Abstract
To understand the connection between alveolar mechanics and key biochemical events such as surfactant secretion, one first needs to characterize the underlying mechanical properties of the lung parenchyma and its cellular constituents. In this study, the mechanics of three major cell types from the neonatal rat lung were studied; primary alveolar type I (AT1) and type II (AT2) epithelial cells and lung fibroblasts were isolated using enzymatic digestion. Atomic force microscopy indentation was used to map the three-dimensional distribution of apparent depth-dependent pointwise elastic modulus. Histograms of apparent modulus data from all three cell types indicated non-Gaussian distributions that were highly skewed and appeared multimodal for AT2 cells and fibroblasts. Nuclear stiffness in all three cell types was similar (2.5 ± 1.0 kPa in AT1 vs. 3.1 ± 1.5 kPa in AT2 vs. 3.3 ± 0.8 kPa in fibroblasts; n = 10 each), whereas cytoplasmic moduli were significantly higher in fibroblasts and AT2 cells (6.0 ± 2.3 and 4.7 ± 2.9 kPa vs. 2.5 ± 1.2 kPa). In both epithelial cell types, actin was arranged in sparse clusters, whereas prominent actin stress fibers were observed in lung fibroblasts. No systematic difference in actin or microtubule organization was noted between AT1 and AT2 cells. Atomic force microscope elastography, combined with live-cell fluorescence imaging, revealed that the stiffer measurements in AT2 cells often colocalized with lamellar bodies. These findings partially explain reported heterogeneity of alveolar cell deformation during in situ lung inflation and provide needed data for better understanding of how mechanical stretch influences surfactant release.
Keywords: atomic force microscope, pneumocytes, cell mechanics, lung elasticity
it is thought that extracellular matrix (ECM) proteins, such as collagen, elastin, and proteoglycans, are the most significant load-bearing elements of the highly heterogeneous lung tissue (37). In fact, some findings suggest that cellular components of the lung parenchyma may have a negligible contribution to macroscopic pulmonary biomechanics (45). However, this does not imply that mechanical properties of alveolar cells do not impact lung function.
On the contrary, multiscale model studies suggest the apparent elastic aggregate modulus of a cell plays a vital role in the transfer of mechanical cues between the tissue level extracellular environment and the molecular machinery within the cell (13). The function of specific cytoskeletal components under different modes of deformation, such as actin under tension or intermediate filaments and microtubules under shear or compression (17, 26, 32), is also critical for proper mechanotransduction. Understanding the relationship between bulk cell mechanical properties and the distribution of cytoskeletal stiffness requires further investigation (8). Nevertheless, changes in cell mechanical properties, usually associated with alterations in cytoskeletal components, have been related to diseases in a number of organ systems, including the lungs (26, 32, 39). Furthermore, mechanical changes that directly alter nuclear deformation may be responsible for modifying the genotype of a cell (10, 20).
The detailed mechanisms by which such factors affect mechanotransduction are not known. Even though a number of previous studies have highlighted the relationship between mechanical forces and alveolar cell function (6, 14, 22, 34), the mechanism by which physical forces are transformed into biochemical signals that are necessary for release of surfactant from type II pneumocytes remains unclear. Improved understanding of this process could bring critical insights into the design of novel therapeutics for a number of diseases that affect lung biomechanics, such as cystic fibrosis and emphysema.
It is now widely recognized that the interplay of alveolar type I (AT1) and type II (AT2) epithelial cells is critical for lung function (5). Isakson and colleagues (18, 19) have used an original experimental setup to show the importance of intercellular communication between the two epithelial cell types via gap junctions. Ashino et al. (3), using an in situ preparation, reported that AT1 cells play a critical role in surfactant secretion through mechanically triggered intercellular Ca2+ signaling. On a similar note, Patel et al. (29) showed that addition of type I-like cells to a culture of AT2 cells increased surfactant secretion by threefold. In that study, they further showed that ATP synthesis by AT1 cells was also critical for this cross talk, and that AT1 cells differ from AT2 cells in their response to similar amounts of stretch. Most recently, Perlman and Bhattacharya (30), using optical sectioning microscopy, showed that AT1 and AT2 cells experienced different mechanical stretches under identical in situ transpulmonary pressures, such that AT2 cells stretched less than AT1 cells, regardless of their anatomical location within the alveolus.
This evidence of localized variation in alveolar deformation, together with the preceding data, suggests that mechanical heterogeneity might play a central role in mechanotransduction and intercellular signaling in the lung. Such differences in alveolar micromechanics could be due to preferential localization of different cell types within the complex alveolar structures (45), regional heterogeneity of ECM protein content (35), or phenotypic differences in biomechanical properties of the cells themselves. Although prior studies have characterized the viscoelastic properties of lung epithelial cells (2, 4, 21, 31), none has specifically examined potential differences between primary AT1 and AT2 cell phenotypes. Therefore, it is the goal of this study to characterize the mechanical properties of three major cell types isolated from the lung parenchyma, namely AT1 and AT2 epithelial cells and lung fibroblasts, and to examine the intracellular distribution of mechanical properties in these cells to better understand the source of heterogeneity in lung cell mechanics and its role in mechanotransduction in the pulmonary alveolus.
MATERIALS AND METHODS
All chemicals were obtained from Sigma (St Louis, MO), unless noted otherwise. Hoechst 33258 and Lysotracker green (LTG) [used for fluorescence tagging of live cell markers for nucleus and lamellar bodies (LBs)] and cy5-phalloidin were obtained from Invitrogen (Carlsbad, CA). In addition, anti-p180 (lamellar transport protein), anti-aquaporin-1, anti-α-tubulin, and anti-vimentin antibodies were obtained from Covance (Berkeley, CA), Abcam (Cambridge, MA), and Sigma, respectively. Cells were isolated from neonatal rat lungs following a protocol modified from Dobbs et al. (11) and Fraslon-Vanhulle et al. (12). All animals received humane care in compliance with the “Principles of Laboratory Animal Care” formulated by the National Society for Medical Research and the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences and published by the National Institutes of Health (NIH Publication No. 85–23, revised 1985). Furthermore, the Institutional Animal Care and Use Committee at Columbia University reviewed and approved the protocol followed in this study.
