Abstract
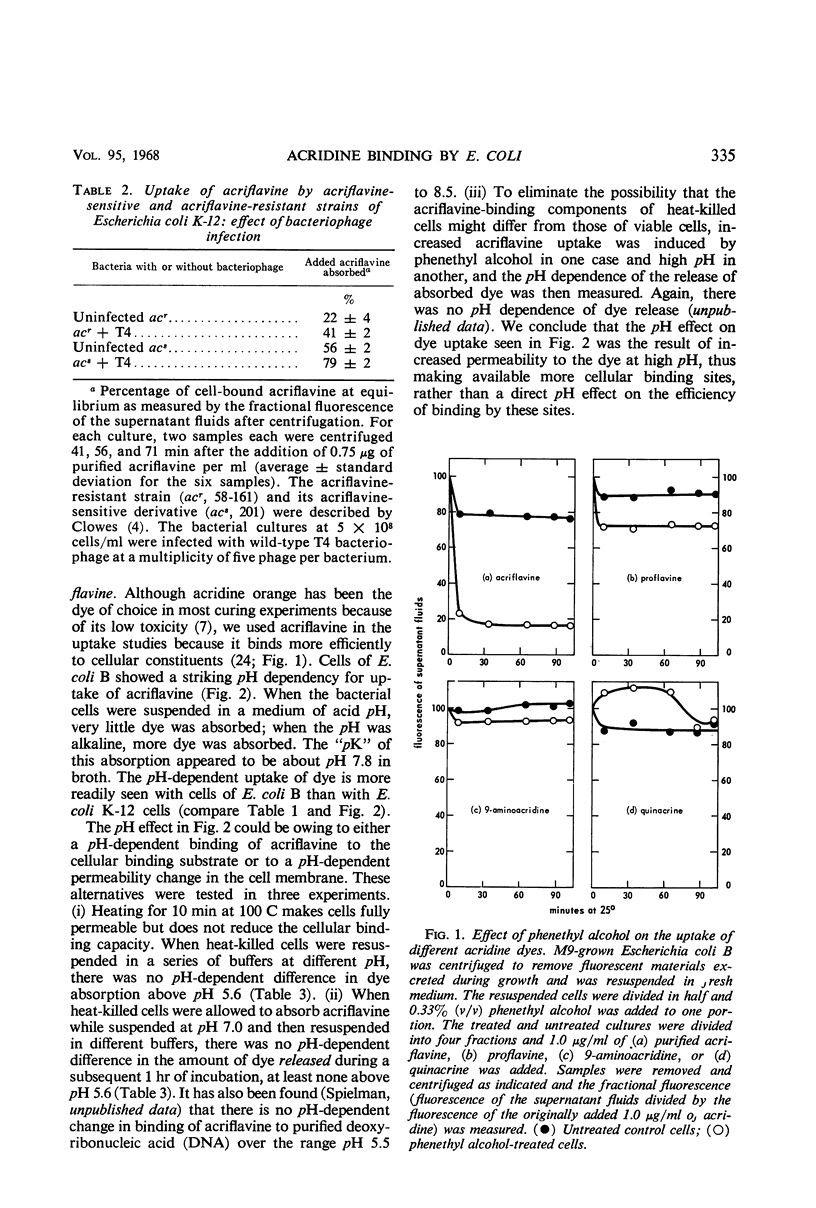
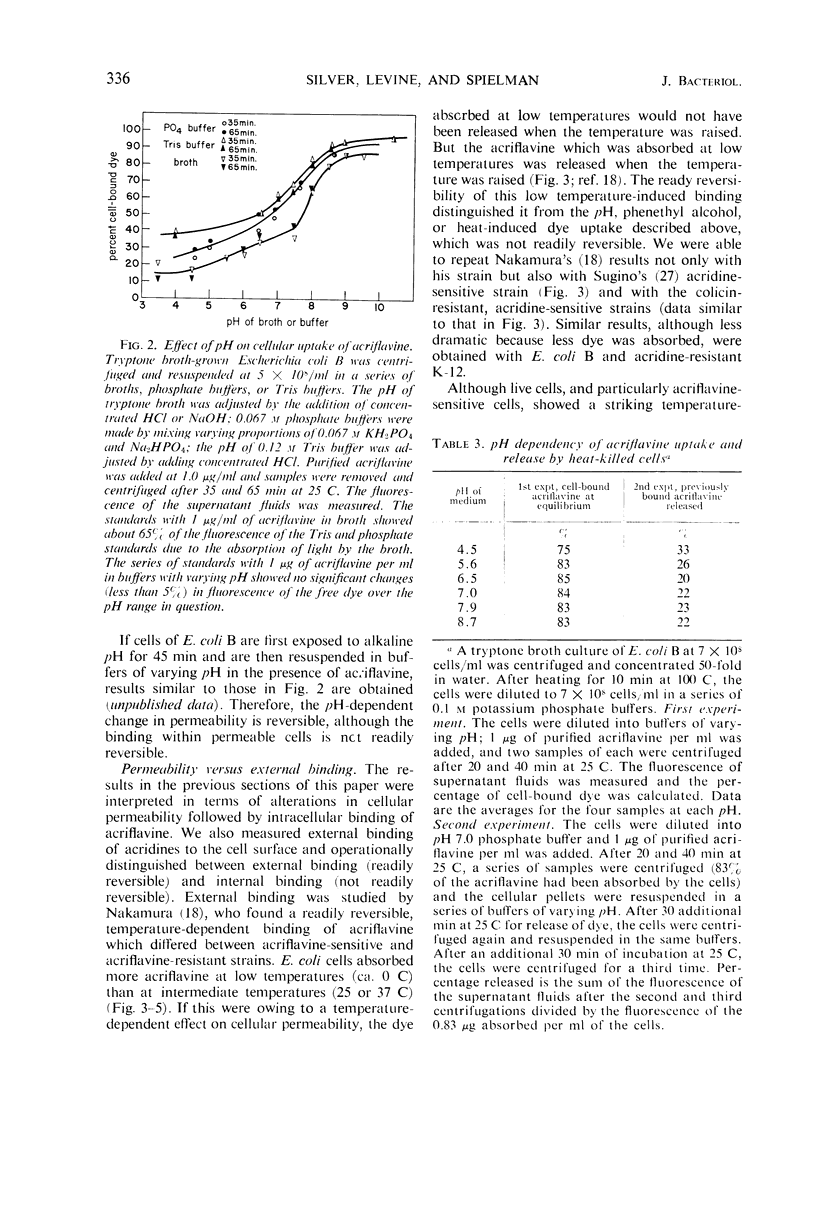
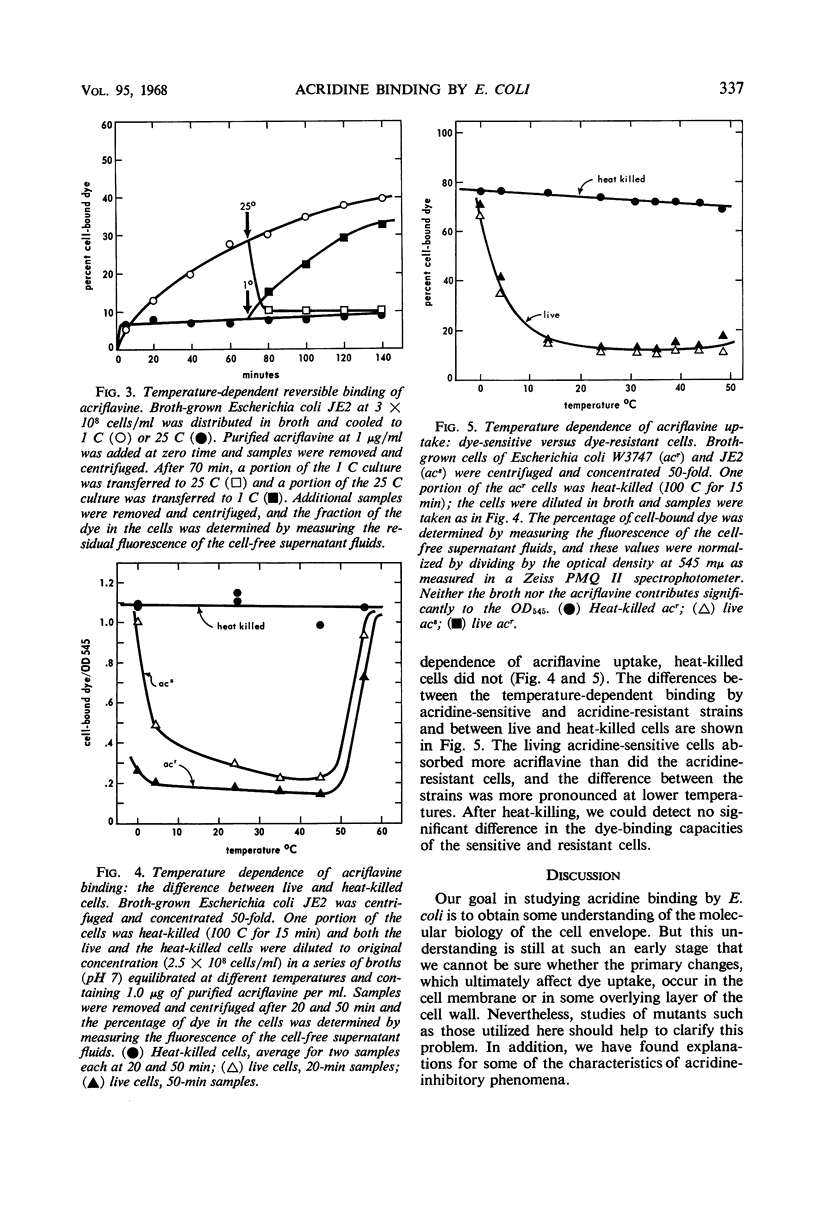
Acridine dye binding by cells of Escherichia coli has been characterized in terms of a number of parameters. There is a temperature-dependent, readily reversible binding of acriflavine which occurs to a greater extent with acridine-sensitive mutants of E. coli K-12 than with wild-type E. coli B or K-12. There is an essentially irreversible internal binding of acriflavine which occurs when the cellular permeability barriers are destroyed or altered by heat-treatment, elevated pH, treatment with toluene or phenethyl alcohol, or infection with bacteriophage T2 or T4. Both the reversible and the irreversible binding of acridines occurs more effectively with the acridine dye acriflavine than with the related dye proflavine, and still less effectively with 9-aminoacridine and quinacrine. These properties of acridine binding can be correlated with various inhibitory effects of the dyes on the cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayer M. E., Anderson T. F. The surface structure of Escherichia coli. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1592–1599. doi: 10.1073/pnas.54.6.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster R. A. An Analysis of the Action of Proflavine on Bacteriophage Growth. J Bacteriol. 