Abstract
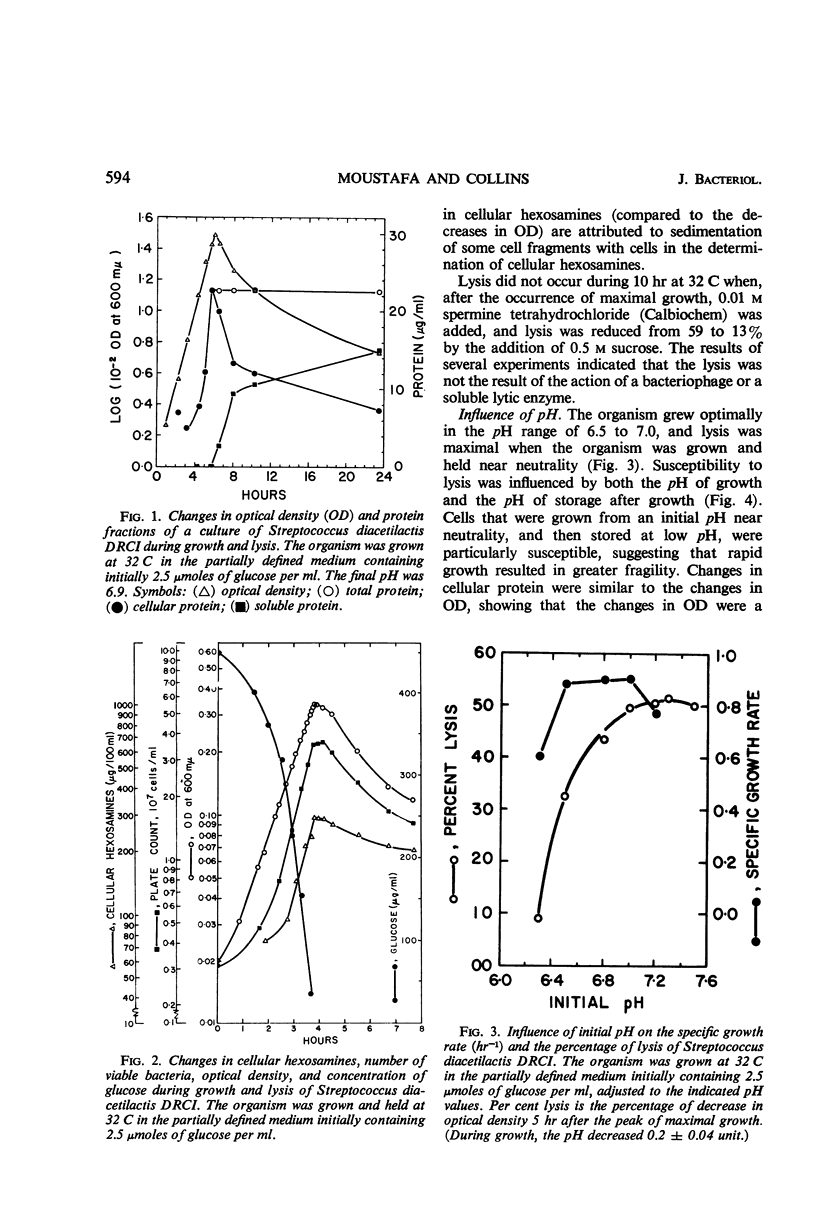