Lungs were isolated from day 3 neonatal Sprague-Dawley rats (Taconic Farms, Germantown, NY) and placed in ice-cold PBS with 0.1% heparin and 2% penicillin-streptomycin. They were then washed with Krebs-bicarbonate buffer containing 5 mM EDTA-EGTA and 0.1% heparin, minced finely, and placed in 4 U/ml of elastase buffer (Worthington Biochemical, Lakewood, NJ) for enzymatic digestion. After 20 min of elastase digestion at 37°C in a shaker bath, the reaction was quenched with fetal bovine serum, and larger tissue pieces were broken down with further agitation using DNase I (Roche, Indianapolis, IN). This whole process was repeated until minced tissue was dissolved completely. Following digestion, cells were centrifuged and resuspended in Tris·HCl buffer with 0.83% ammonium chloride to lyse remaining red blood cells, and were finally panned on rat IgG coated bacteriological dishes (Fisher Scientific, Pittsburgh, PA) for 60 min to eliminate white blood cells. After this step, the supernate with suspended cells was preplated twice on polystyrene dishes for 25 min, during which a majority of lung fibroblasts was separated from alveolar epithelial cells. The final cell suspension was collected, counted with a hemacytometer, and plated on 60-mm polystyrene tissue culture dishes at ∼50% confluency.
Cells were cultured in standard DMEM with 10% fetal bovine serum, 1% penicillin-streptomycin, and 0.2% amphotericin-B before testing with the atomic force microscope (AFM); the culture period was limited to 12–48 h so as to minimize dedifferentiation. To demarcate the nuclei and LBs of type II cells, all live cells were simultaneously stained with Hoechst 33258 and LTG for 30 min before testing; morphology was used to confirm phenotype of -negative cells, i.e., squamous AT1 cells vs. polarized and polygonal fibroblasts. A Bioscope AFM (Veeco Metrology, Santa Barbara, CA), coupled with an inverted confocal microscope (Olympus, Center Valley, PA) and a heated stage, was used for mechanical measurements at a physiological 37°C temperature. A 100-μm2 region was tested in each cell via an 11-by-11 force-volume array (121 indentations per cell) with 1-μm spacing between each indentation using a standard blunt-pyramidal tip silicon nitride cantilever with a nominal spring constant of 0.03 N/m. All indentations were performed at a scan rate of 10 μm/s; force curves at a given point were repeatable for the duration of the experiment. The regions of interest for the three cell types consistently targeted a portion of the nuclear area and its perimeter, as confirmed by live fluorescence microscopy. This site was selected for its proximity to the perinuclear LBs in type II pneumocytes. The indentation experiments were restricted to 35–45 min to measure one to three cells per dish (30 cells, 10 of each type, were tested from 17 dishes obtained via 7 different lung isolations).
AFM force curves were analyzed using a non-Hertzian, pointwise analysis method, as previously described (7, 9). Briefly, the contact point was first determined by fitting a bidomain polynomial function to the raw AFM force curve. The cell indentation response was then determined in the usual way from the relationship between indentation force, obtained by multiplying probe deflection by the spring constant, and indentation depth, obtained from the difference between probe z-position and deflection. The indentation analysis assumed the cell to be an isotropic, elastic half-space, using a blunt cone approximation of the AFM tip geometry. A “pointwise” apparent elastic modulus was then calculated from each individual force-depth datum measured during the AFM indentation test, yielding a depth-dependent modulus that can reveal deviations from solutions based on classical “Hertzian” contact theory (9). The resulting three-dimensional stiffness distributions were rendered at a representative indentation depth of 300 nm, yielding an elastography image for each cell that could be overlaid onto the fluorescent images taken during or after the indentations and correlated with the underlying cellular regions (8). Because indentations were applied using a standard pyramidal AFM tip targeting a perinuclear region with a typical thickness of 3–5 μm, no explicit correction for rigid substrate effects was made, and no evidence of depth-dependent stiffening due to the substrate was observed. All indentations were pooled into one of two regional groups, nuclear or cytoplasmic, based on their physiological location, as determined by phase contrast and fluorescence microscopy. Modulus values from each region of the cell were used to calculate mean nuclear and cytoplasmic stiffness values for each cell, and these values were averaged to obtain mean values for each region in each of the three cell types.
To examine phenotypic differences in intracellular structure, lung cells were also cultured on glass coverslips under conditions otherwise identical to those tested on polystyrene, fixed with 10% formalin for 10 min, and washed with PBS. They were then stained for key structural elements, namely, actin, vimentin, microtubules, and nuclei, as well as p180 lamellar transport protein and/or aquaporin-1 to confirm the presence of AT2 and AT1 epithelial cell types, respectively. For the antibody-based stains, the cells were permeabilized with 0.05% Triton X, blocked with 2% bovine serum albumin, and sequentially incubated in primary and secondary antibodies. Filamentous actin and nuclei were labeled using cy5-phalloidin and 4,6-diamidino-2-phenylindole, respectively, immediately after secondary antibody incubation. Fluorescent images were obtained with a ×100 oil immersion objective. Boundaries of AT1 and AT2 cells were traced manually based on their differential interference contrast images, and mean normalized fluorescence gray-scale values for p180, actin, and α-tubulin were measured to determine the relative expression levels of these key molecules as a function of epithelial cell phenotype.
All values are reported as means ± SD, except for the averages of pooled moduli from different locations, which are presented as means ± SE. A two-way ANOVA with a post hoc Tukey's least significant difference test was used to determine statistical significance among the three cell types and the corresponding indentation locations, unless noted otherwise. Significance of Pearson correlations between fluorescent expressions of different molecules was examined with independent two-tailed t-tests. Statistical significance was attained with a P value of 0.05.
