Abstract
Connexin (Cx) proteins are known to play a role in cell-to-cell communication via intercellular gap junction channels or transiently open hemichannels. Previous studies have identified several connexin isoforms in the juxtaglomerular apparatus (JGA), but the vascular connexin isoform Cx45 has not yet been studied in this region. The present work aimed to identify in detail the localization of Cx45 in the JGA and to suggest a functional role for Cx45 in the kidney using conditions where Cx45 expression or function was altered. Using mice that express lacZ coding DNA under the control of the Cx45 promoter, we observed β-galactosidase staining in cortical vasculature and glomeruli, with specific localization to the JGA region. Renal vascular localization of Cx45 was further confirmed with the use of conditional Cx45-deficient (Cx45fl/fl:Nestin-Cre) mice, which express enhanced green fluorescence protein (EGFP) instead of Cx45 only in cells that, during development, expressed the intermediate filament nestin. EGFP fluorescence was found in the afferent and efferent arteriole smooth muscle cells, in the renin-producing juxtaglomerular cells, and in the extra- and intraglomerular mesangium. Cx45fl/fl:Nestin-Cre mice exhibited increased renin expression and activity, as well as higher systemic blood pressure. The propagation of mechanically induced calcium waves was slower in cultured vascular smooth muscle cells (VSMCs) from Cx45fl/fl:Nestin-Cre mice and in control VSMC treated with a Cx45 gap mimetic peptide that inhibits Cx45 gap junctional communication. VSMCs allowed the cell-to-cell passage of the gap junction permeable dye Lucifer yellow, and calcium wave propagation was not altered by addition of the ATP receptor blocker suramin, suggesting that Cx45 regulates calcium wave propagation via direct gap junction coupling. In conclusion, the localization of Cx45 to the JGA and functional data from Cx45fl/fl:Nestin-Cre mice suggest that Cx45 is involved in the propagation of JGA vascular signals and in the regulation of renin release and blood pressure.
Keywords: gap junction, Cre/loxP technique, lacZ
the juxtaglomerular apparatus (JGA) is an important anatomical component of the renin-angiotensin system (RAS), and it plays a major role in regulating body fluid and electrolyte homeostasis and blood pressure. The JGA consists of a tubular component (the macula densa, MD), the extraglomerular mesangium, and a vascular component that includes the terminal part of the afferent arteriole containing the renin-producing juxtaglomerular (JG) cells. Two major regulatory functions are performed by the JGA: the high distal tubular [NaCl]-induced afferent arteriolar vasoconstriction (tubuloglomerular feedback, TGF) and the low tubular [NaCl]-induced renin release. Several connexin (Cx) isoforms have already been studied in the JGA. Cx37, Cx40, and Cx43 have all been identified in the endothelium of the proximal afferent arteriole (2, 17). Only Cx43 has been found within the efferent arteriole endothelium (42). JG cells express Cx37 and Cx40, and these Cxs are also found in the intra- and extraglomerular mesangial cells (42). Cx43 is also expressed within the glomerulus (35). These three isoforms, along with Cx45, are considered to be the predominant vascular connexins (39), yet Cx45 has not been studied within the renal vasculature and JGA.
Cx45 is expressed in several organ systems during embryogenesis and is essential for the proper development of the cardiac and vascular system (19, 20). In the adult animal, expression is reduced (1, 7) but is known to continue both in conductive cardiomyocytes (14) and distinct neuronal subpopulations in the adult brain (33). Although Cx45 has been previously found in the kidney, these studies focused on its expression in the developing kidney and in kidney-derived cell lines (5, 32). In the mature kidney, however, exact and detailed cellular localization of Cx45 was hampered by the lack of specific detection methods. Recently, transgenic mouse techniques have been developed that provide not only a localization tool but also tissue- or cell-specific deletion of connexins (19, 23). To aid in localization of Cx45 in all organ systems, a transgenic mouse, in which one copy of the Cx45 coding DNA is replaced by the lacZ reporter coding DNA (Cx45+/−), was developed and studied (19). Additionally, since general deletion of Cx45 proves to be lethal during embryogenesis (19, 20), the Cre/loxP technique was recently used to generate a mouse with a Cx45 gene deletion that is restricted to cells expressing the protein nestin during development (23). This method has previously been used to achieve conditional expression of a variety of genes in the kidney (4, 15). By creating a mouse with the Cre recombinase gene under the control of kidney-specific promoters, gene deletions have been produced in renal structures, including the vasculature and glomeruli, both of which express nestin during development (8).
The present work details the localization of Cx45 in the JGA of adult mouse kidney using genetic techniques. We then identified its relevance in renal (patho)physiology. Our data show that Cx45 is expressed in the vascular component of JGA and is involved in the propagation of JGA vascular signals and in the regulation of renin release and blood pressure.
MATERIALS AND METHODS
Animals.
C57BL/6 mice were bred in house and were fed standard diets (Harlan Teklad, Madison, WI) and provided drinking water ad libitum or water supplemented with 500 mg/l captopril for 1 wk. Cx45+/+ and Cx45+/− mice, where the coding region of the Cx45 gene was replaced with the β-galactosidase (lacZ) reporter gene, were previously generated and described (17). Cx45fl/fl:Nestin-Cre mice were kindly provided by Dr. Marla Feller at the University of California in San Diego and have previously been described (23). The Cx45fl/fl:Nestin-Cre mice were developed from two mouse strains: Cx45fl/fl mice, which were generated in C57BL/6 mice and backcrossed to C57BL/6 mice for at least three generations, and Nestin-Cre mice, which were generated in B6SJLF2 mice and backcrossed to C57BL/6 mice for at least six generations (Jackson Laboratory, Bar Harbor, ME). By breeding these two mouse lines, the background similarity to C57BL/6 was more than 90% percent (23). In these mice, the Cx45 coding DNA has been replaced by the enhanced green fluorescence protein (EGFP) reporter gene in cells that expressed nestin during development by way of the Cre/loxP site specific recombination system. Because of this recombination, EGFP gene transcription comes under the control of the Cx45 promoter. Therefore, EGFP staining will only occur in cells that expressed nestin during development and that normally express Cx45. All animal protocols have been approved by the Institutional Animal Care and Use Committee at the University of Southern California.
β-Gal staining.
Kidneys from Cx45+/+ and Cx45+/− mice were frozen on dry ice, embedded in Tissue-Tec (Sakura, Zoeterwoude, Netherlands), sectioned (10–20 μm) on a cryostat (MICROM HM 500 OM), and transferred onto Superfrost plus slides (Menzel, Braunschweig, Germany). Sections were fixed with 0.2% glutaraldehyde in 0.1 M PBS, rinsed three times in lacZ washing buffer (0.1 M phosphate buffer, pH 7.4, 1.25 mM MgCl2, 5 mM EGTA, 0.2% Nonidet P-40, and 0.01% sodium deoxycholate), and stained in lacZ substrate buffer (lacZ washing buffer supplemented with 0.4 mg/ml X-Gal [5-brom-4-chloro-3-indoly-β-D-glactopyranoside], 5 mM potassium ferrocyanide, and 5 mM potassium ferricyanide) overnight at 37°C. Sections were then washed in PBS, stained in 0.1% eosin for 5 min, rinsed in water, and mounted in Entellan (Merck, Darmstadt, Germany).
