Abstract
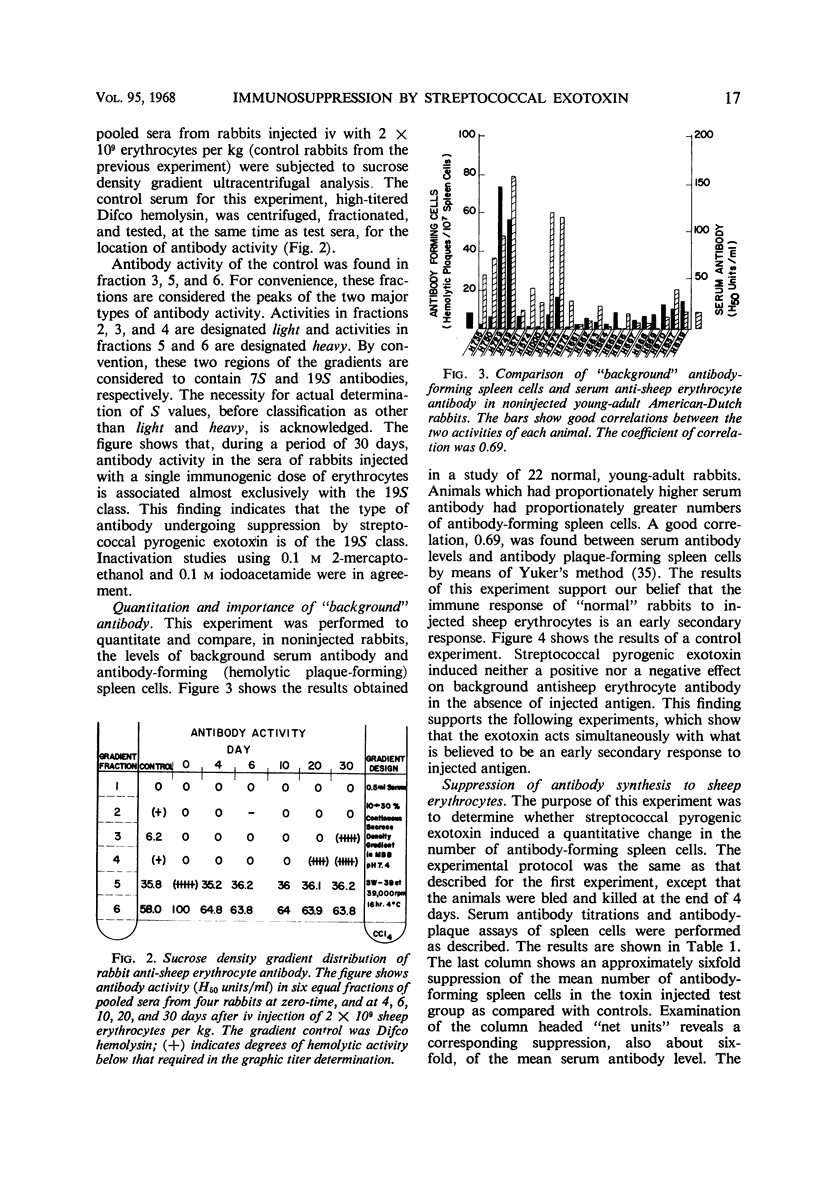
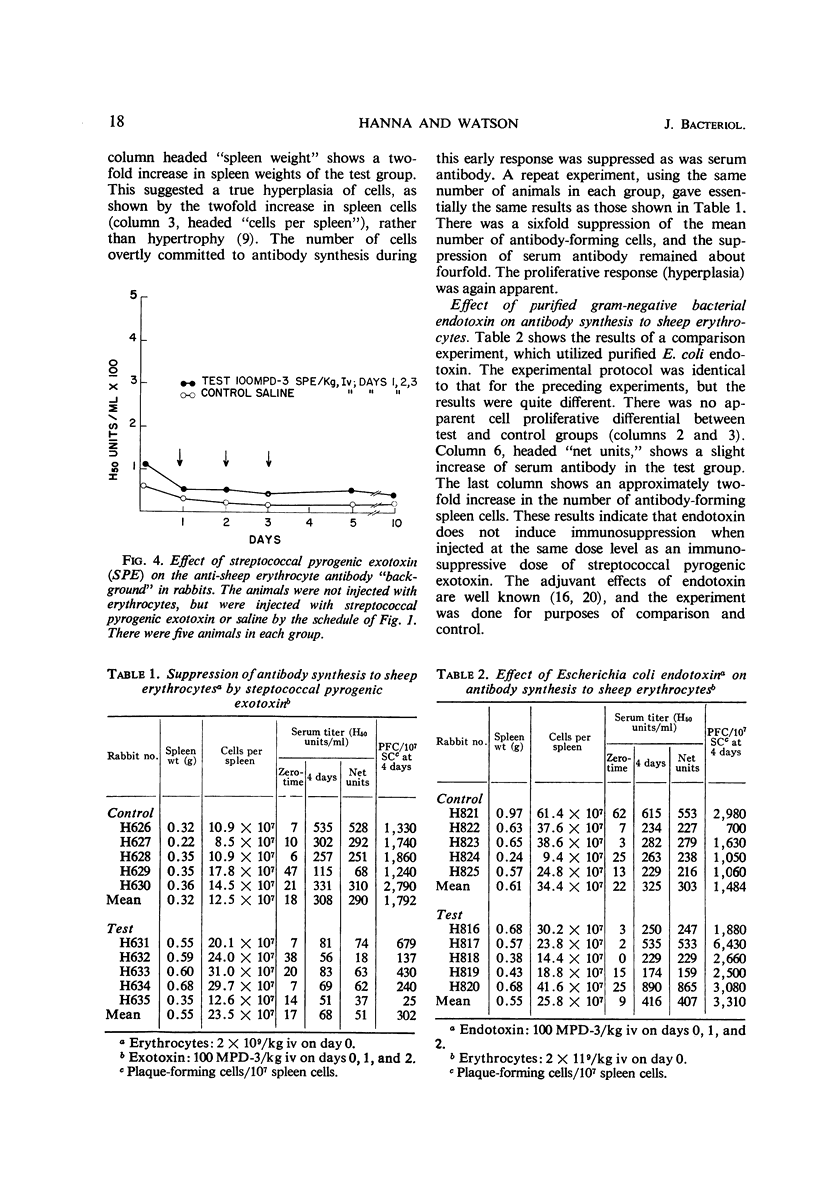
In rabbits, purified streptococcal pyrogenic exotoxin, at 0.002 of the ld50 dose, suppressed the antibody response to injected sheep erythrocytes. The antibody suppressed was determined by density gradient ultracentrifugal analysis to be of the 19S class. Background serum antibody (50% hemolytic units), as determined photometrically, correlated well with background antibody-forming spleen cells, as determined by the hemolytic-plaque technique. The exotoxin induced neither positive nor negative changes in background antibody levels, but suppressed the early secondary response to injected antigen. A comparison and control experiment showed that purified gram-negative bacterial endotoxin at identical protocol did not induce antibody suppression, but did induce the well-known adjuvant effect. Because streptococcal pyrogenic exotoxin is known to inhibit the phagocytic function of the reticuloendothelial system (RES), these data strongly support the concept that antigen is processed by cells of the RES before it evokes a secondary immune response. The results also demonstrated that streptococcal pyrogenic exotoxin may play a unique role in lowering the acquired defense of the host against infection. If the anamnestic immune response of the host is temporarily suppressed, then the host-parasite balance would be upset in favor of the parasite.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AITKEN I. D. DEVELOPMENT OF NATURAL ANTI-FORSSMAN HEMOLYSIN IN YOUNG RABBITS. J Infect Dis. 1964 Apr;114:174–178. doi: 10.1093/infdis/114.2.174. [DOI] [PubMed] [Google Scholar]
- BRADLEY S. G., WATSON D. W. SUPPRESSION BY ENDOTOXIN OF THE IMMUNE RESPONSE TO ACTINOPHAGE IN THE MOUSE. Proc Soc Exp Biol Med. 1964 Nov;117:570–572. doi: 10.3181/00379727-117-29640. [DOI] [PubMed] [Google Scholar]
- CARLSON A. S., KELLNER A., BERNHEIMER A. W., FREEMAN E. B. A streptococcal enzyme that acts specifically upon diphosphopyridine nucleotide: characterization of the enzyme and its separation from streptolysin O. J Exp Med. 1957 Jul 1;106(1):15–26. doi: 10.1084/jem.106.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CREMER N., WATSON D. W. Host-parasite factors in group A streptococcal infections. A comparative study of streptococcal pyrogenic toxins and gram-negative bacterial endotoxin. J Exp Med. 1960 Dec 1;112:1037–1053. doi: 10.1084/jem.112.6.