RESULTS
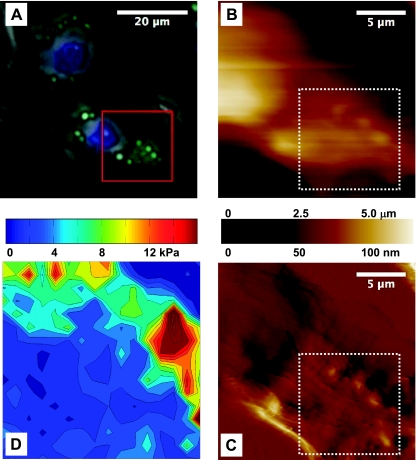
Contact-mode-AFM and live fluorescence images of a representative AT2 cell during testing are shown in Fig. 1, indicating the site of the indentation array on a typical region of interest, which, in this case, covered a distinct cluster of LBs. The apparent elastic modulus for each indentation location was evaluated at an indentation depth of 300 nm, and the resultant elastography image for the targeted subregion of the contact mode image is plotted in Fig. 1D.
Fig. 1.
A: fluorescent image of representative alveolar type II (AT2) cells indicating lamellar bodies (LBs) (green) and nuclei (blue), combined with a phase-contrast image. Superimposed is the 20 × 20-μm region of interest (red square) that was scanned with the atomic force microscope (AFM) in contact mode. LBs are visible in the resultant height (B) and deflection (C) AFM images. A 10 × 10-μm area within the region of interest (white dotted square) was probed mechanically with 121 indentations executed in an 11 × 11 array, with 1-μm spacing along both x- and y-axes. D: the resultant elastography map, rendered at an indentation depth of 300 nm, highlights the stiffer peaks that colocalize with LBs.
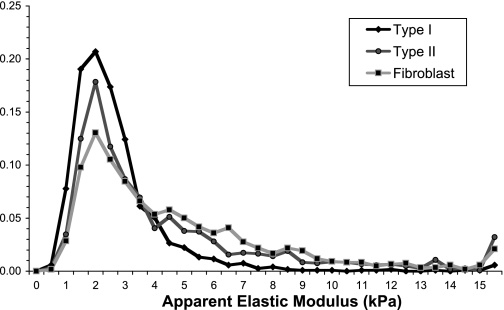
When the apparent modulus values, combined from all 121 indentations of each cell at an indentation depth of 300 nm, were viewed as histograms with 0.5-kPa bins, the contrast between mechanical properties of different cell types became apparent (Fig. 2). Overall, none of the three cell types had a normal Gaussian stiffness distribution. In particular, stiffness values in type I epithelial cells were concentrated in the 1- to 4-kPa range, with a relatively narrow band of variation, and had fewer hits in the 4- to 15-kPa range compared with both fibroblasts and AT2 cells. As a result, the median and the mean of modulus values of AT1 cells were within 10% of each other and were not significantly different. By contrast, AT2 cells and fibroblasts had wider, more skewed distributions, which resulted in a significant difference between their median and mean measurements (P < 0.01 each). This reflects the non-Gaussian distribution of stiffness measurements in these two cell types and represents mechanical contributions from multiple populations of structural elements within the cell. Notably, the modulus distribution from each of the three cell types had a mode of 2 kPa, suggesting a common cellular element contributing to the elastic response.
Fig. 2.
Overall distribution of apparent elastic modulus at 300-nm depth from 121 indentations in each cell for type I and type II pneumocytes, and lung fibroblasts (n = 10 each). All cells seemingly share common structural elements within the 1- to 4-kPa stiffness range; the main difference between distributions lies within the stiffness range of 4–15 kPa.
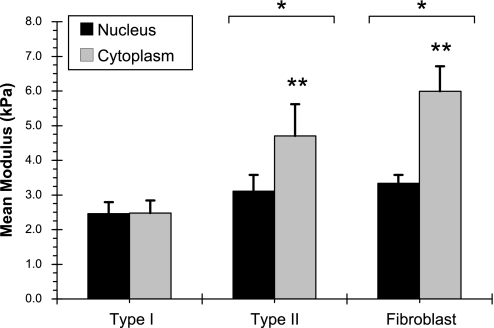
To better understand the underlying reason behind these observations, indentations were pooled into two separate groups for each cell type based on physiological location, namely nuclear or cytoplasmic (Fig. 3). Mean nuclear stiffness in three cell types was similar (2.5 ± 1.0, 3.1 ± 1.5, and 3.3 ± 0.8 kPa for AT1, AT2, and fibroblast cells, respectively) with no statistical differences. Whereas cytoplasmic stiffness for AT2 cells and fibroblasts was significantly higher than that of AT1 cells (4.7 ± 2.9 and 6.0 ± 2.3 kPa in AT2 cells and fibroblasts vs. 2.5 ± 1.2 kPa in AT1 cells; P < 0.05). One-half of all indentations in three cell types were over the nucleus. While the impact of nucleus to the measured aggregate modulus was slightly lower in AT2 cells, the number of indentations over each site was adequate to reveal the various populations of material in each cell type (at least 16 indentations on each site, except for one cell, which had eight indentations over its nucleus). The ratio of nucleus to cytoplasmic indentations was 34 ± 19% in type II cells vs. 64 ± 23% in type I cells (P < 0.05) and 52 ± 12% in fibroblasts (P = not significant).
Fig. 3.
Apparent elastic moduli of nucleus and cytoplasm in the three respective cell types (mean ± SE). Two-way ANOVA showed that fibroblasts and type II cells had significantly higher cytoplasmic stiffness compared with their respective nuclei (**P < 0.05). These values were significantly higher than cytoplasmic and nuclear stiffness of type I cells as well (*P < 0.05). The three mean nuclear moduli were not statistically different (P = not significant).
