Abstract
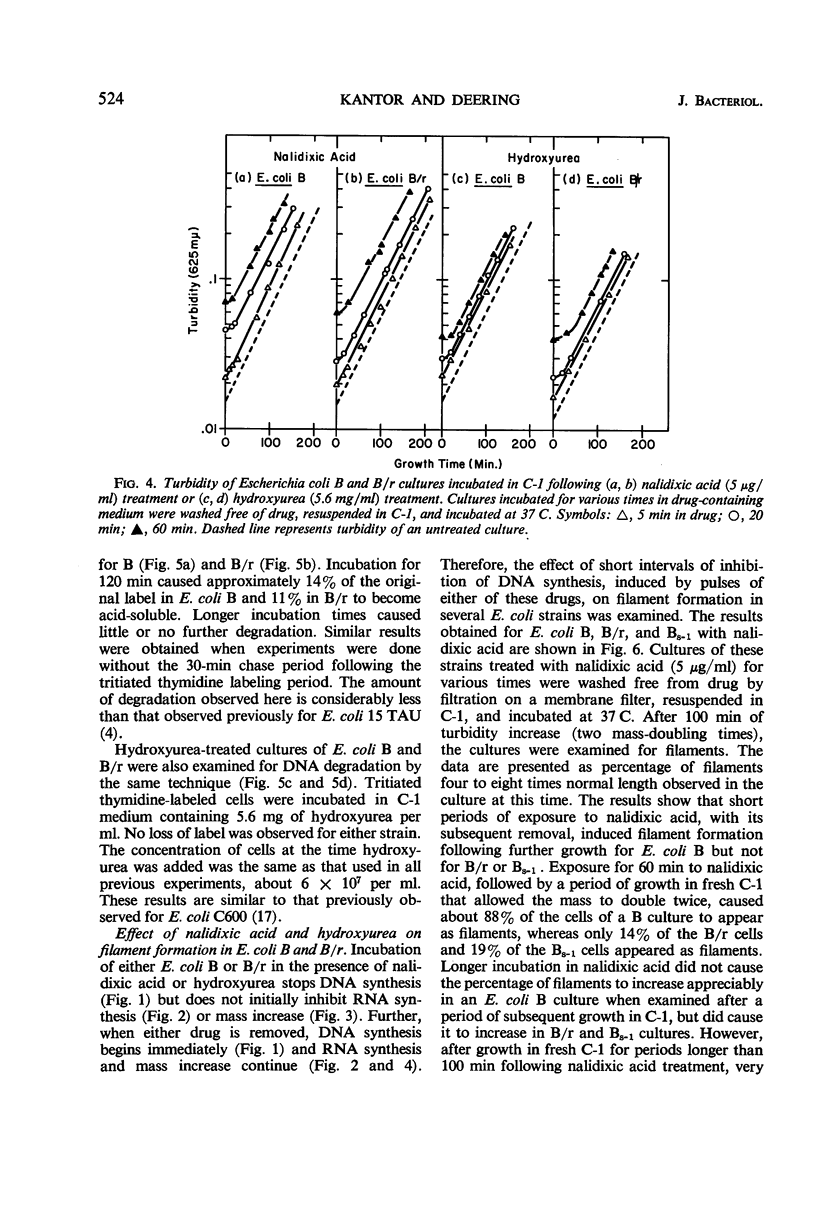
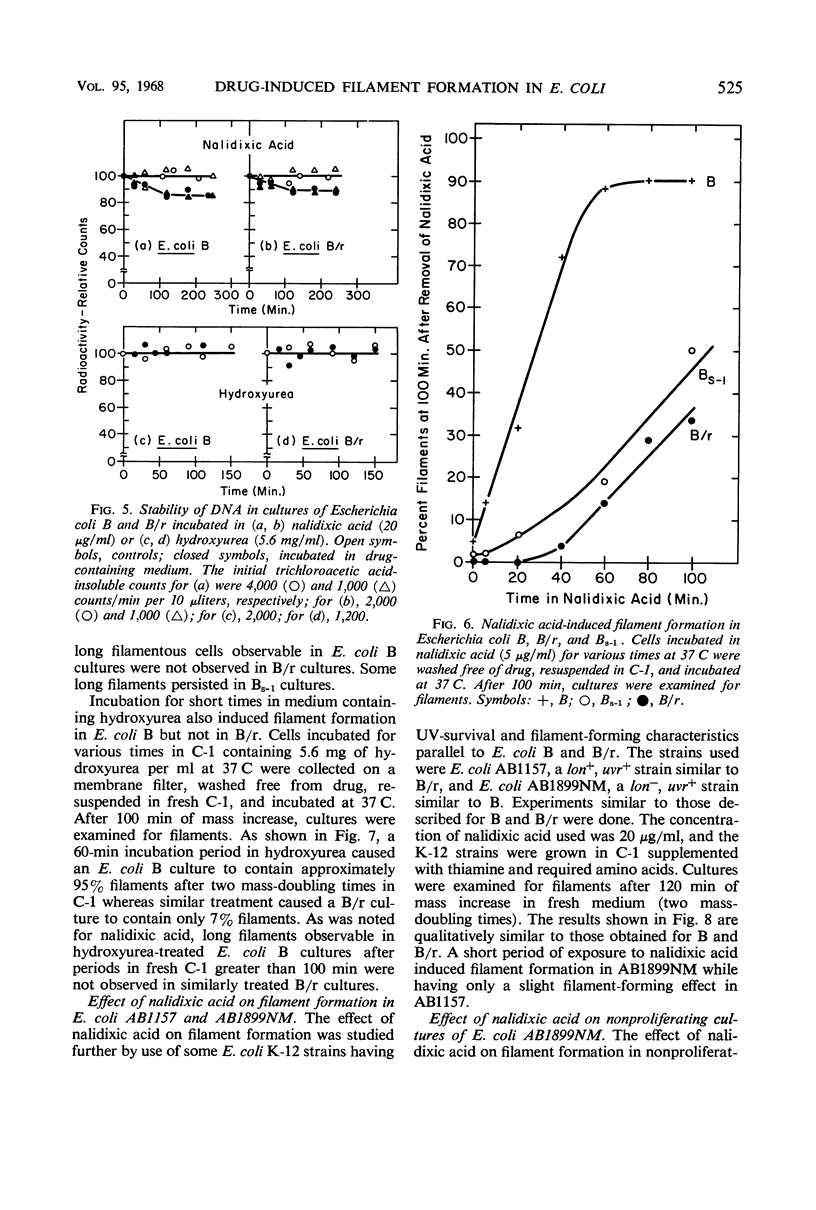
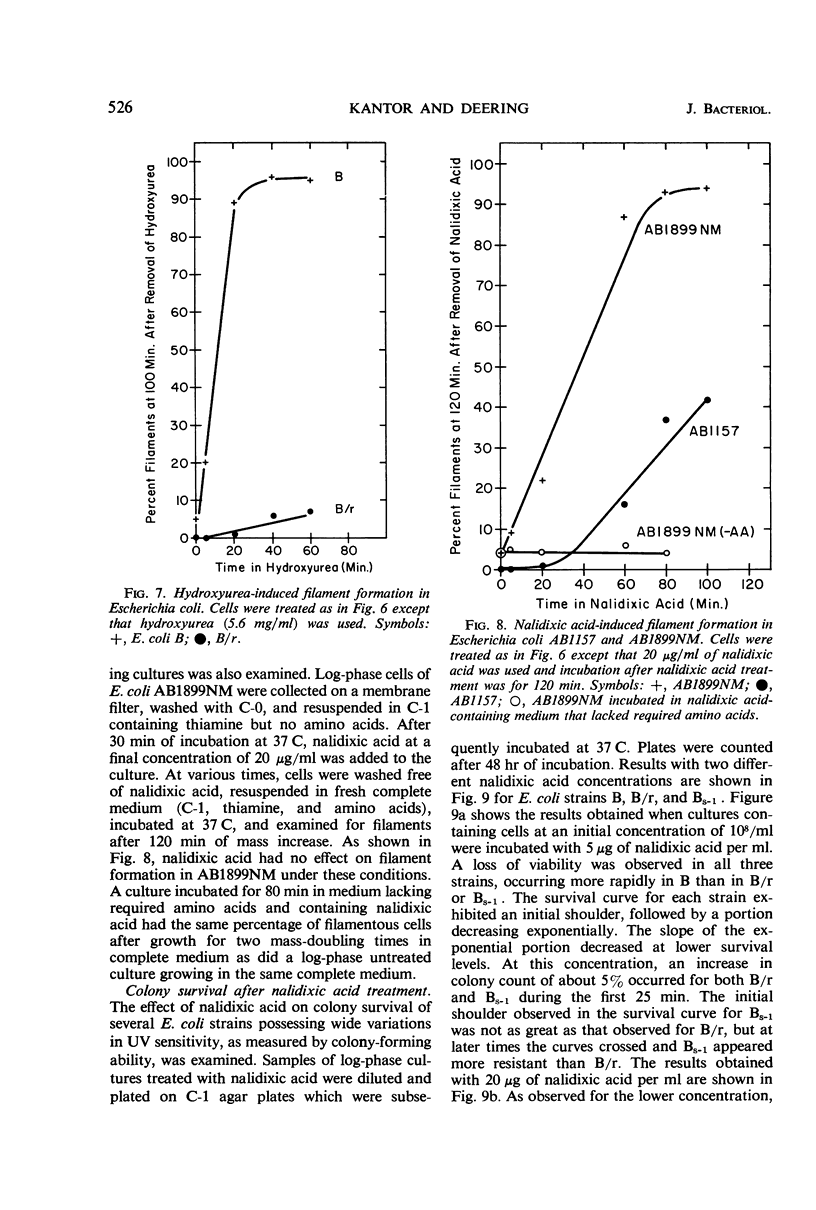
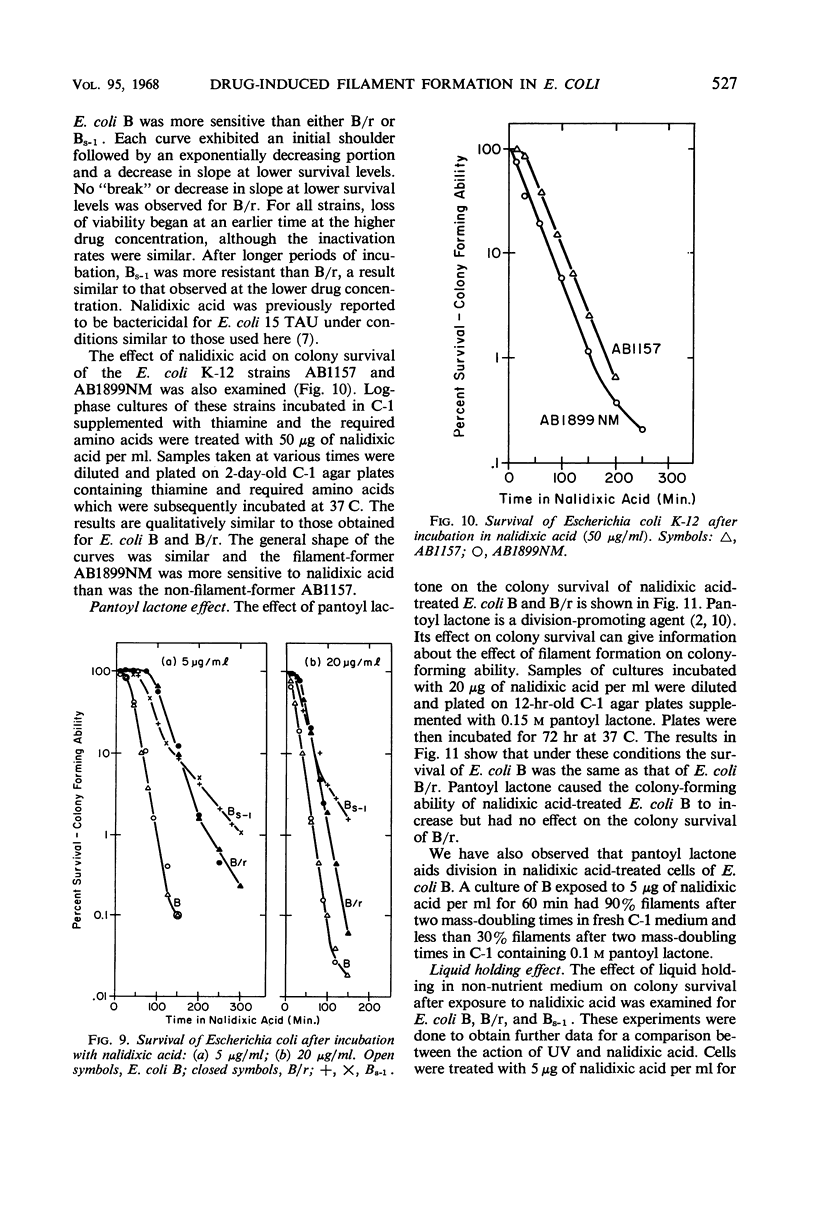
Short periods of incubation in medium containing nalidixic acid or hydroxyurea, followed by a return to normal growth conditions, induced filament formation in Escherichia coli B (fil+) and AB1899NM (lon−) but not in B/r (fil−) and AB1157 (lon+). These drugs reversibly stopped deoxyribonucleic acid (DNA) synthesis with little or no effect on ribonucleic acid (RNA) synthesis or mass increase. The initial imbalance caused by incubation in these drugs was the same for B and B/r as was macromolecular synthesis following a return to normal growth conditions. DNA degradation caused by nalidixic acid was measured and found to be the same for B and B/r. Hydroxyurea caused no DNA degradation in these two strains. Survival curves as determined under various conditions by colony formation suggested that the property of filament formation was responsible for the extrasensitivity of fil+ and lon− strains to either nalidixic acid or hydroxyurea. E. coli B was more sensitive to either drug than was B/r or Bs-1. Pantoyl lactone or liquid holding treatment aided division and colony formation of