1948 Dec;56(6):795–809. doi: 10.1128/jb.56.6.795-809.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOSHINO T. The influence of the host character of E. coli upon its lysis by bacteriophage. II. The influence of host character on the acriflavine tolerance of bacteriophage T3. Jpn J Exp Med. 1954 Apr;24(2):63–68. [PubMed] [Google Scholar]
- Hirota Y. THE EFFECT OF ACRIDINE DYES ON MATING TYPE FACTORS IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1960 Jan;46(1):57–64. doi: 10.1073/pnas.46.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkas J. D., Türler H., Chargaff E. Studies on the specification of accessory biochemical characters, as exemplified by the fatty acid patterns of various strains of Escherichia coli. Biochim Biophys Acta. 1965 Nov 15;111(1):96–109. doi: 10.1016/0304-4165(65)90476-9. [DOI] [PubMed] [Google Scholar]
- LERMAN L. S. The structure of the DNA-acridine complex. Proc Natl Acad Sci U S A. 1963 Jan 15;49:94–102. doi: 10.1073/pnas.49.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITSUHASHI S., MORIMURA M., KONO K., OSHIMA H. ELIMINATION OF DRUG RESISTANCE OF STAPHYLOCOCCUS AUREUS BY TREATMENT WITH ACRIFLAVINE. J Bacteriol. 1963 Jul;86:162–164. doi: 10.1128/jb.86.1.162-164.