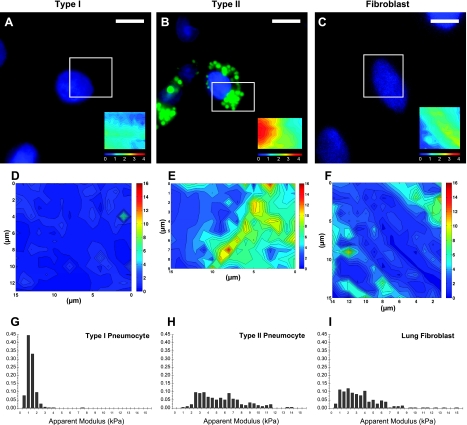
A spatial map of stiffness was overlaid onto the structural fluorescent images obtained in living cells. In Fig. 4, such an “elastography map” at an indentation depth of 300 nm is shown for representative AT1, AT2, and fibroblast cells. When the image is juxtaposed with the fluorescence image of the corresponding region, stiff peaks were clearly correlated with the perinuclear LBs stained positive for LTG in the AT2 cell. No such discrete sites of elevated stiffness were observed in AT1 cells, for which the stiffness was nearly homogeneous. In lung fibroblasts, parallel linear bands of elevated stiffness were observed, consistent with the arrangement of actin stress fibers within these cells, as described below. Despite substantial differences in the topography of the three cell types, in which AT2 cells exhibited a prominent nucleus, whereas AT1 cells and fibroblasts were more flattened, the elastography maps were poorly correlated with cell topography. The normalized stiffness histogram obtained from the elastography map of each cell clearly shows the non-Gaussian nature of the distributions, particularly for the AT2 and fibroblast cells, which appear to represent multiple populations of cytoskeletal material.
Fig. 4.
Correlation of mechanics with the underlying intracellular structural elements using high-resolution AFM elastography imaging in a representative alveolar type I (AT1) cell (A, D, G), an AT2 cell (B, E, H), and a lung fibroblast (C, F, I). Size and density of these spatial stiffness maps depended on the shape and size of the target cell of interest. A–C: fluorescent images of the three cell types (blue = nucleus, green = LBs, scale bars = 10 μm) with the region of interest probed for elastography imaging indicated by a white box. Inset: a topographical map of the boxed region of interest, obtained by identifying the contact point of each force curve within the indentation array, highlights the differences in shape of the three cells. The 4-μm color scale is relative, since the height was obtained relative to the AFM piezo above the cell. No obvious difference in relative height can be observed in or around the LBs, suggesting that they are well beneath the cell membrane. D–F: two-dimensional elastography maps at an indentation depth of 300 nm (all color scales are 0–16 kPa). E: elastography map from the AT2 cell reveals concentrated stiff perinuclear peaks that colocalize with LBs. G–I: normalized histograms of apparent elastic modulus obtained from the regions of interest showing a more normal distribution in the AT1 cell and highly skewed distributions with multiple populations of material evident in the AT2 cell and the fibroblast.
To further investigate the underlying intracellular structures that might be responsible for the observed mechanical differences, cells were stained for the major structural elements; high-resolution fluorescent images for representative alveolar epithelial cells and lung fibroblasts are shown in Figs. 5 and 6. Microtubule expression in all three cell types was similar. Expression of actin filaments in the two epithelial cell types showed localized actin clusters with no consistent organizational differences between AT1 and AT2 cells, whereas lung fibroblasts did not contain such actin clusters and typically exhibited prominent linear actin stress fibers. There was no significant correlation between expressions of either p180 lamellar transport protein vs. actin (n = 30, P = 0.34, r = 0.18) or p180 vs. α-tubulin (n = 30, P = 0.70, r = 0.07), demonstrating that expressions of these two molecules were not dependent on epithelial phenotype.
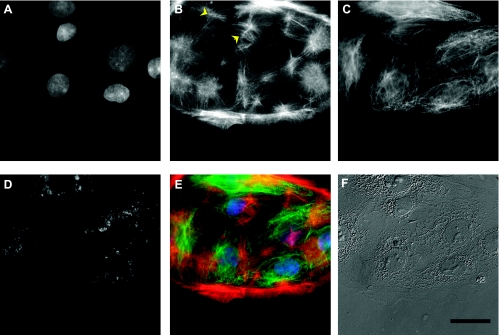
Fig. 5.
Coculture of primary alveolar epithelial cells as visualized via fluorescence (A–E) and differential interference contrast (DIC) microscopy (F), showing nuclei (A), filamentous actin (B), α-tubulin (C), AT2-specific LBs (D), and the resulting pseudocolor image combining A–C (E). Both type I and type II epithelial cells contained clusters of actin filaments (yellow arrows). A semiquantitative correlation of LB marker p180 and actin led to a nonsignificant weak relationship, suggesting that actin was not responsible for the observed mechanical difference between the two cell types. Scale bar = 20 μm.
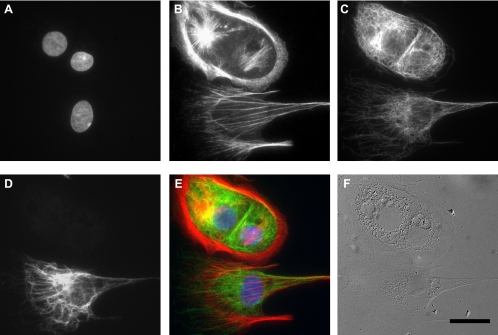
Fig. 6.
Coculture of primary alveolar epithelial cells and lung fibroblasts, as visualized via fluorescence (A–E) and DIC microscopy (F), showing nuclei (A), filamentous actin (B), α-tubulin (C), fibroblast-specific vimentin (D), and the resulting composite image (E). In particular, lung fibroblasts (positive for vimentin) were typically rich in linear actin stress fibers, whereas AT1 and AT2 epithelial cells (negative for vimentin) were mostly devoid of stress fibers, exhibiting a more diffuse actin distribution. Scale bar = 20 μm.