Antibodies.
The rabbit polyclonal renin antibodies for immunofluorescence studies were provided by Dr. Joël Menard (Institut National de la Sante et de la Recherche Medicale, Paris, France), whereas rabbit polyclonal renin antibodies for immunoblots were a kind gift from Dr. Tadashi Inagami (Vanderbilt University, Nashville, TN). Both renin antibodies were characterized in previous publications (6, 25). Rabbit polyclonal anti-Cx45 was kindly provided by Dr. Ulrike Janssen-Bienhold, University of Oldenburg, Germany and was previously characterized (10).
Immunofluorescence labeling of Cx45fl/fl:Nestin-Cre mouse kidney tissue.
Kidneys were fixed in situ by perfusion with periodate-lysine-paraformaldehyde (PLP). Coronal kidney sections were incubated overnight in PLP at 4°C before overnight cryoprotection in 2.3 M sucrose. Tissue was embedded in optimal cutting temperature embedding medium (Sakura) over dry ice. Thin sections were cut on a Leica CM cryostat (Leica Microsystems, Bannockburn, IL). Sections were fixed with 4% paraformaldehyde for 10 min, permeabilized for 10 min with 0.1% Triton X-100 in PBS, and subsequently incubated in a solution of 5% normal goat serum in PBS for 30 min to block nonspecific binding. Additionally, some sections were also probed with antibodies against renin, at a dilution of 1:100 for 1 h, followed by incubation with secondary Alex Fluor 594 goat-anti rabbit antibodies (Invitrogen, Carlsbad, CA) at a 1:500 dilution for 1 h. Following a final wash step, all sections were mounted with Vectashield mounting media containing the nuclear stain DAPI (Vector Laboratories, Burlingame, CA) and examined with a Leica TCS SP2 confocal microscope.
Measuring plasma renin activity.
Renin activity of mouse plasma was measured with a fluorescence resonance energy transfer (FRET)-based 5-FAM-conjugated renin substrate (Anaspec, San Jose, CA) and a cuvette-based spectrofluorometer (Quantamaster-8; PTI, Birmingham, NJ). In the native state of the FRET peptide, the fluorescence of 5-FAM is quenched by QXL-520. Upon cleavage of the substrate into two fragments by renin, 5-FAM will fluoresce. A similar method utilizing a FRET-based 5-(2-aminoethyl)aminonaphthalene-1-sulfonic acid-conjugated renin substrate has been described before (16, 35). Briefly, 0.33 μM of the renin substrate in Krebs-Ringer (pH 7.4) was loaded into the cuvette and heated to 37°C. After taking a baseline reading, we mixed 30 μl of mouse plasma with the renin substrate in the 37°C chamber, and the emitted fluorescence signal as an index of angiotensin I (ANG I) generation was measured at 520 nm in response to excitation at 490 nm for a period of 800 s. The initial rate of the increase in 5-FAM fluorescence was then analyzed as a measure of renin activity using the FeliX32 software (PTI).
Blood pressure measurement.
C57BL/6 mice and Cx45fl/fl:Nestin-Cre mice were anesthetized with a combination of Inactin (100 mg/kg body wt) and ketamine (100 mg/kg body wt) intraperitoneally. To measure systemic blood pressure, a cannula was inserted into the left carotid artery, and, with the use of an analog single-channel transducer signal conditioner and transducer, data were collected using data acquisition system QUAD-161 (World Precision Instruments, Sarasota, FL). Statistical significance was tested using an unpaired t-test, and data are shown as means + SE.
Isolation and culture of vascular smooth muscle cells from mouse kidneys.
Kidneys were collected from C57BL/6 and Cx45fl/fl:Nestin-Cre mice anesthetized with 100 mg/kg body wt Inactin. The terminal afferent arteriole was manually dissected on ice under a microscope from sagittal slices of kidney in DMEM culture medium containing 3% FBS (Invitrogen). The afferent arteriole was cut into short segments and transferred to tissue culture dishes containing circular glass coverslips. Explants gave rise to vascular smooth muscle cells (VSMC) ∼2–3 days after attachment. Isolated VSMC were then grown to 90% confluence on the glass coverslips in the following media: DMEM with 25 mM d-glucose with the addition of 3.7 g NaHCO3, 20% FBS, and 1% penicillin-streptomycin. Cells were bathed in a modified Krebs-Ringer HCO3 buffer during dye incubation and subsequent experiments. This buffer was composed of: 115 mM NaCl, 5 mM KCl, 25 mM NaHCO3, 960 μM NaH2PO4, 240 μM Na2HPO4, 1.2 mM MgSO4, 2 mM CaCl2, 5.5 mM d-glucose, and 100 μM l-arginine. All solutions were adjusted to pH 7.4 using HCl and NaOH.
Immunoblotting of C57BL/6 and Cx45fl/fl:Nestin-Cre mouse and rat kidneys.
Mice were anesthetized with 100 mg/kg Inactin, and kidneys were perfused with ice-cold PBS to remove blood. Tissue was then homogenized with a rotor-stator homogenizer in a buffer containing 20 mM Tris·HCl, 1 mM EGTA, pH 7.0, and a protease inhibitor cocktail (BD Biosciences, San Jose, CA). Samples were centrifuged at low speed to pellet cellular debris, and supernatant was collected and assayed. Forty micrograms of protein were run per lane, separated on a 4–20% SDS-polyacrylamide gel (Bio-Rad, Hercules, CA), and then transferred to a polyvinylidene difluoride membrane (Millipore, Billerica, MA). After we blocked the membrane in blocking buffer (Li-Cor, Lincoln, NE), blots were probed with rabbit polyclonal antibodies to Cx45 at a dilution of 1:1,000 overnight. Reactivity of the primary antibodies was detected with IR680-labeled goat anti-rabbit antibodies (1:15,000 dilutions, Li-Cor). Blots were imaged with the Odyssey Infrared Imaging System (Li-Cor) and accompanying software. The blot was reprobed with a mouse monoclonal antibody to GAPDH (Ambion, Austin, TX) at a dilution of 1:4,000 for 1 h (Santa Cruz Biotechnology, Santa Cruz, CA) to test for protein loading and quality of transfer. Labeling was detected and imaged with an IR800-labeled goat anti-mouse antibody as described above (Li-Cor system).
Immunoblotting of VSMCs.
Afferent arteriole VSMCs isolated from C57BL/6 and Cx45fl/fl:Nestin-Cre mice were grown to confluence in plates as described above. Cells were removed from the plates by scraping and lysed with CellLytic-M lysis buffer (Sigma-Aldrich, St. Louis, MO) according to manufacturer's instructions. Protein concentration was assayed by a modified Bradford method (Quick Start Bradford protein assay, Bio-Rad). Seven-microgram samples were blotted and analyzed for Cx45 and GAPDH as described above.
