. Tashiro. 2000. Recovery of pathogenic measles virus from cloned cDNA. J. Virol. 746643-6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Takeuchi, K., S. I. Kadota, M. Takeda, N. Miyajima, and K. Nagata. 2003. Measles virus V protein blocks interferon (IFN)-alpha/beta but not IFN-gamma signaling by inhibiting STAT1 and STAT2 phosphorylation. FEBS Lett. 545177-182. [DOI] [PubMed] [Google Scholar]
- 70.Takeuchi, K., M. Takeda, N. Miyajima, Y. Ami, N. Nagata, Y. Suzaki, J. Shahnewaz, S. Kadota, and K. Nagata. 2005. Stringent requirement for the C protein of wild-type measles virus for growth both in vitro and in macaques. J. Virol. 797838-7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Takeuchi, O., and S. Akira. 2007. Recognition of viruses by innate immunity. Immunol. Rev. 220214-224. [DOI] [PubMed] [Google Scholar]
- 72.Talon, J., C. M. Horvath, R. Polley, C. F. Basler, T. Muster, P. Palese, and A. Garcia-Sastre. 2000. Activation of interferon regulatory factor 3 is inhibited by the influenza A virus NS1 protein. J. Virol. 747989-7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tatsuo, H., N. Ono, K. Tanaka, and Y. Yanagi. 2000. SLAM (CDw150) is a cellular receptor for measles virus. Nature 406893-897. [DOI] [PubMed] [Google Scholar]
- 74.Tober, C., M. Seufert, H. Schneider, M. A. Billeter, I. C. Johnston, S. Niewiesk, V. ter Meulen, and S. Schneider-Schaulies. 1998. Expression of measles virus V protein is associated with pathogenicity and control of viral RNA synthesis. J. Virol. 728124-8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Valsamakis, A., H. Schneider, P. G. Auwaerter, H. Kaneshima, M. A. Billeter, and D. E. Griffin. 1998. Recombinant measles viruses with mutations in the C, V, or F gene have altered growth phenotypes in vivo. J. Virol. 727754-7761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wansley, E. K., P. J. Dillon, M. D. Gainey, J. Tam, S. D. Cramer, and G. D. Parks. 2005. Growth sensitivity of a recombinant simian virus 5 P/V mutant to type I interferon differs between tumor cell lines and normal primary cells. Virology 335131-144. [DOI] [PubMed] [Google Scholar]
- 77.Witko, S. E., C. Kotash, M. S. Sidhu, S. A. Udem, and C. L. Parks. 2006. Inhibition of measles virus minireplicon-encoded reporter gene expression by V protein. Virology 348107-119. [DOI] [PubMed] [Google Scholar]