DISCUSSION
In this study, the mechanical properties of three different primary cell types isolated from the neonatal rat lung were characterized using AFM elastography. Interestingly, AT1 and AT2 epithelial cells and lung fibroblasts all appeared to share some common cellular material, having an apparent elastic modulus of ∼2 kPa, which corresponded to a nuclear component that was similar in all cell types. However, both AT2 cells and fibroblasts were observed to have significantly stiffer cytoplasmic constituents than AT1 cells. The results suggest that the difference in expression of a common cytoskeletal component, such as actin, may contribute to the elevated elastic moduli in fibroblasts. LB-rich sites within AT2 cells were also found to be stiffer than other parts of the cell. The fact that AT1 cells were softer overall than their globular cousins may partially explain the recent observation that AT1 cells undergo larger deformations than AT2 cells when subjected to identical transpulmonary pressures. In their recent study, Perlman and Bhattacharya (30) report that, regardless of their anatomical location within the alveolus, type II cells show ∼50% less maximum deformation compared with the rest of the alveolus. The independence of this observation from geometry suggests that the underlying mechanical properties of alveolar constituents (in proximity to type II cells) are a governing factor in determining the magnitude of local deformation. Our findings, which suggest that the cytoplasmic stiffness of AT1 cells is ∼50% of AT2 cells, support this hypothesis.
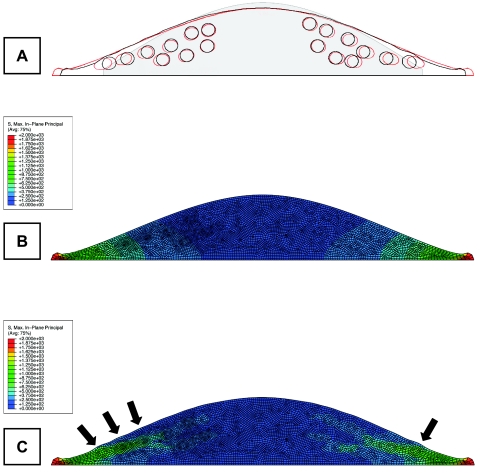
Differences in intercellular stiffness would lead to inhomogeneous alveolar deformations under homogeneous transpulmonary pressure fields. Subcellular heterogeneities, as observed in this study, would lead to alterations in intracellular strains, which, in turn, may impact mechanosensitive signaling pathways critical for pulmonary physiology. To better understand such a phenomenon, simple two-dimensional finite-element models of AT1 and AT2 cells on a deformable substrate were generated using the data obtained from AFM indentations (ABAQUS; Simulia, Providence, RI). The two models were identical, except, in the AT2 model, 15% of the elements with cytoplasmic stiffness (1.5 kPa) were replaced with stiffer ones (7.5 kPa) within 18 circular locations. When the AT2 cell was stretched to a basal lateral tension of 2,000 Pa, the stiffer bodies not only moved closer to the apical surface (7% decrease in granule-to-surface distance compared with the AT1 cell model), but also experienced elevated principal stresses that may influence local mechanosensitive signaling (Fig. 7). These observations suggested that an increase in local material properties of LBs further assists in physically delivering surfactant to the cell surface during alveolar inflation. Furthermore, lateral extension was reduced by 17% due to an increase in the mean modulus of the cell, which was akin to the increase in the aggregate apparent modulus of the cell as observed in this study. Even though this model was too simple to draw detailed conclusions, it further supported the hypothesis that internal mechanical heterogeneities of type II cells due to stiff surfactant carrying LBs may play a role in cellular mechanotransduction of surfactant release.
Fig. 7.
Two-dimensional finite-element modeling studies suggest that elevated stiffness of LBs may influence cellular signaling via increased local stresses and facilitate surfactant secretion by pushing LBs outward during alveolar stretch. A: the outline of the deformed model geometry with and without stiff LBs (black and red lines, respectively) superimposed over the un-deformed outline of the cell (gray shade). B: contour plot of maximum principal stress in a basally loaded AT1 cell with no stiff granules. C: the same plot for a AT2 cell exhibits elevated stresses around stiff LBs. Arrows point to granular sites where the LBs begin to protrude due to their increased stiffness.
The physiological significance of the AT2 cell stiffness might also be considered in the context of alveolar protective mechanisms against pulmonary edema formation. Pulmonary edema results when increased lung microvascular pressure increases fluid filtration, causing alveolar liquid entry. Typically, microvascular pressure increases occur during increases in lung blood flow as in exercise, or in congestive heart failure associated with increased left atrial pressure. However, alveolar surfactant prevents accumulation of alveolar liquid (43), thereby providing a safety factor against alveolar flooding. Increased microvascular pressure stimulates surfactant secretion (44), a protective response that is attributable to the compressive effect of vascular congestion on the alveolus. AT2 cell mechanics might sensitize this compressive response in that surfactant secretion might be more effectively induced in the relatively stiff AT2 cell. Thus AT2 stiffness might facilitate mechanical coupling between congested lung vessels and the alveolus in blocking pressure-induced pulmonary edema.
The exact function of AT1 cells in lung mechanobiology is still heavily debated. Recent findings suggest critical roles for AT1 cells in the intricate cellular mechanotransduction network within the alveolus (29). Potentially, these roles might encompass increased chemical stimulation via gap-junctional calcium signaling (18), direct stimulation of AT2 cells via mechanical tethering (36), or enhanced AT2 cell sensitivity to mechanical stimulation via other intercellular mechanisms (3). It is likely that the exact means of conveyance involves a combination of these factors. In any case, the response of AT1 cells to mechanical stretch in an in vivo setting will be a key parameter in understanding their function, and to elucidate this we first have to understand how mechanical forces are perceived by these cells.