RT-PCR of VSMCs.
Total RNA was purified from confluent afferent arteriolar VSMCs derived from C57BL/6 and Cx45fl/fl:Nestin-Cre mouse kidneys using a total RNA Mini kit in accordance with manufacturer's instructions (Bio-Rad). RNA was then quantified using spectrophotometry and reverse transcribed to single-strand cDNA with the use of avian reverse transcriptase and random hexamers according to manufacturer's instructions (Thermoscript RT-PCR systems, Invitrogen). Two microliters of cDNA were amplified with a master mix containing Taq polymerase (Invitrogen) and the following primers: Cx37 forward: 5′-GTCACAGATGGTTCTGGAAT-3′; Cx37 reverse: 5′-ACAAGACATCAACCAGTTCC-3′; Cx40 forward: 5′-ATCTCCCACATTCGTTATTG-3′; Cx40 reverse: 5′-AGGAAGATCCCATAGAGGAG-3′; Cx43 forward: 5′-TTCATTTTCAGAATCCTGCT-3′; Cx43 reverse: 5′-GGATGCTGATGATGTAGGTT-3′; β-actin sense: 5′-GGTGTGATGGTGGGAATGGGTC-3′; β-actin anti-sense: 5′-ATGGCGTGAGGGAGAGCATAGC-3′. All primer sequences were based on previous publications (36, 24).
Measurement of calcium wave propagation in VSMCs.
Coverslips containing the VSMC monolayer were mounted to a chamber of the Leica TCS SP2 confocal microscope system and imaged in the absence of any dyes to establish any EGFP fluorescence (excitation at 488 nm, emission at 520 ± 20 nm). VSMCs were then loaded for 20 min with the ratiometric calcium dyes fluo-4 AM (excitation at 488 nm, emission at 520 ± 20 nm) and fura red AM (excitation at 488 nm, emission at >600 nm) (Invitrogen) at a final concentration of 1 μM and 5 μM, respectively. A transmitted light detector and differential interference contrast imaging were used to visualize the position of the pipette before and during mechanical stimulation. All experiments were performed using the same instrument settings, and data acquisition and analysis were done using the Leica LCS imaging software (LCS 2.61.1537). Calcium wave velocity was calculated using the formula: velocity = distance/time, where distance was defined as the length between the point of mechanical stimulation and the center of a cell with increased intracellular calcium concentration ([Ca2+]i). For each experimental group, n = 6. Statistical significance was calculated by a one-way ANOVA analysis followed by Dunnett's post hoc comparison with data shown as means + SE.
Mechanical stimulation of VSMCs.
A single VSMC of the monolayer was stimulated with a glass micropipette (Drummond Scientific, Broomall, PA) pulled to 2–3 μm diameter using a micropipette puller (PP-830; Narishige, Tokyo, Japan). A micromanipulator (ROE-200; Sutter Instruments, Novato, CA) was used to position and lower the micropipette to contact the monolayer.
Pharmacological treatment of VSMCs.
In cell calcium wave experiments, the gap junction uncoupling agent 18α-glycyrrhetinic acid (18α-GA, 25 μM) (Sigma-Aldrich) was used as a nonspecific gap junction inhibitor. To specifically block Cx45 in the same experiments, a Cx45 gap mimetic peptide of sequence QVHPFYVCSRLPCPHK (amino acids 202–217) was synthesized (USC/Norris Cancer Center DNA Core Facility, Los Angeles, CA) on the basis of the work of Li and Simard (21). Cell monolayers were incubated with the gap mimetic peptide at a concentration of 500 μM for 3 h at 37°C, as previously described. The nonselective purinergic receptor antagonist suramin was applied to cell monolayers at a concentration of 50 μM for 10 min at 37°C.
Dye-spreading assay.
Coverslips containing a confluent VSMC monolayer were mounted to a chamber of the Leica confocal microscope system and bathed with 1 ml of modified Krebs-Ringer HCO3 buffer. Hoechst 33342 (10 μM, Invitrogen) was added to the bath before the experiment to identify nuclei. A single cell within the VSMC monolayer was then injected with a micropipette loaded with Lucifer yellow (700 μM, Invitrogen), and the dye was allowed to diffuse to adjacent cells for 5 min. Images were recorded every 15 s. Both Hoechst 33342 (emission between 400 and 450 nm) and Lucifer yellow (emission >550 nm) were excited using two-photon excitation at 800 nm by a MaiTai laser (Spectra-Physics, Mountain View, CA).
RESULTS
LacZ localization in the renal cortex of Cx45+/− mice.
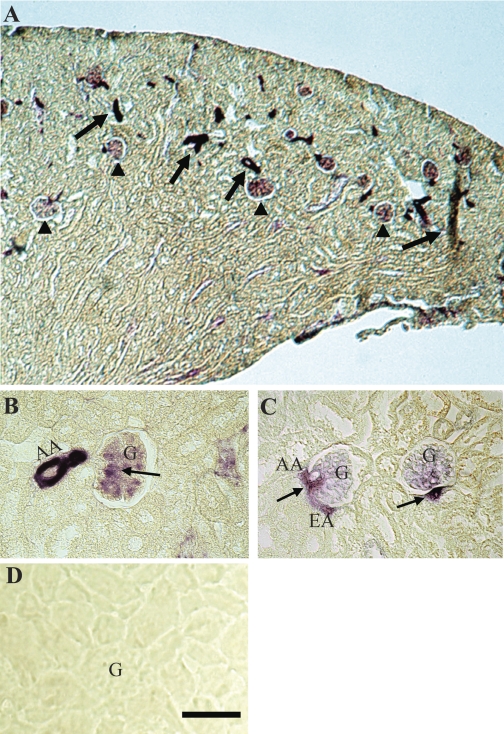
Kidneys from Cx45+/− mice, where one copy of the Cx45 gene is replaced by the lacZ reporter gene, were sectioned and stained with X-Gal to determine Cx45 renal expression. Expression of a transgenic reporter gene was used for localization instead of immunohistochemistry since specific antibodies were not available. LacZ staining was found in the renal cortex, specifically in blood vessels and glomeruli (Fig. 1A). Although these results confirm previously published data on the gross renal localization of Cx45 (19), Cx45 expression within these structures was not investigated in earlier studies. Using higher power magnification, we were able to further elucidate the localization of Cx45 in the JGA. Both afferent and efferent arterioles were positive for lacZ, as well as the extra- and intraglomerular mesangium (Fig. 1, B and C). No lacZ labeling was detected in sections stained in parallel from Cx45+/+ mice, which served as a negative control for endogenous galactosidase activity (Fig. 1D). These results indicate that Cx45 is expressed throughout the vascular components of JGA.
Fig. 1.