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRAPER L. R., SUSSDORF D. H. The serum hemolysin response in intact and splenectomized rabbits following immunization by various routes. J Infect Dis. 1957 Mar-Apr;100(2):147–161. doi: 10.1093/infdis/100.2.147. [DOI] [PubMed] [Google Scholar]
- Dresser D. W., Wortis D. H. Use of an antiglobulin serum to detect cells producing antibody with low haemolytic efficiency. Nature. 1965 Nov 27;208(5013):859–861. doi: 10.1038/208859a0. [DOI] [PubMed] [Google Scholar]
- FISHMAN M. Antibody formation in vitro. J Exp Med. 1961 Dec 1;114:837–856. doi: 10.1084/jem.114.6.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss R. J. Hypertrophy versus hyperplasia. Science. 1966 Sep 30;153(3744):1615–1620. doi: 10.1126/science.153.3744.1615. [DOI] [PubMed] [Google Scholar]
- HANNA E. E., WATSON D. W. HOST-PARASITE RELATIONSHIPS AMONG GROUP A STREPTOCOCCI. 3. DEPRESSION OF RETICULOENDOTHELIAL FUNCTION BY STREPTOCOCCAL PYROGENIC EXOTOXINS. J Bacteriol. 1965 Jan;89:154–158. doi: 10.1128/jb.89.1.154-158.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HYDE R. M., WATSON D. W. Antigenic relationship of streptococcal diphosphopyridine nucleotidases. Proc Soc Exp Biol Med. 1963 Apr;112:825–827. doi: 10.3181/00379727-112-28179. [DOI] [PubMed] [Google Scholar]
- JOHNSON A. G., GAINES S., LANDY M. Studies on the O antigen of Salmonella typhosa. V. Enhancement of antibody response to protein antigens by the purified lipopolysaccharide. J Exp Med. 1956 Feb 1;103(2):225–246. doi: 10.1084/jem.103.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIM Y. B., BRADLEY S. G., WATSON D. W. CHARACTERIZATION OF EARLY 19 S AND LATE 7 S IMMUNOGLOBULINS IN THE MOUSE. J Immunol. 1964 Nov;93:798–806. [PubMed] [Google Scholar]
- Kim Y. B., Bradley S. G., Watson D. W. Ontogeny of the immune response. IV. The role of antigen elimination in the true primary immune response in germfree, colostrum-deprived piglets. J Immunol. 1967 Aug;99(2):320–326. [PubMed] [Google Scholar]
- MERRITT K., JOHNSON A. G. STUDIES ON THE ADJUVANT ACTION OF BACTERIAL ENDOTOXINS ON ANTIBODY FORMATION. V. THE INFLUENCE OF ENDOTOXIN AND 5-FLUORO-2-DEOXYURIDINE ON THE PRIMARY ANTIBODY RESPONSE OF THE BALB MOUSE TO A PURIFIED PROTEIN ANTIGEN. J Immunol. 1963 Aug;91:266–272. [PubMed] [Google Scholar]