Recent studies by Perlman and Bhattacharya (30) uncover some highly heterogeneous deformation patterns for the alveolar network during in situ transpulmonary inflation. Reasons for such heterogeneity within the lung parenchyma can be examined under three major subcategories: architectural, extracellular, and cellular. Architectural complexity that stems from a mechanically inhomogeneous alveolar structure; variability of mechanical properties that arise from multifarious ECM components such as proteoglycans, elastin, and collagen; and finally the differences in elastic properties of the constituent cell types themselves could all contribute to heterogeneity in lung mechanics. In their in vitro study of isolated parenchymal strips, Yuan et al. (45) found that rendering the cells unviable had no effect on the viscoelastic properties of the tissue. Even though their method of cell elimination (which involved leaving the tissue overnight in a room temperature tissue bath) may not have successfully removed cells as structural constituents, their experimental techniques were rigorous, and their conclusion that “the contribution of cell stiffness is minimal” was still reasonable. The major question is whether the cells were eliminated from the alveolar niche as mechanical components, or were they simply rendered dysfunctional in terms of their responsiveness to a biochemical challenge (the method employed to test cell viability in this particular study). The unknown in this scenario is whether cells, encased within the ECM, could still act as intermediary structural elements, even after their membrane integrity was compromised.
The actin cytoskeleton in vivo is interconnected with the ECM via integrins, and it is known to be the most mechanically significant intracellular structural element (33, 42). We have previously shown that actin stress fibers in the midcytoplasmic region of human endothelial cells produce a signature peak in stiffness distribution histograms that is centralized around 3–10 kPa. When cells were treated with actin polymerization antagonist cytochalasin-D, this peak was shown to disappear from the same region of interest (8). Furthermore, Oswari et al. (28) has shown actin to be a likely player in the dedifferentiation process of AT2 cells into AT1-like cells and critically affects the injury response of these cells. Knowing this direct link between actin and the mechanical response of cells, the amount of polymerized actin in type I and type II cells, as quantified from fluorescent images, was compared and found to be independent of p180 expression (Fig. 5), demonstrating that differences in cytoplasm mechanics were not due to actin. Following a previous paper reporting on “actin envelopes” around the LBs (41), colocalization between actin and p180 was sought. However, no persistent colocalization was observed, further supporting the hypothesis that LBs were directly influencing the local cytoplasmic mechanical response in our cultures.
The mean cytoplasmic modulus values reported here were, in general, stiffer than previously reported moduli of other epithelial cell types (2). It should be noted, however, that these measurements were from primary cells and that previous studies focused on a central location within the cell, which is more likely to be dominated by nuclear properties. Because none of the tested lung cell types in this study exhibited normal (Gaussian) distributions of stiffness, it follows that averaging of local mechanical measurements could inaccurately represent the bulk properties of the whole cell, consistent with our laboratory's previous studies of vascular endothelial cells (8). Identification of mechanically distinct subcellular structures suggests that, even if the bulk mechanical response to uniform deformations was similar for different cell types, the local manifestations of such homogeneous loads could still be heterogeneous, leading to complex intracellular deformation patterns. These findings point to a possible direct connection between heterogeneous distributions of organelles or cytoskeletal proteins and the complex intracellular signaling pathways that are known to behave discretely and with wide spatial variation (15). Previously, this disjointed behavior of (mostly cytoskeleton-related) cell signaling has been explained with spring-and-cable models of force transduction instead of simpler continuum models (38). This was attributed to the potential noncontinuum nature of the cell and was further supported by the inability of homogeneous cell models to reproduce observed mechanical phenomena (16). By introducing intracellular mechanical heterogeneity, however, continuum models can actually be employed to predict cellular responses to physical forces with greater accuracy and efficiency (25). Building on the data obtained from the present study, future computational models focusing on such heterogeneities may help to resolve some of the details about pulmonary mechanotransduction and related physiological processes.
The technique of AFM elastography, as employed in this study, is not without limitations. First, only elastic properties of the cells were measured. Cells, like most other biological tissues, exhibit viscoelastic behavior that can also be characterized with the AFM (2, 23, 24). Since the alveolar inflation studies that inspired the present work were performed in a quasi-static manner (30), we initially focused on phenotypic differences in the apparent equilibrium elastic modulus of alveolar cells; further characterization of viscoelastic properties may be an area for future investigation. To minimize viscoelastic artifacts and produce repetitive force curves, indentation velocity was limited to 10 μm/s during testing at 37°C (1). Cells may also exhibit anisotropic properties, such that the in-plane stiffness differs from the transverse stiffness. However, the deformation resulting from an indentation test does include reciprocal in-plane extension to conserve the volume of the sample. Therefore, regions that appear stiffer in the AFM elastography maps are likely to also deform less due to in-plane forces.
Another limitation of the study was the motion of intracellular components, especially the LBs, during the time span of the experimental protocol. Such dynamics, likely augmented by the 37°C physiological temperature, probably increased spatial localization errors, as well as overall experimental variability. To minimize associated inaccuracies, fluorescent and phase contrast images were obtained before, during, and after the experiment, and cells that exhibited substantial morphological changes during testing were excluded from the analysis. Thus, while we are confident that our results and conclusions were not adversely affected by active cell reorganization, they may be biased toward cells that were intrinsically more stable in culture. In addition, even though all type II cells tested in this study contained clearly identifiable globular LBs, the fact remains that they were cultured alveolar cells known to undergo rapid phenotypic transition. The LTG-negative squamous AT1-like epithelial cells tested in this study could either be native type I cells or type II cells that have dedifferentiated during the first 48 h of the isolation (27). In addition, the tested cells were not confluent, which may have affected intracellular dynamics (40), and by extension biomechanics, differently in AT1 and AT2 cells. Despite these limitations, the characterizations performed in this study have significant value as the first quantitative biomechanical measurements on freshly isolated mixed-population alveolar cells, forming a foundation for future studies of type I and type II cocultures.