nalidixic acid-treated B but had no effect on B/r. Likewise, the filament-former AB1899NM was more sensitive to nalidixic acid than was the non-filament-former AB1157. The sensitivity of B/r and Bs-1 to nalidixic acid was nearly the same except at longer times in nalidixic acid, when Bs-1 appeared more resistant. Even though nalidixic acid, hydroxyurea, and ultraviolet light may produce quite different molecular alterations in E. coli, they all cause a metabolic imbalance resulting in a lowered ratio of DNA to RNA and protein. We propose that it is this imbalance per se rather than any specific primary chemical or photochemical alterations which leads to filament formation by some genetically susceptible bacterial strains such as lon− and fil+.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADLER H. I., HARDIGREE A. A. ANALYSIS OF A GENE CONTROLLING CELL DIVISION AND SENSITIVITY TO RADIATION IN ESCHERICHIA COLI. J Bacteriol. 1964 Mar;87:720–726. doi: 10.1128/jb.87.3.720-726.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook T. M., Deitz W. H., Goss W. A. Mechanism of action of nalidixic acid on Escherichia coli. IV. Effects on the stability of cellular constituents. J Bacteriol. 1966 Feb;91(2):774–779. doi: 10.1128/jb.91.2.774-779.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings D. J., Mondale L. Thymineless death in Escherichia coli: strain specificity. J Bacteriol. 1967 Jun;93(6):1917–1924. doi: 10.1128/jb.93.6.1917-1924.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEERING R. A. Studies on division inhibition and filament formation of Escherichia coli by ultraviolet light. J Bacteriol. 1958 Aug;76(2):123–130. doi: 10.1128/jb.76.2.123-130.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitz W. H., Cook T. M., Goss W. A. Mechanism of action of nalidixic acid on Escherichia coli. 3. Conditions required for lethality. J Bacteriol. 1966 Feb;91(2):768–773. doi: 10.1128/jb.91.2.768-773.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERRERA M. Induction of filamentous forms in ultraviolet-irradiated E. coli B. Br J Radiol. 1954 Jan;27(313):76–80. doi: 10.1259/0007-1285-27-313-76. [DOI] [PubMed] [Google Scholar]
- GOSS W. A., DEITZ W. H., COOK T. M. MECHANISM OF ACTION OF NALIDIXIC ACID ON ESCHERICHIA COLI.II. INHIBITION OF DEOXYRIBONUCLEIC ACID SYNTHESIS. J Bacteriol. 1965 Apr;89:1068–1074. doi: 10.1128/jb.89.4.1068-1074.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRULA E. A., GRULA M. M. Cell division in a species of Erwinia III. Reversal of inhibition of cell division caused by D-amino acids, penicillin, and ultraviolet light. J Bacteriol. 