- Makinodan T., Albright J. F., Perkins E. H., Nettesheim P. Suppression of immunologic responses. Med Clin North Am. 1965 Nov;49(6):1569–1596. doi: 10.1016/s0025-7125(16)33247-3. [DOI] [PubMed] [Google Scholar]
- ROWLEY D. A. The effect of splenectomy on the formation of circulating antibody in the adult male albino rat. J Immunol. 1950 Apr;64(4):289–295. [PubMed] [Google Scholar]
- STERN K. INHIBITION IN VIVO OF RETICULO-ENDOTHELIAL PHAGOCYTOSIS BY SYNTHETIC POLYAMINO ACIDS. Proc Soc Exp Biol Med. 1963 Nov;114:321–325. doi: 10.3181/00379727-114-28664. [DOI] [PubMed] [Google Scholar]
- Sterzl J., Ríha I. Detection of cells producing 7S antibodies by the plaque technique. Nature. 1965 Nov 27;208(5013):858–859. doi: 10.1038/208858a0. [DOI] [PubMed] [Google Scholar]
- TALIAFERRO W. H., TALIAFERRO L. G. ENHANCEMENT OF NATURAL HEMOLYSIN IN ADULT RABBITS AFTER RADIATION. Proc Natl Acad Sci U S A. 1965 Jan;53:139–146. doi: 10.1073/pnas.53.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TERRES G., WOLINS W. Enhanced immunological sensitization of mice by the simultaneous injection of antigen and specific antiserum. I. Effect of varying the amount of antigen used relative to the antiserum. J Immunol. 1961 Apr;86:361–368. [PubMed] [Google Scholar]
- WANNAMAKER L. W. The differentiation of three distinct desoxyrlbonucleases of group A Streptococci. J Exp Med. 1958 Jun 1;107(6):797–812. doi: 10.1084/jem.107.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATSON D. W. Host-parasite factors in group A streptococcal infections. Pyrogenic and other effects of immunologic distinct exotoxins related to scarlet fever toxins. J Exp Med. 1960 Feb 1;111:255–284. doi: 10.1084/jem.111.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATSON D. W., KIM Y. B. MODIFICATION OF HOST RESPONSES TO BACTERIAL ENDOTOXINS. I. SPECIFICITY OF PYROGENIC TOLERANCE AND THE ROLE OF HYPERSENSITIVITY IN PYROGENICITY, LETHALITY, AND SKIN REACTIVITY. J Exp Med. 1963 Sep 1;118:425–446. doi: 10.1084/jem.118.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOLES W. R., DILUZIO N. R. RETICULOENDOTHELIAL FUNCTION AND THE IMMUNE RESPONSE. Science. 1963 Nov 22;142(3595):1078–1080. doi: 10.1126/science.142.3595.1078. [DOI] [PubMed] [Google Scholar]


