Abstract
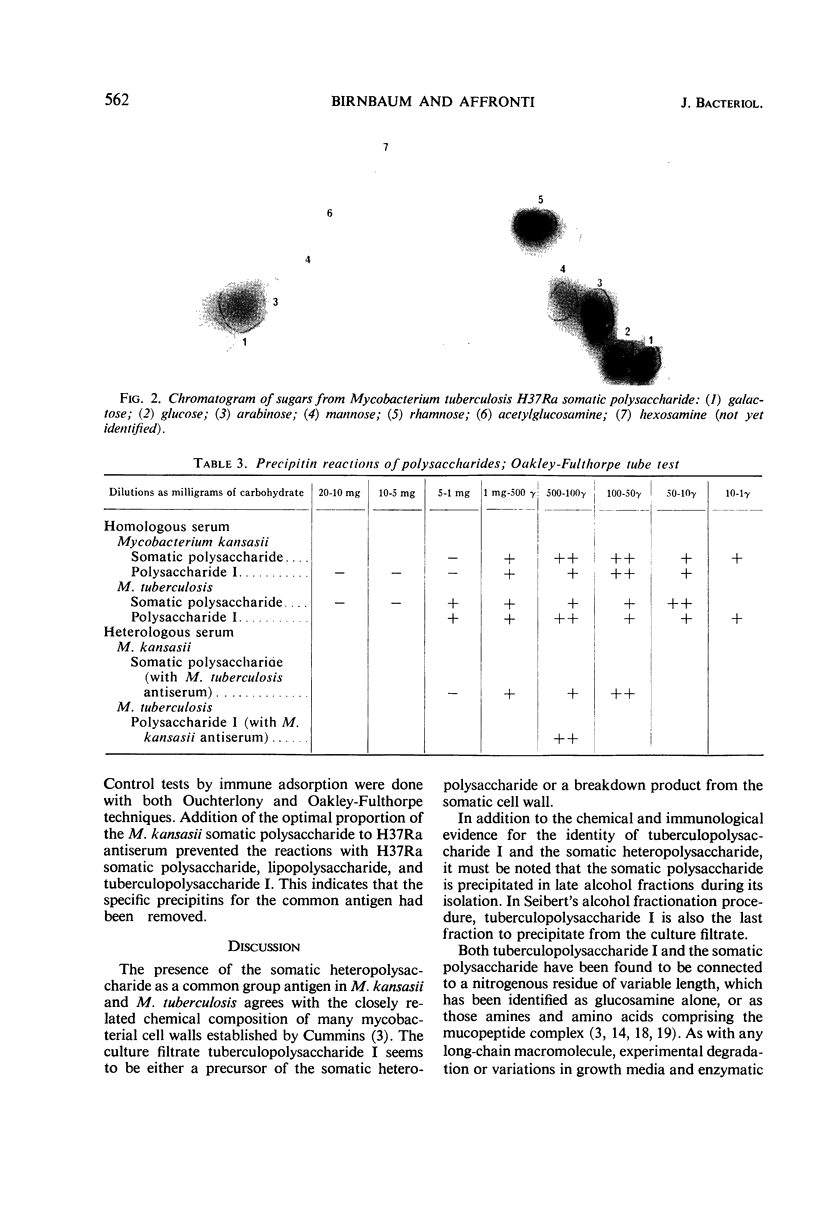
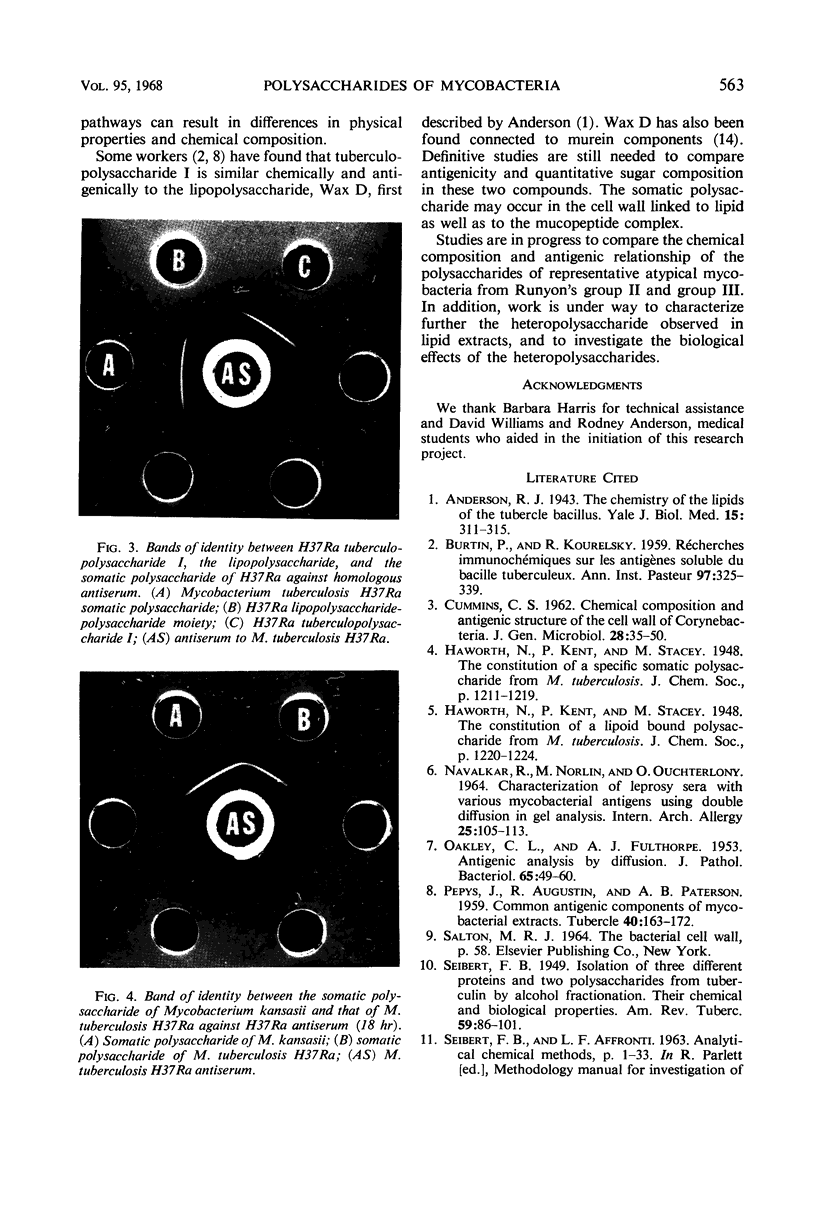
The identity of a heteropolysaccharide from cell walls of Mycobacterium tuberculosis H37Ra with Seibert's tuberculopolysaccharide I was demonstrated by thin-layer chromatography, chemical analysis, and antigenic tests. The polysaccharide of M. kansasii was shown to be identical with that of M. tuberculosis. Defatted cells were disintegrated by ultrasonic treatment in the presence of glass beads; cell walls were obtained by differential ultracentrifugation. Ethyl alcohol-precipitated carbohydrate extracts were analyzed for protein and nucleic acid; these impurities were removed. Tuberculopolysaccharide I from the mycobacterial culture filtrate is probably derived from a lipopolysaccharide of the cell wall, which is partially removed by chloroform in the intact state. Alkaline extraction releases additional polysaccharide, in varying degrees of association with cell wall murein.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTIN P. [Immunochemical research on the soluble antigens of the tubercle bacillus. II. Immunochemical study of 2 polysaccharides present in the filtrates of culture of the tubercle bacillus]. Ann Inst Pasteur (Paris) 1959 Sep;97:325–339. [PubMed] [Google Scholar]
- CUMMINS C. S. Chemical composition and antigenic structure of cell walls of Corynebacterium, Mycobacterium, Nocardia, Actinomyces and Arthrobacter. J Gen Microbiol. 1962 Apr;28:35–50. doi: 10.1099/00221287-28-1-35. [DOI] [PubMed] [Google Scholar]