Localization of Connexin (Cx)45 transcription in Cx45+/− mouse kidney sections by X-Gal staining. A: β-gal was detected in the renal cortex, predominantly in vasculature (arrow) and glomeruli (arrowhead). B and C: within the juxtaglomerular apparatus (JGA), β-gal staining was observed in the afferent (AA) and efferent arterioles (EA), as well as the extra- and intraglomerular mesangial cells (arrows). D: no β-gal staining was detected in sections from Cx45+/+ mouse kidneys; G, glomerulus. Bar = 50 μm.
EGFP localization in the JGA of Cx45fl/fl:Nestin-Cre mice.
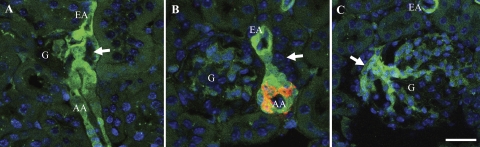
To provide a further line of evidence that Cx45 is expressed in the JGA, the expression of EGFP in kidney sections of Cx45fl/fl:Nestin-Cre mice was analyzed (Fig. 2). In these mice, the coding region of Cx45 was flanked by loxP sites. Additionally, these mice expressed Cre recombinase in cells expressing the intermediate filament protein nestin during development. In the nestin-positive cells, Cre recombinase excised the floxed Cx45 coding region, leaving the EGFP reporter gene under the control of the Cx45 promoter. Therefore EGFP expression indicates Cx45 expression only in cells that express nestin during development and express Cx45 in the adult.
Fig. 2.
Detection and localization of enhanced green fluorescence protein (EGFP) in Cx45fl/fl:Nestin-Cre mouse kidney sections. EGFP expression is controlled by the Cx45 promoter only in cells that expressed nestin during development. A: EGFP (green) was detected in the AA and EA and in the extraglomerular mesangial cells. G, glomerulus; arrow, macula densa. B: sections were labeled for renin (red) in a consecutive serial section. EGFP was colocalized to the renin-positive region of the AA. C: EGFP was also found in the extraglomerular mesangium (arrow). Bar = 20 μm.
At the JGA, strong EGFP signals were observed in VSMCs of both the afferent and efferent arterioles (Fig. 2, A–B) and presumably in extraglomerular and intraglomerular mesangial cells (Fig. 2C). To verify JGA localization, a consecutive section was colabeled with renin antibodies (Fig. 2B). The renin labeling was consistent with the anticipated JGA location in the afferent arteriole. As predicted, the EGFP signal overlapped with the renin labeling confirming the expression of Cx45 in renin-producing JG cells. EGFP was not detected in any endothelial cells.
Effects of Cx45 on renin expression and blood pressure.
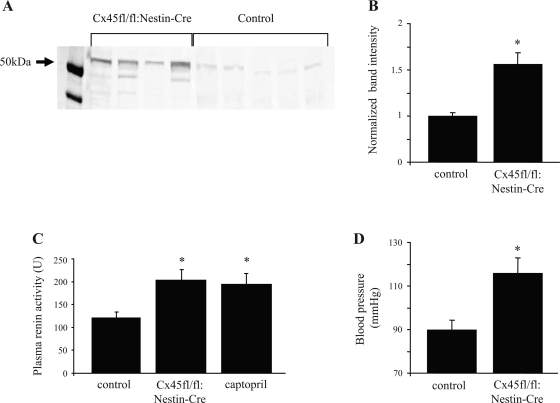
Since Cx45 was expressed in the JGA, it may play a role in renin expression and blood pressure regulation. Samples of whole kidney homogenate from Cx45fl/fl:Nestin-Cre mice (n = 4) and C57BL/6 mice (control) (n = 5) were run on SDS-PAGE gels, transferred, and blotted for renin (Fig. 3A). Densitometry analysis (Fig. 3B) revealed a significant upregulation (∼50%) of renin expression in Cx45fl/fl:Nestin-Cre mice compared with C57BL/6 mice (P < 0.05).
Fig. 3.
Renal renin expression, plasma renin activity, and systemic blood pressure in C57BL/6 and Cx45fl/fl:Nestin-Cre mice. A: C57BL/6 (control, n = 5) and Cx45fl/fl:Nestin-Cre (n = 4) kidney homogenate samples were blotted with renin antibodies. B: densitometric analysis of immunoblot indicated that the expression of renin in Cx45fl/fl:Nestin-Cre mice was significantly higher than that of C57BL/6 mice (*P < 0.05). C: plasma renin activity was measured in real-time using a fluorescence resonance energy transfer-based fluorogenic renin substrate. Renin activity was significantly higher in Cx45fl/fl:Nestin-Cre mice compared with C57BL/6 mice, and this increase was comparable to the increase observed in captopril-treated mice (*P < 0.05, n = 5 per group). D: mean arterial blood pressure was significantly increased by ∼30% in Cx45fl/fl:Nestin-Cre mice (*P < 0.05, n = 3 per group). Values are means + SE.
With the use of spectrofluorometry, plasma renin activity was analyzed in C57BL/6 and Cx45fl/fl:Nestin-Cre mice (Fig. 3C). Plasma samples were mixed with a fluorogenic renin substrate, and the emitted FRET signal (representative of the generation of ANG I) was detected. Renin activity (ANG I generation) was plotted as a function of time, and the initial activity was quantified. Plasma renin activity was significantly higher by ∼70% in Cx45fl/fl:Nestin-Cre mice compared with control mice (control: 121 ± 10; Cx45fl/fl:Nestin-Cre: 204 ± 24, n = 5, P < 0.05). This increase in plasma renin activity was comparable to the increase observed in captopril-treated mice (194 ± 14, P < 0.05 vs. control, P > 0.05 vs. Cx45fl/fl:Nestin-Cre).
The blood pressure of control and Cx45fl/fl:Nestin-Cre mice was measured by pressure transducer catheterization. C57BL/6 mice had an average mean arterial blood pressure (MAP) of 90 ± 2 mmHg (n = 3), whereas Cx45fl/fl:Nestin-Cre had an average MAP of 116 ± 5 mmHg (n = 3) (Fig. 3D). The 28% increase in MAP in Cx45fl/fl:Nestin-Cre mice was found to be significant (P < 0.05)
Characterization of VSMCs from C57BL/6 and Cx45fl/fl:Nestin-Cre mice.
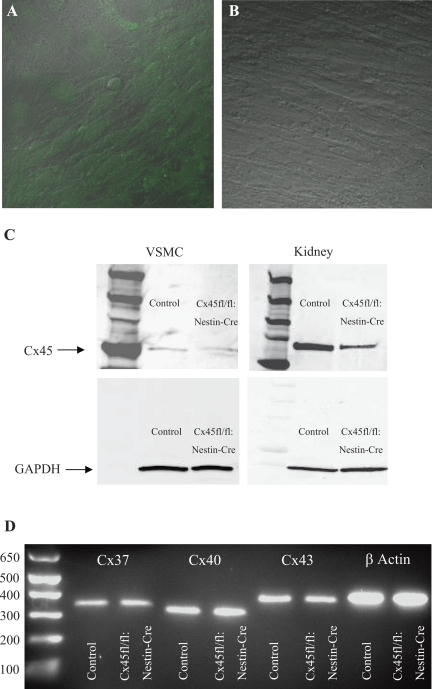
VSMCs derived from Cx45fl/fl:Nestin-Cre mice were positive for EGFP (Fig. 4A), indicating that the Cx45 coding region was excised in these cells. Under the same microscope and laser settings, no EGFP signal was observed in VSMCs cultured from C57BL/6 (control VSMC) mice (Fig. 4B).