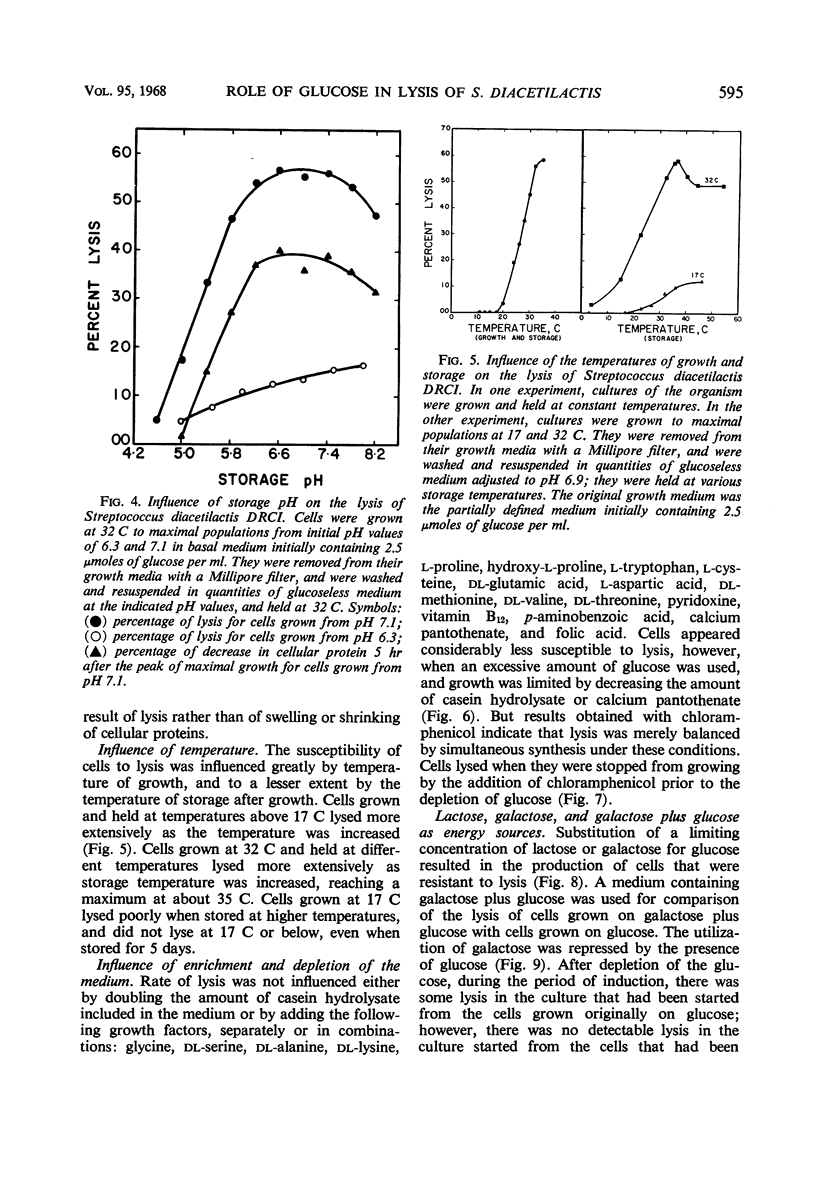