In summary, herein we present the first study that examines the mechanics of different types of primary alveolar cells from the mammalian lung. The initial findings suggest significant differences in the distribution of apparent elastic properties between the two primary alveolar epithelial cell types, as well as lung fibroblasts. While type I pneumocytes were found to be more homogeneous and compliant, type II pneumocytes and fibroblasts were stiffer with wider modulus distributions reflecting cytoplasmic moduli that were significantly stiffer than nuclei. Spatially, the stiffer material regions in AT2 cells often colocalized with perinuclear LBs, the functional units of these cells that secrete pulmonary surfactant. These findings provide needed data for modeling the complex mechanotransduction processes within the alveoli to improve our understanding of physiological and pathological pulmonary function.
GRANTS
Funding for this study was provided by the National Science Foundation (CAREER Award BES-0239138; K. D. Costa), the National Heart, Lung, and Blood Institute (HL64896; J. Bhattacharya), and a fellowship from the Stony Wold-Herbert Fund (E. U. Azeloglu).
Acknowledgments
We acknowledge Rengin Soydaner Azeloglu for technical assistance in fluorescence staining and microscopy, and Dr. Carrie E. Perlman, Keyue Shen, and Vikrum A. Thimmappa for technical assistance and useful discussions.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.A-Hassan E, Heinz WF, Antonik MD, D'Costa NP, Nageswaran S, Schoenenberger CA, Hoh JH. Relative microelastic mapping of living cells by atomic force microscopy. Biophys J 74: 1564–1578, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alcaraz J, Buscemi L, Grabulosa M, Trepat X, Fabry B, Farre R, Navajas D. Microrheology of human lung epithelial cells measured by atomic force microscopy. Biophys J 84: 2071–2079, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashino Y, Ying X, Dobbs LG, Bhattacharya J. [Ca2+]i oscillations regulate type II cell exocytosis in the pulmonary alveolus. Am J Physiol Lung Cell Mol Physiol 279: L5–L13, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Berrios JC, Schroeder MA, Hubmayr RD. Mechanical properties of alveolar epithelial cells in culture. J Appl Physiol 91: 65–73, 2001. [DOI] [PubMed] [Google Scholar]
- 5.Boitano S, Safdar Z, Welsh DG, Bhattacharya J, Koval M. Cell-cell interactions in regulating lung function. Am J Physiol Lung Cell Mol Physiol 287: L455–L459, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Cavanaugh KJ, Margulies SS. Measurement of stretch-induced loss of alveolar epithelial barrier integrity with a novel in vitro method. Am J Physiol Cell Physiol 283: C1801–C1808, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Costa KD Imaging and probing cell mechanical properties with the atomic force microscope. Methods Mol Biol 319: 331–361, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Costa KD, Sim AJ, Yin FC. Non-Hertzian approach to analyzing mechanical properties of endothelial cells probed by atomic force microscopy. J Biomech Eng 128: 176–184, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Costa KD, Yin FC. Analysis of indentation: implications for measuring mechanical properties with atomic force microscopy. J Biomech Eng 121: 462–471, 1999. [DOI] [PubMed] [Google Scholar]
- 10.Dahl KN, Engler AJ, Pajerowski JD, Discher DE. Power-law rheology of isolated nuclei with deformation mapping of nuclear substructures. Biophys J 89: 2855–2864, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dobbs LG, Gonzalez R, Williams MC. An improved method for isolating type II cells in high yield and purity. Am Rev Respir Dis 134: 141–145, 1986. [DOI] [PubMed] [Google Scholar]
- 12.Fraslon-Vanhulle C, Chailley-Heu B, Batenburg JJ, Elfring R, Bourbon JR. Ontogeny of surfactant proteins and lipid-synthesizing enzymes in cultured fetal lung epithelial cells. Am J Physiol Lung Cell Mol Physiol 267: L375–L383, 1994. [DOI] [PubMed] [Google Scholar]
- 13.Guilak F, Mow VC. The mechanical environment of the chondrocyte: a biphasic finite element model of cell-matrix interactions in articular cartilage. J Biomech 33: 1663–1673, 2000. [PubMed] [Google Scholar]
- 14.Gutierrez JA, Gonzalez RF, Dobbs LG. Mechanical distension modulates pulmonary alveolar epithelial phenotypic expression in vitro. Am J Physiol Lung Cell Mol Physiol 274: L196–L202, 1998. [DOI] [PubMed] [Google Scholar]
- 15.Helmke BP, Goldman RD, Davies PF. Rapid displacement of vimentin intermediate filaments in living endothelial cells exposed to flow. Circ Res 86: 745–752, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Hu S, Chen J, Fabry B, Numaguchi Y, Gouldstone A, Ingber DE, Fredberg JJ, Butler JP, Wang N. Intracellular stress tomography reveals stress focusing and structural anisotropy in cytoskeleton of living cells. Am J Physiol Cell Physiol 285: C1082–C1090, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Ingber DE Tensegrity: the architectural basis of cellular mechanotransduction. Annu Rev Physiol 59: 575–599, 1997. [DOI] [PubMed] [Google Scholar]
- 18.Isakson BE, Evans WH, Boitano S. Intercellular Ca2+ signaling in alveolar epithelial cells through gap junctions and by extracellular ATP. Am J Physiol Lung Cell Mol Physiol 280: L221–L228, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Isakson BE, Seedorf GJ, Lubman RL, Evans WH, Boitano S. Cell-cell communication in heterocellular cultures of alveolar epithelial cells. Am J Respir Cell Mol Biol 29: 552–561, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Lammerding J, Schulze PC, Takahashi T, Kozlov S, Sullivan T, Kamm RD, Stewart CL, Lee RT. Lamin A/C deficiency causes defective nuclear mechanics and mechanotransduction. J Clin Invest 113: 370–378, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laurent VM, Henon S, Planus E, Fodil R, Balland M, Isabey D, Gallet F. Assessment of mechanical properties of adherent living cells by bead micromanipulation: comparison of magnetic twisting cytometry vs optical tweezers. J Biomech Eng 124: 408–421, 2002. [DOI] [PubMed] [Google Scholar]