Fig. 4.
Characterization of vascular smooth muscle cell (VSMC) primary cultures from C57BL/6 and Cx45fl/fl:Nestin-Cre mice. A: EGFP expression (green) was detected in VSMC derived from Cx45fl/fl:Nestin-Cre mice. B: under the same microscope and laser settings, VSMCs derived from C57BL/6 mice (control VSMC) were negative for the EGFP signal. Differential interference-contrast background was added and merged with fluorescence in A and B; ×40 magnification. C: immunoblots with protein samples from C57BL/6 VSMCs and kidney (control) and Cx45fl/fl:Nestin-Cre VSMCs and kidney probed for Cx45 and the loading control GAPDH. Cx45 expression was detected in all 4 samples, but expression was reduced in Cx45fl/fl:Nestin-Cre VSMCs and kidney extracts. D: Cx37, 40, and 43 mRNA was detected in C57BL/6 (control) and Cx45fl/fl:Nestin-Cre VSMCs by RT-PCR. mRNA from all 3 Cxs at their expected bands sizes (344, 323, and 394 bp, respectively) was observed. β-actin (400 bp) served as a positive control.
To quantify the excision of the Cx45 coding region from Cx45fl/fl:Nestin-Cre VSMCs, protein extracts from C57BL/6 (control) and Cx45fl/fl:Nestin-Cre VSMCs were run on SDS-PAGE gels, transferred to membranes, and probed with Cx45 antibodies (Fig. 4C). Whole kidney homogenate from C57BL/6 and Cx45fl/fl:Nestin-Cre mice were also analyzed by immunoblot to provide a means to compare Cx45 expression in a cell culture with total renal Cx45 expression. A single band ∼50 kDa in size appeared on all blots probed with Cx45 antibodies. Cx45 expression was ∼50% lower in both VSMCs and kidneys from the Cx45fl/fl:Nestin-Cre mouse compared with control VSMCs and kidneys on the basis of densitometry (not shown), indicating effective Cx45 excision in a significant cell population of Cx45fl/fl:Nestin-Cre VSMCs and kidneys. Both blots were also probed with GAPDH as a confirmation of equal protein loading (Fig. 4C).
Since VSMCs are subject to phenotypic changes in culture, we sought to identify any differences in Cx expression that may have arisen during the culture process. Total RNA was purified from confluent C57BL/6 (control) and Cx45fl/fl:Nestin-Cre VSMC and amplified by RT-PCR with primers for Cxs 37, 40, and 43. Bands at the predicted band size (344, 323, and 391 bp) were detected for both control and Cx45fl/fl:Nestin-Cre VSMC samples (Fig. 4D). Amplification with a β-actin primer pair, which served as a positive control for the experiment, also produced bands of the expected size (400 bp) for both cell culture types.
Effects of Cx45 on calcium wave propagation in VSMCs.
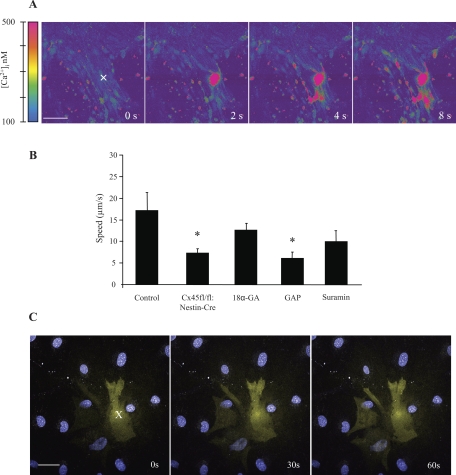
When control and Cx45fl/fl:Nestin-Cre VSMCs were loaded with fluo-4/fura red and one VSMC in the center of the microscope field was mechanically stimulated with a glass micropipette, an increase in [Ca2+]i in the stimulated cell was observed (Fig. 5A). This increase in [Ca2+]i propagated to adjacent cells (Fig. 5A).
Fig. 5.
Calcium wave propagation in VSMCs from C57BL/6 and Cx45fl/fl:Nestin-Cre mice. A: representative pseudocolor fluo-4/fura red ratio images from control VSMC shown at different time points (0–8 s) after the stimulation of a single cell (indicated by X); [Ca2+]i, intracellular calcium concentration; bar = 20 μm. B: speed of calcium wave propagation in control VSMC, Cx45fl/fl:Nestin-Cre VSMC, and control VSMC treated with 18α- glycyrrhetinic acid (18α-GA), Cx45 gap mimetic peptide (GAP), or suramin. Calcium wave propagation was significantly slower in Cx45fl/fl:Nestin-Cre and GAP-treated VSMC (n = 6, *P < 0.05). C: gap junction permeable dye Lucifer yellow was loaded by microinjection into a single VSMC (indicated by X) and spread to adjacent cells within 60 s. Nuclei were labeled blue with Hoechst 33342; bar = 10 μm.
The propagation speed (μm/s) of the calcium wave was analyzed in control, Cx45fl/fl:Nestin-Cre, and pharmacologically treated control VSMCs (n = 6 for all experimental groups) (Fig. 5B). In control VSMCs, the propagation speed was 17 ± 5 μm/s. The speed of propagation in Cx45fl/fl:Nestin-Cre VSMCs was significantly lower (7 ± 1 μm/s, P < 0.05). The nonspecific gap junction inhibitor 18α-GA failed to significantly lower the speed of calcium wave propagation in control VSMCs (12.4 ± 2.0 μm/s, P > 0.05); however, control VSMCs treated with a Cx45-specific gap mimetic peptide (GAP) did exhibit a significant reduction in calcium wave propagation speed (5.7 ± 1.6 μm/s, P < 0.05).
Since these experiments pointed to a role for Cx45 in calcium wave propagation, we sought to establish whether Cx45 regulates calcium wave propagation indirectly by an extracellular agent such as ATP or directly via intercellular coupling. Generally, calcium wave speeds above 100 μm/s are thought to be due to direct coupling between cells (28, 31), whereas slower calcium wave speeds (as we observed) are associated with the release of ATP via Cx hemichannels (9, 36). To test whether ATP played a role in the calcium wave propagation, control VSMCs were treated with the nonselective purinergic receptor antagonist suramin (Fig. 5B). This treatment did not significantly reduce calcium propagation speed compared with control (10.3 ± 2.4 μm/s, P > 0.05). To determine whether VSMCs were directly coupled, a dye-spreading assay was conducted. A single VSMC was loaded by microinjection with Lucifer yellow (Fig. 5C). Lucifer yellow, which does not cross cell membranes but does permeate gap junctions, spread to adjacent VSMCs within 60 s of injection, indicating that cultured VSMCs were coupled by gap junctions.