- DEWIJS H., JOLLES P. CELL WALLS OF THREE STRAINS OF MYCOBACTERIA (MYCOBACTERIUM PHLEI, MYCOBACTERIUM FORTUITUM AND MYCOBACTERIUM KANSASII). PREPARATION, ANALYSIS AND DIGESTION BY LYSOZYMES OF DIFFERENT ORIGINS. Biochim Biophys Acta. 1964 Nov 1;83:326–332. [PubMed] [Google Scholar]
- NAVALKAR R. G., NORLIN M., OUCHTERLONY O. CHARACTERIZATION OF LEPROSY SERA WITH VARIOUS MYCOBACTERIAL ANTIGENS USING DOUBLE DIFFUSION-IN-GEL ANALYSIS. A PRELIMINARY REPORT. Int Arch Allergy Appl Immunol. 1964;25:105–113. doi: 10.1159/000229513. [DOI] [PubMed] [Google Scholar]
- OAKLEY C. L., FULTHORPE A. J. Antigenic analysis by diffusion. J Pathol Bacteriol. 1953 Jan;65(1):49–60. doi: 10.1002/path.1700650105. [DOI] [PubMed] [Google Scholar]
- PEPYS J., AUGUSTIN R., PATERSON A. B. Common antigenic components of mycobacterial extracts. Tubercle. 1959 Jun;40:163–172. doi: 10.1016/s0041-3879(59)80035-0. [DOI] [PubMed] [Google Scholar]
- STACEY M. Mycobacterium tuberculosis polysaccharides. Bibl Tuberc. 1955;9:7–17. [PubMed] [Google Scholar]
- TANAKA A., KITAGAWA M. FRACTIONATION AND CHARACTERIZATION OF WAX D, A MACROMOLECULAR PEPTIDOGLYCOLIPID OF MYCOBACTERIUM TUBERCULOSIS. I. BIOCHEMICAL INVESTIGATIONS OF WAX D OF HUMAN STRAIN H37RA. Biochim Biophys Acta. 1965 Feb 1;98:182–193. [PubMed] [Google Scholar]
- VOMHOF D. W., TUCKER T. C. THE SEPARATION OF SIMPLE SUGARS BY CELLULOSE THIN-LAYER CHROMATOGRAPHY. J Chromatogr. 1965 Feb;17:300–306. doi: 10.1016/s0021-9673(00)99872-8. [DOI] [PubMed] [Google Scholar]
- YAMAMURA Y., OKADA Y., NAGAMATSU S., IMADA T. THE ISOLATION FROM TUBERCULIN OF TWO DIFFERENT POLYSACCHARIDES HAVING ANAPHYLACTIC ACTIVITY. THEIR CHEMICAL AND BIOLOGIC PROPERTIES. Am Rev Respir Dis. 1965 Jun;91:839–845. doi: 10.1164/arrd.1965.91.6.839. [DOI] [PubMed] [Google Scholar]