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURRAY R. G., STEED P., ELSON H. E. THE LOCATION OF THE MUCOPEPTIDE IN SECTIONS OF THE CELL WALL OF ESCHERICHIA COLI AND OTHER GRAM-NEGATIVE BACTERIA. Can J Microbiol. 1965 Jun;11:547–560. doi: 10.1139/m65-072. [DOI] [PubMed] [Google Scholar]
- Nagel de Zwaig R., Luria S. E. Genetics and physiology of colicin-tolerant mutants of Escherichia coli. J Bacteriol. 1967 Oct;94(4):1112–1123. doi: 10.1128/jb.94.4.1112-1123.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H. Acriflavine-binding capacity of Escherichia coli in relation to acriflavine sensitivity and metabolic activity. J Bacteriol. 1966 Nov;92(5):1447–1452. doi: 10.1128/jb.92.5.1447-1452.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H. Gene-Controlled Resistance to Acriflavine and Other Basic Dyes in Escherichia coli. J Bacteriol. 1965 Jul;90(1):8–14. doi: 10.1128/jb.90.1.8-14.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H. Phenethyl alcohol sensitivity in Escherichia coli. J Bacteriol. 1967 Mar;93(3):1183–1184. doi: 10.1128/jb.93.3.1183-1184.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M., Witten C. Interaction of colicins with bacterial cells. 3. Colicin-tolerant mutations in Escherichia coli. J Bacteriol. 1967 Oct;94(4):1093–1111. doi: 10.1128/jb.94.4.1093-1111.