DISCUSSION
Here we report that Cx45 is found in the renal cortex of the adult mouse kidney, specifically in the vasculature and glomeruli. In the JGA, Cx45 was expressed in the mesangium and the smooth muscle cells of the afferent and efferent arterioles, and in renin-producing JG cells. Both renal renin expression and plasma renin activity were markedly increased in Cx45fl/fl:Nestin-Cre mice, which are considered deficient in their JGA expression of Cx45. VSMCs cultured from afferent arterioles of Cx45fl/fl:Nestin-Cre mice had reduced calcium wave propagation speed compared with VSMCs cultured from C57BL/6 mice. This decrease in speed was replicated in control VSMCs treated with a Cx45-specific gap mimetic peptide. The nonspecific gap junction blocker 18α-GA did not significantly reduce propagation speed.
Because of the lack of specific antibodies against Cx45, two transgenic mice with reporter gene constructs were studied to determine the intrarenal localization of Cx45. Using mice expressing the lacZ gene under the control of the Cx45 promoter, Cx45 was detected in glomeruli and vascular structures of the renal cortex, which affirms previous findings in these mice (19) (Fig. 1A). In addition, we observed β-gal staining under higher magnification and identified positive structures as the afferent and efferent arterioles, glomeruli, and mesangial cells (Fig. 1, B–C). We utilized a second method to substantiate these findings. Cx45fl/fl:Nestin-Cre mice have a deletion of the Cx45 coding region in cells expressing nestin during embryogenesis. Although nestin is typically thought of as a neuronal marker, it is also expressed during development by the metanephric mesenchyme, the progenitor to all renal cell types except collecting duct epithelial cells (8). Therefore Cx45 is functionally knocked out in JGA cells, and its expression is replaced by EGFP expression. By detecting EGFP signal in Cx45fl/fl:Nestin-Cre mice, we observed a continuous Cx45 labeling in VSMCs of the afferent and efferent arteriole and the adjacent extraglomerular mesangial cells (Fig. 2, A–C). These localization data suggest the possibility of fast and direct coupling between the afferent and efferent arterioles and the mesangium. There is indeed evidence for simultaneous propagation of the TGF calcium wave into these areas (26). The colocalization of EGFP with renin in the afferent arteriole (Fig. 2B) suggests that this Cx isoform may also play a role in renin synthesis and release.
The importance of Cx45 to the vasculature was already evident from previous studies. Cx45-deficient mice are characterized by abnormal development of the vasculature, and this genetic modification proves to be lethal (19). Functional Cx45 gap junctions have also been discovered in the VSMCs of cerebral arteries, where it has been suggested that they play a role in the regulation of blood flow in the nervous system (21, 22). However, the appearance of Cx45 in the renal vasculature does not allow us to presume a functional role for the protein.
Therefore, to ascertain the physiological relevance of Cx45 in the kidney, in vivo and in vitro experiments were performed with Cx45fl/fl:Nestin-Cre mice. These mice had increased renin expression and activity and increased MAP (Fig. 3). These changes in blood pressure and the RAS, as well as the localization, mirror those observed in another Cx transgenic mouse, the Cx40-deficient mouse (Cx40−/−) (18, 38). Cx40−/− mice have renin-dependent hypertension. Wagner et al. (38) concluded that this hypertension is due to a failure to properly engage the ANG II and intrarenal blood pressure negative feedback loops on renin. Clearly, both mouse model studies indicated that Cxs exert an effect on renal blood pressure regulation.
There are also differences between our study and those performed in the Cx40−/− model. Although Cx45fl/fl:Nestin-Cre mice had a significant elevation in blood pressure, they were not hypertensive. This limited blood pressure increase may be due to a restriction of the gene knockout to nestin-expressing cells, as opposed to the systemic knockout in the Cx40−/− mouse model. As with Cx40−/−, the elevated blood pressure we observed does appear to be RAS dependent in nature, given the increase in renin levels and activity. However, the precise feedback mechanism through which renin is dysregulated in Cx45fl/fl:Nestin-Cre mice requires further study. Blood pressure regulation and renin secretion can also be regulated by the renal sympathetic nerve (12). Therefore, we cannot exclude the possibility that the observed elevations in blood pressure and renin were due to the effects of a conditional knockout in other Cx45- and nestin-expressing cells, including those of the nervous system (33, 39, 43). However, the effects of Cx45 loss in isolated VSMC primary cultures on calcium waves do suggest that Cx45 plays a role in JGA function at the local level and is independent of sympathetic innervation.
In addition to in vivo and in vitro analysis of Cx45fl/fl:Nestin-Cre mice, we also attempted to block Cx45 function pharmacologically. Surprisingly, the nonspecific gap junction inhibitor 18α-GA did not significantly reduce VSMC calcium wave propagation in control VSMC. Recently published data in mouse embryonic stem cells suggest that 18α-GA may not inhibit Cx45 (40). In these cells (which express Cx31, 43, and 45), inhibition of gap junction intracellular communication by 18α-GA required the expression of Cx43. It has been established that Cx45 and Cx43 can interact together to form functional gap junctions (11, 40), but only Cx43 mRNA, and not protein, has been found in the smooth muscle cells of the renal vasculature (2, 42). Therefore, it seems unlikely that Cx43 and Cx45 form heteromeric channels in VSMCs, and the lack of these channels could explain the inability of 18α-GA to reduce calcium wave propagation we observed.
A previously developed Cx45 gap mimetic peptide was also used to inhibit of Cx45 (21). The Cx45 peptide was designed to be homologous to earlier identified Cx43 and Cx40 blocking peptides. By patch-clamping smooth muscle cell pairs, it was demonstrated that the Cx45 peptide altered conductance in a manner that was consistent with channel blockade (21). In applying the Cx45 peptide to control VSMC, we were able to reduce calcium wave propagation to levels observed with Cx45fl/fl:Nestin-Cre VSMC. However, our study marks the first attempt to use this mimetic peptide in a cell culture model, and the results, therefore, should be interpreted with caution.
One possible mechanism through which Cx45 could affect renin regulation and blood pressure is calcium signaling. Basolateral ATP released from the MD (3) initiates a propagating calcium wave in the JGA and beyond (26) during TGF that controls renal blood flow and glomerular filtration rate (29). The increase in [Ca2+]i accomplishes two mechanisms: inhibition of renin release from JG cells (30) and contraction of VSMCs in the afferent arterioles (41). Gap junctions are known to be instrumental in calcium wave propagation in several cell types (9, 13, 34), and it has been previously demonstrated that the calcium wave of TGF can be abolished by gap junction blockers (26).