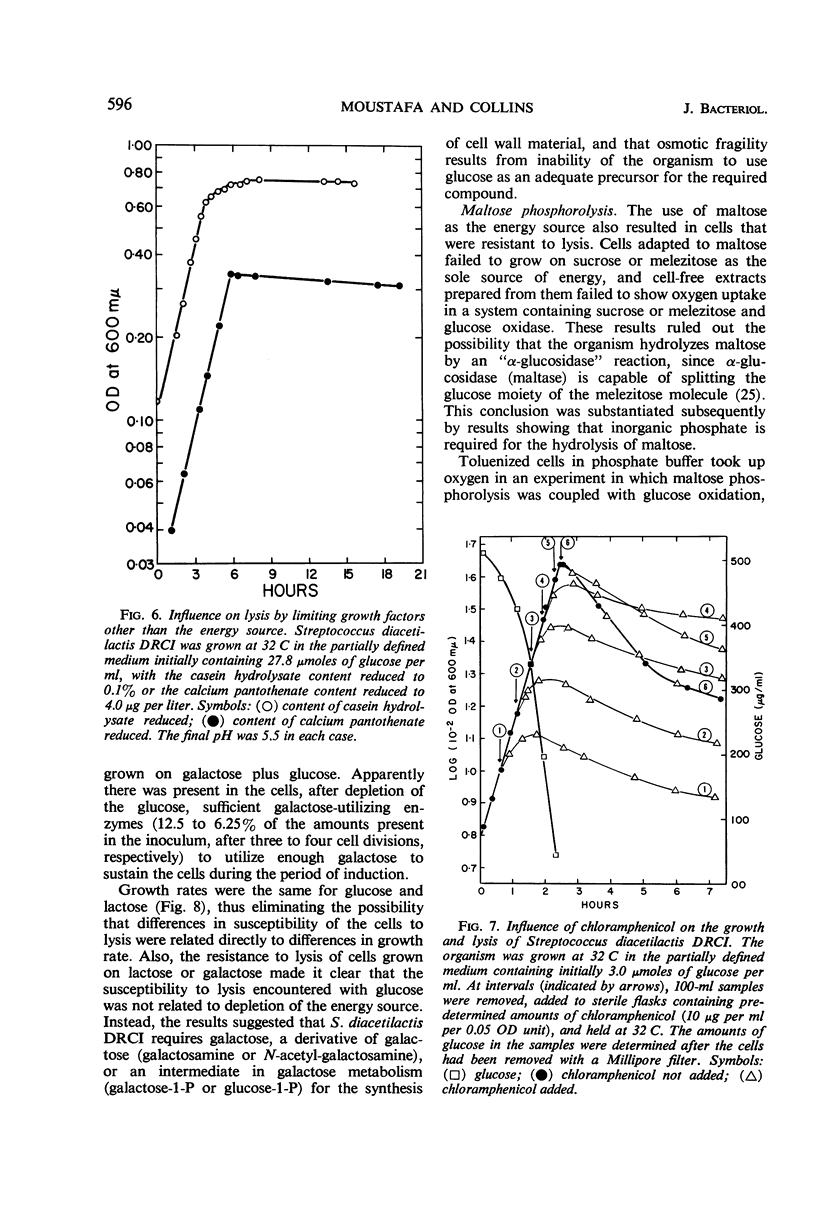
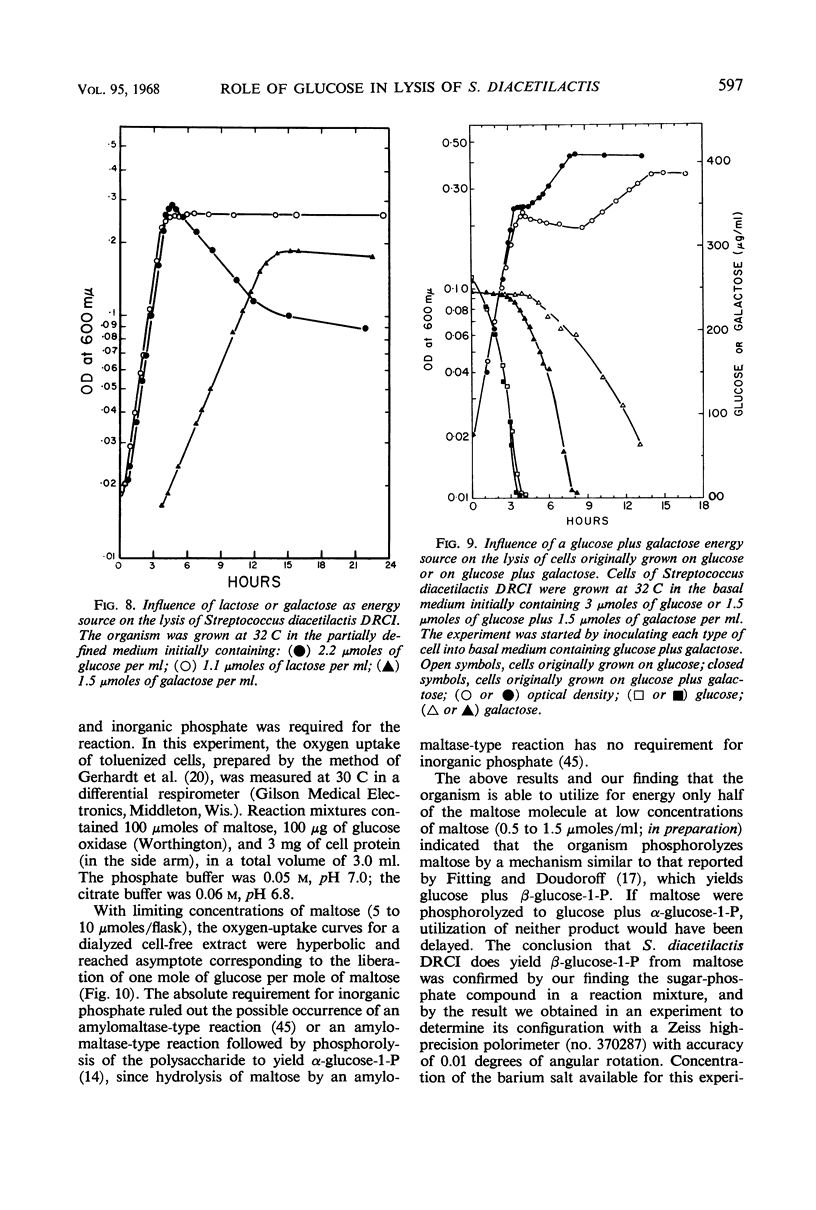