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORGEL A., BRENNER S. Mutagenesis of bacteriophage T4 by acridines. J Mol Biol. 1961 Dec;3:762–768. doi: 10.1016/s0022-2836(61)80081-8. [DOI] [PubMed] [Google Scholar]
- SILVER S. ACRIFLAVINE RESISTANCE: A BACTERIOPHAGE MUTATION AFFECTING THE UPTAKE OF DYE BY THE INFECTED BACTERIAL CELLS. Proc Natl Acad Sci U S A. 1965 Jan;53:24–30. doi: 10.1073/pnas.53.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver S. Acridine sensitivity of bacteriophage T2: a virus gene affecting cell permeability. J Mol Biol. 1967 Oct 14;29(1):191–202. doi: 10.1016/0022-2836(67)90190-8. [DOI] [PubMed] [Google Scholar]
- Silver S., Wendt L. Mechanism of action of phenethyl alcohol: breakdown of the cellular permeability barrier. J Bacteriol. 1967 Feb;93(2):560–566. doi: 10.1128/jb.93.2.560-566.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino Y. Mutants of Escherichia coli sensitive to methylene blue and acridines. Genet Res. 1966 Feb;7(1):1–11. doi: 10.1017/s0016672300009423. [DOI] [PubMed] [Google Scholar]
- WATANABE T., FUKASAWA T. Episome-mediated transfer of drug resistance in Enterobacteriaceae. II. Elimination of resistance factors with acridine dyes. J Bacteriol. 1961 May;81:679–683. doi: 10.1128/jb.81.5.679-683.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]