Our observation that calcium wave propagation in VSMCs is dependent on the expression and function of Cx45 (Fig. 5B) suggests that Cx45 may play a role in the propagation of TGF calcium wave (26). Calcium propagation involving Cxs occurs by two mechanisms: either by intercellular gap junction communication or via the release of an extracellular mediator such as ATP. Calcium wave propagation speeds above 100 μm/s have previously been found in intact preglomerular smooth muscle cells (28). In the present study, however, the VSMC calcium propagation speeds measured were at least fivefold slower, suggesting that the calcium wave in cultured VSMCs does not rely solely on fast and direct gap junctional coupling but instead involves an extracellular mediator, such as ATP. A recently published paper from Toma et al. (36) examined the role of Cx40 on calcium wave propagation in a glomerular endothelial cell (GENC) culture. Calcium wave propagation speeds similar to those we presently observed in VSMCs were recorded in GENCs, and the authors concluded that the control of calcium wave propagation by Cx40 was mediated by ATP (36). It is well established that extracellular ATP can cause cell-to-cell calcium signaling via Cxs (9).
However, when we tested this slow wave hypothesis in cultured VSMCs, our data pointed toward gap junction intercellular communication as the mechanism behind the calcium wave. The purinergic receptor antagonist suramin failed to significantly reduce calcium wave propagation speeds, and a dye-spreading assay using Lucifer yellow provided evidence of direct VSMC coupling. Our findings are supported by several other studies that have reported intercellular coupling in both smooth muscle cultures and preparations (27, 31). The reason for the slow calcium wave propagation in VSMCs despite the presence of direct gap junctional coupling is unknown, but the discrepancy could be possibly explained by the different techniques (cultured VSMCs vs. intact vessels) used. Future investigation of the TGF calcium wave in intact preglomerular vessels from control and Cx45fl/fl:Nestin-Cre mice could further support the physiological significance of Cx45 in the propagation of calcium waves in the JGA.
In comparing our findings to the recent work on Cx40 in GENCs (36), Cxs appear to be a critical factor for calcium signaling in the JGA, but they seem to use at least two different mechanisms to achieve calcium wave propagation: direct gap junctional coupling (Cx45) and cell-to-cell signaling via extracellular ATP (Cx40). The different characteristics of the two cell types (endothelial cells vs. smooth muscle cells) or the functional differences that exist between the Cx40 and 45 isoforms may explain why both indirect and direct methods of calcium wave propagation occur in the JGA.
In conclusion, in this study we reported the localization of Cx45 to the renal cortical vasculature, glomeruli, and the JGA region. In the JGA, the afferent and efferent arterioles and intra- and extraglomerular mesangial cells were all Cx45 positive. Renin expression, plasma renin activity, and blood pressure were all increased significantly in Cx45fl/fl:Nestin-Cre mice, which have reduced JGA Cx45 expression. The speed of calcium wave propagation in VSMC cultured from Cx45fl/fl:Nestin-Cre mice was significantly lower than in control VSMCs. Treatment of control VSMCs with a Cx45-specific gap mimetic peptide also reduced calcium wave propagation. Blockade of purinergic receptors failed to reduce calcium wave propagation, whereas a dye-spreading assay provided evidence of cell-to-cell coupling between VSMCs. The localization of Cx45, its effects on renin, and calcium wave propagation all suggest a role for Cx45 in TGF, renin regulation, and systemic blood pressure maintenance. Although the precise mechanism through which Cx45 controls these regulatory systems remains to be determined, a model that utilizes intercellular gap junction communication is likely involved.
GRANTS
These studies were supported by grants DK64324 and DK74754 from the National Institutes of Health and by an Established Investigator Award 0640056N from the American Heart Association to J. Peti-Peterdi. Work in the Bonn laboratory was supported by grants of the German Research Association (SFB 645, B2, and Wi270/29–1) to K. Willecke.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Alcolea S, Theveniau-Ruissy M, Jarry-Guichard T, Marics I, Tzouanacou E, Chauvin JP, Briand JP, Moorman AF, Lamer WH, Gros DB. Down-regulation of connexin 45 gene products during mouse heart development. Circ Res 84: 1365–1379, 1999. [DOI] [PubMed] [Google Scholar]
- 2.Arensback B, Mikkelsen HB, Gustafsson F, Christensen T, Holstein-Rathlou NH. Expression of connexin 37, 40, and 43 mRNA and protein in renal preglomerular arterioles. Histochem Cell Biol 115: 479–487, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Bell PD, Lapointe JY, Sabirov R, Hayashi S, Peti-Peterdi J, Manabe K, Kovacs G, Okada Y. Macula densa cell signaling involves ATP release through a maxi anion channel. Proc Natl Acad Sci USA 100: 4322–4327, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouchard M, Souabni A, Busslinger M. Tissue-specific expression of Cre recombinase from the Pax8 locus. Genesis 38: 105–109, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Butterweck A, Gerg U, Elfgang C, Willecke K, Traub O. Immunochemical characterization of the gap junction protein connexin45 in mouse kidney and transfected human HeLa cells. J Membr Biol 141: 247–256, 1994. [DOI] [PubMed] [Google Scholar]
- 6.Camilleri JP, Phat VN, Bariety J, Corvol P, Menard J. Use of a specific antiserum for renin detection in human kidney. J Histochem Cytochem 28: 1343–1346, 1980. [DOI] [PubMed] [Google Scholar]
- 7.Chang Q, Gonzalez M, Pinter MJ, Balice-Gordon RJ. Gap junctional coupling and patterns of connexin expression among neonatal rat lumbar spinal motor neurons. J Neurosci 19: 10813–10828, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen J, Boyle S, Zhao M, Su W, Takahashi K, Davis L, DeCaestecker M, Takahashi T, Breyer MD, Hao CM. Differential expression of the intermediate filament protein nestin during renal development and its localization in adult podocytes. J Am Soc Nephrol 17: 1283–1291, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Cotrina ML, Lin JH, Alves-Rodrigues A, Liu S, Li J, Azmi-Ghadimi H, Kang J, Naus CC, Nedergaard M. Connexins regulate calcium signaling by controlling ATP release. Proc Natl Acad Sci USA 95: 15735–15740, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dedek K, Schultz K, Pieper M, Dirks P, Maxeiner S, Willecke K, Weiler R, Janssen-Bienhold U. Localization of heterotypic gap junction composed of connexin45 and connexin36 in the rod pathway of the mouse retina. Eur J Neurosci 24: 1675–1686, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Desplantez T, Halliday D, Dupont E, Weingart R. Cardiac connexins Cx43 and Cx45: formation of diverse gap junction channels with diverse electrical properties. Pflügers Arch 448: 363–375, 2004. [DOI] [PubMed] [Google Scholar]