1962 May;83:981–988. doi: 10.1128/jb.83.5.981-988.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWARD-FLANDERS P., SIMSON E., THERIOT L. A LOCUS THAT CONTROLS FILAMENT FORMATION AND SENSITIVITY TO RADIATION IN ESCHERICHIA COLI K-12. Genetics. 1964 Feb;49:237–246. doi: 10.1093/genetics/49.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harm W. The role of host-cell repair in liquid-holding recovery of u.v.-irradiated Escherichia coli. Photochem Photobiol. 1966 Sep;5(9):747–760. doi: 10.1111/j.1751-1097.1966.tb05824.x. [DOI] [PubMed] [Google Scholar]
- Kantor G. J., Deering R. A. Recovery of division ability in ultraviolet-irradiated Escherichia coli induced by photoreactivation, photoprotection, and liquid holding treatment. J Bacteriol. 1967 Dec;94(6):1946–1950. doi: 10.1128/jb.94.6.1946-1950.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantor G. J., Deering R. A. Ultraviolet radiation studies of filamentous Escherichia coli B. J Bacteriol. 1966 Oct;92(4):1062–1069. doi: 10.1128/jb.92.4.1062-1069.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R. B., Aldous E. RECOVERY FROM ULTRAVIOLET IRRADIATION IN ESCHERICHIA COLI. J Bacteriol. 