- 22.Liu M, Tanswell AK, Post M. Mechanical force-induced signal transduction in lung cells. Am J Physiol Lung Cell Mol Physiol 277: L667–L683, 1999. [DOI] [PubMed] [Google Scholar]
- 23.Mahaffy RE, Park S, Gerde E, Kas J, Shih CK. Quantitative analysis of the viscoelastic properties of thin regions of fibroblasts using atomic force microscopy. Biophys J 86: 1777–1793, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mathur AB, Collinsworth AM, Reichert WM, Kraus WE, Truskey GA. Endothelial, cardiac muscle and skeletal muscle exhibit different viscous and elastic properties as determined by atomic force microscopy. J Biomech 34: 1545–1553, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Na S, Sun Z, Meininger GA, Humphrey JD. On atomic force microscopy and the constitutive behavior of living cells. Biomech Model Mechanobiol 3: 75–84, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishimura S, Nagai S, Katoh M, Yamashita H, Saeki Y, Okada J, Hisada T, Nagai R, Sugiura S. Microtubules modulate the stiffness of cardiomyocytes against shear stress. Circ Res 98: 81–87, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Olsen CO, Isakson BE, Seedorf GJ, Lubman RL, Boitano S. Extracellular matrix-driven alveolar epithelial cell differentiation in vitro. Exp Lung Res 31: 461–482, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Oswari J, Matthay MA, Margulies SS. Keratinocyte growth factor reduces alveolar epithelial susceptibility to in vitro mechanical deformation. Am J Physiol Lung Cell Mol Physiol 281: L1068–L1077, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Patel AS, Reigada D, Mitchell CH, Bates SR, Margulies SS, Koval M. Paracrine stimulation of surfactant secretion by extracellular ATP in response to mechanical deformation. Am J Physiol Lung Cell Mol Physiol 289: L489–L496, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Perlman CE, Bhattacharya J. Alveolar expansion imaged by optical sectioning microscopy. J Appl Physiol 103: 1037–1044, 2007. [DOI] [PubMed] [Google Scholar]
- 31.Puig-De-Morales M, Grabulosa M, Alcaraz J, Mullol J, Maksym GN, Fredberg JJ, Navajas D. Measurement of cell microrheology by magnetic twisting cytometry with frequency domain demodulation. J Appl Physiol 91: 1152–1159, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Ridge KM, Linz L, Flitney FW, Kuczmarski ER, Chou YH, Omary MB, Sznajder JI, Goldman RD. Keratin 8 phosphorylation by protein kinase C delta regulates shear stress-mediated disassembly of keratin intermediate filaments in alveolar epithelial cells. J Biol Chem 280: 30400–30405, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Rotsch C, Jacobson K, Radmacher M. Dimensional and mechanical dynamics of active and stable edges in motile fibroblasts investigated by using atomic force microscopy. Proc Natl Acad Sci USA 96: 921–926, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanchez-Esteban J, Wang Y, Filardo EJ, Rubin LP, Ingber DE. Integrins beta1, alpha6, and alpha3 contribute to mechanical strain-induced differentiation of fetal lung type II epithelial cells via distinct mechanisms. Am J Physiol Lung Cell Mol Physiol 290: L343–L350, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Sannes PL Differences in basement membrane-associated microdomains of type I and type II pneumocytes in the rat and rabbit lung. J Histochem Cytochem 32: 827–833, 1984. [DOI] [PubMed] [Google Scholar]
- 36.Sirianni FE, Chu FS, Walker DC. Human alveolar wall fibroblasts directly link epithelial type 2 cells to capillary endothelium. Am J Respir Crit Care Med 168: 1532–1537, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Suki B, Ito S, Stamenovic D, Lutchen KR, Ingenito EP. Biomechanics of the lung parenchyma: critical roles of collagen and mechanical forces. J Appl Physiol 98: 1892–1899, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Sultan C, Stamenovic D, Ingber DE. A computational tensegrity model predicts dynamic rheological behaviors in living cells. Ann Biomed Eng 32: 520–530, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Trepat X, Grabulosa M, Buscemi L, Rico F, Farre R, Navajas D. Thrombin and histamine induce stiffening of alveolar epithelial cells. J Appl Physiol 98: 1567–1574, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Trepat X, Puig F, Gavara N, Fredberg JJ, Farre R, Navajas D. Effect of stretch on structural integrity and micromechanics of human alveolar epithelial cell monolayers exposed to thrombin. Am J Physiol Lung Cell Mol Physiol 290: L1104–L1110, 2006. [DOI] [PubMed] [Google Scholar]
- 41.van Weeren L, de Graaff AM, Jamieson JD, Batenburg JJ, Valentijn JA. Rab3D and actin reveal distinct lamellar body subpopulations in alveolar epithelial type II cells. Am J Respir Cell Mol Biol 30: 288–295, 2004. [DOI] [PubMed] [Google Scholar]
- 42.Wakatsuki T, Schwab B, Thompson NC, Elson EL. Effects of cytochalasin D and latrunculin B on mechanical properties of cells. J Cell Sci 114: 1025–1036, 2001. [DOI] [PubMed] [Google Scholar]
- 43.Wang PM, Ashino Y, Ichimura H, Bhattacharya J. Rapid alveolar liquid removal by a novel convective mechanism. Am J Physiol Lung Cell Mol Physiol 281: L1327–L1334, 2001. [DOI] [PubMed] [Google Scholar]
- 44.Wang PM, Fujita E, Bhattacharya J. Vascular regulation of type II cell exocytosis. Am J Physiol Lung Cell Mol Physiol 282: L912–L916, 2002. [DOI] [PubMed] [Google Scholar]
- 45.Yuan H, Ingenito EP, Suki B. Dynamic properties of lung parenchyma: mechanical contributions of fiber network and interstitial cells. J Appl Physiol 83: 1420–1431, 1997. [DOI] [PubMed] [Google Scholar]