- 12.DiBona GF Neural control of renal function: cardiovascular implications. Hypertension 13: 539–548, 1989. [DOI] [PubMed] [Google Scholar]
- 13.Gomes P, Srinivas SP, Van Driessche W, Vereecke J, Himpens B. ATP release through connexin hemichannels in corneal endothelial cells. Invest Ophthalmol Vis Sci 46: 1208–1218, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Gros DB, Jongsma HJ. Connexins in mammalian heart function. Bioessays 18: 719–760, 1996. [DOI] [PubMed] [Google Scholar]
- 15.Igarashi P Kidney-specific gene targeting. J Am Soc Nephrol 15: 2237–2239, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Hanner F, Chambrey R, Bourgeois S, Meer E, Mucsi I, Rosivall L, Shull GE, Lorenz JN, Eladari D, Peti-Peterdi J. Increased renal renin content in mice lacking the Na+/H+ exchanger NHE2. Am J Physiol Renal Physiol 294: F937–F944, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hwan Seul K, Beyer EC. Heterogeneous localization of connexin40 in the renal vasculature. Microvasc Res 59: 140–148, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Krattinger N, Capponi A, Mazzolai L, Aubert JF, Caille D, Nicod P, Waeber G, Meda P, Haefliger JA. Connexin40 regulated renin production and blood pressure. Kidney Int 72: 814–822, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Krüger O, Plum A, Kim JS, Winterhager E, Maxeiner S, Hallas G, Kirchoff S, Traub O, Lamers WH, Willecke K. Defective vascular development in connexin 45-deficient mice. Development 127: 4179–4193, 2000. [DOI] [PubMed] [Google Scholar]
- 20.Kumai M, Nishii K, Nakamura K, Takeda N, Suzuki M, Shibata Y. Loss of connexin45 causes a cushion defect in early cardiogenesis. Development 127: 3501–3512, 2000. [DOI] [PubMed] [Google Scholar]
- 21.Li X, Simard M. Connexin45 gap junction channels in rat cerebral vascular smooth muscle cells. Am J Physiol Heart Circ Physiol 281: H1890–H1898, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Li X, Simard JM. Increase in Cx45 gap junction channels in cerebral smooth muscle cells from SHR. Hypertension 40: 940–946, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Maxeiner S, Dedek K, Janssen-Bienhold U, Ammermüller J, Brune H, Kirsch T, Pieper M, Degen J, Krüger O, Willecke K, Weiler R. Deletion of connexin45 in mouse retinal neurons disrupts the rod/cone signaling pathway between AII amacrine and ON cone bipolar cells and leads to impaired visual transmission. J Neurosci 25: 566–576, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCulloch F, Chambrey R, Eladari D, Peti-Peterdi J. Localization of connexin 30 in the luminal membrane of cells in the distal nephron. Am J Physiol Renal Physiol 289: F1304–F1312, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Norling LL, Gomez RA, Inagami T. Characterization of a synthetic peptide antibody recognizing rat kidney renin and prorenin. Clin Nephrol 43: 232–6, 1995. [PubMed] [Google Scholar]
- 26.Peti-Peterdi J Calcium wave of tubuloglomerular feedback. Am J Physiol Renal Physiol 291: F473–F480, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Sakai N, Tabb T, Garfield RE. Studies of connexin 43 and cell-to-cell coupling in cultured human uterine smooth muscle. Am J Obstet Gynecol 167: 1267–1277, 1992. [DOI] [PubMed] [Google Scholar]
- 28.Salomonsson M, Gustafsson F, Andreasen D, Jensen BL, Holstein-Rathlou NH. Local electric stimulation causes conducted calcium response in rat interlobular arteries. Am J Physiol Renal Physiol 283: F473–F480, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Schnermann J Juxtaglomerular cell complex in the regulation of renal salt excretion. Am J Physiol Regul Integr Comp Physiol 274: R263–R279, 1998. [DOI] [PubMed] [Google Scholar]
- 30.Schweda F, Kurtz A. Cellular mechanism of renin release. Acta Physiol Scand 181: 383–390, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Segal SS, Duling R. Conduction of vasomotor responses in arterioles: a role for cell-to-cell coupling? Am J Physiol Heart Circ Physiol 256: H838–H845, 1989. [DOI] [PubMed] [Google Scholar]
- 32.Silverstein DM, Thornhill BA, Leung JC, Vehaskari VM, Craver RD, Trachtman HA, Chevalier RL. Expression of connexins in the normal and obstructed developing kidney. Pediatr Nephrol 18: 216–224, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Söhl G, Odermatt B, Maxeiner S, Degen J, Willecke K. New insight into the expression and function of neural connexins with transgenic mouse mutants. Brain Res Brain Res Rev 47: 245–259, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Suadicani SO, Vink MJ, Spray DC. Slow intercellular Ca2+ signaling in wild-type and Cx43-null neonatal mouse cardiac myocytes. Am J Physiol Heart Circ Physiol 279: H3076–H3088, 2000. [DOI] [PubMed] [Google Scholar]
- 35.Takenaka T, Inoue T, Kanno Y, Okada H, Hill CE, Suzuki H. Connexins 37 and 40 transduce purinergic signals mediating renal autoregulation. Am J Physiol Regul Integr Comp Physiol 294: R1–R11, 2008. [DOI] [PubMed] [Google Scholar]
- 36.Toma I, Bansal EJ, Meer EJ, Kang JJ, Vargas SL, Peti-Peterdi J. Connexin 40 and ATP-dependent intercellular calcium wave in renal glomerular endothelial cells. Am J Physiol Regul Integr Comp Physiol 294: R1769–R1776, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wagner AJ, Holstein-Rathlou NH, Marsh DJ. Internephron coupling by conducted vasomotor responses in normotensive and spontaneously hypertensive rats. Am J Physiol Renal Physiol 272: F372–F379, 1997. [DOI] [PubMed] [Google Scholar]
- 38.Wagner C, de Wit C, Kurtz L, Grünberger C, Kurtz A, Schweda F. Connexin40 is essential for the pressure control of renin synthesis and secretion. Circ Res 100: 556–563, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Willecke K, Eiberger J, Degen J, Eckardt D, Romualdi A, Güldenagel M, Deutsch U, Söhl G. Structural and functional diversity of connexin genes in the mouse and human genome. Biol Chem 383: 725–737, 2002. [DOI] [PubMed] [Google Scholar]
- 40.Wörsdörfer P, Maxeiner S, Markopoulos C, Kirfel G, Wulf V, Auth T, Urschel S, von Maltzahn J, Willecke K. Connexin expression and functional analysis of gap junctional communication in mouse embryonic stem cells. Stem Cells 26: 431–439, 2008. [DOI] [PubMed] [Google Scholar]
- 41.Yip KP, Marsh DJ. [Ca2+]i in rat afferent arteriole during constriction measured with confocal fluorescence microscopy. Am J Physiol Renal Fluid Electrolyte Physiol 271: F1004–F1011, 1996. [DOI] [PubMed] [Google Scholar]
- 42.Zhang J, Hill CE. Differential connexin expression in preglomerular and postglomerular vasculature: accentuation during diabetes. Kidney Int 68: 1171–1185, 2005. [DOI] [PubMed] [Google Scholar]
- 43.Zimmerman L, Parr B, Lendahl U, Cunningham M, McKay R, Gavin B, Mann J, Vassileva G, McMahon A. Independent regulatory elements in the nestin gene direct transgene expression to neural stem cells or muscle precursors. Neuron 12: 11–24, 1994. [DOI] [PubMed] [Google Scholar]