1949 Mar;57(3):363–375. doi: 10.1128/jb.57.3.363-375.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz H. S., Garro A. J., Levy J. A., Carr H. S. Studies with hydroxyurea. I. The reversible inhibition of bacterial DNA synthesis and the effect of hydroxyurea on the bactericidal action of streptomycin. Biochim Biophys Acta. 1966 Mar 21;114(3):501–515. [PubMed] [Google Scholar]
- SETLOW R. B., SWENSON P. A., CARRIER W. L. THYMINE DIMERS AND INHIBITION OF DNA SYNTHESIS BY ULTRAVIOLET IRRADIATION OF CELLS. Science. 1963 Dec 13;142(3598):1464–1466. doi: 10.1126/science.142.3598.1464. [DOI] [PubMed] [Google Scholar]
- Swenson P. A., Setlow R. B. Effects of ultraviolet radiation on macromolecular synthesis in Escherichia coli. J Mol Biol. 1966 Jan;15(1):201–219. doi: 10.1016/s0022-2836(66)80221-8. [DOI] [PubMed] [Google Scholar]
- VAN DE PUTTE P., WESTENBROEK C., ROERSCH A. THE RELATIONSHIP BETWEEN GENE-CONTROLLED RADIATION RESISTANCE AND FILAMENT FORMATION IN ESCHERICHIA COLI. Biochim Biophys Acta. 1963 Oct 15;76:247–256. doi: 10.1016/0006-3002(63)90037-4. [DOI] [PubMed] [Google Scholar]
- Witkin E. M. Genetics of Resistance to Radiation in ESCHERICHIA COLI. Genetics. 1947 May;32(3):221–248. doi: 10.1093/genetics/32.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]