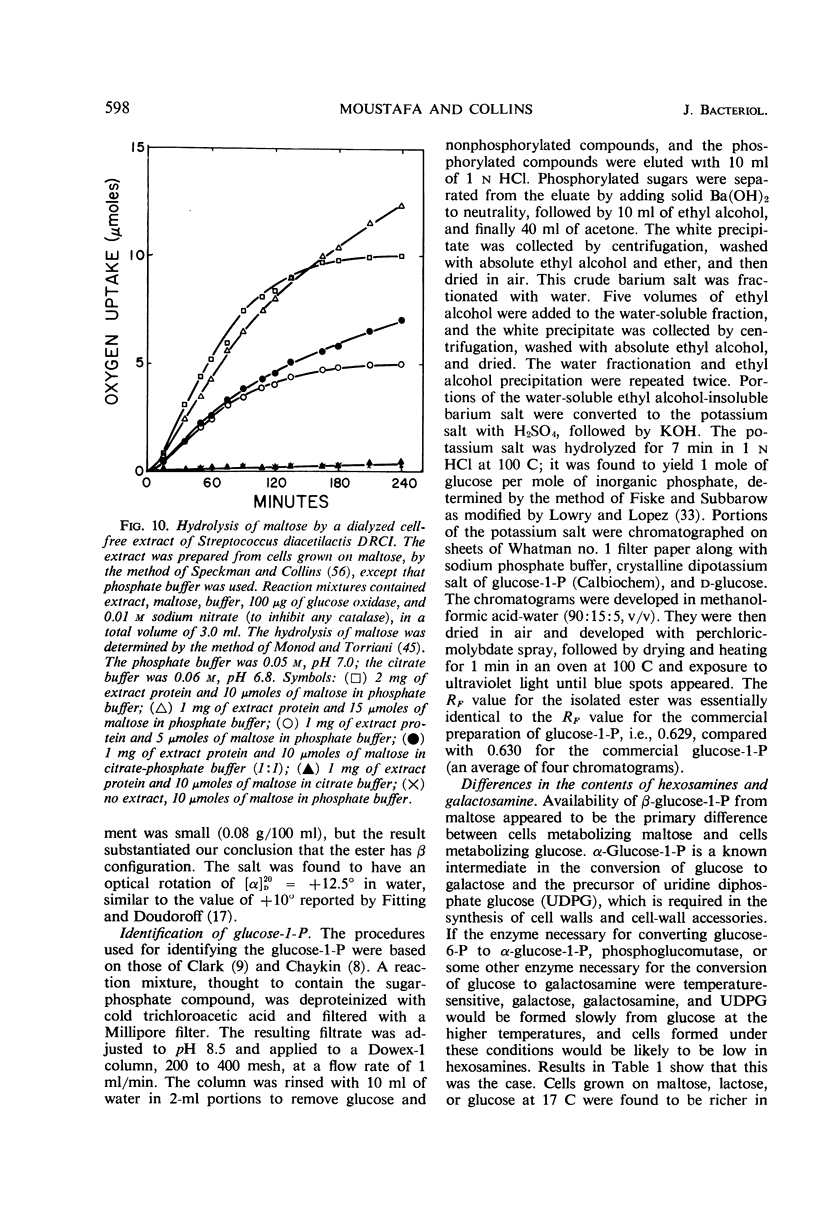
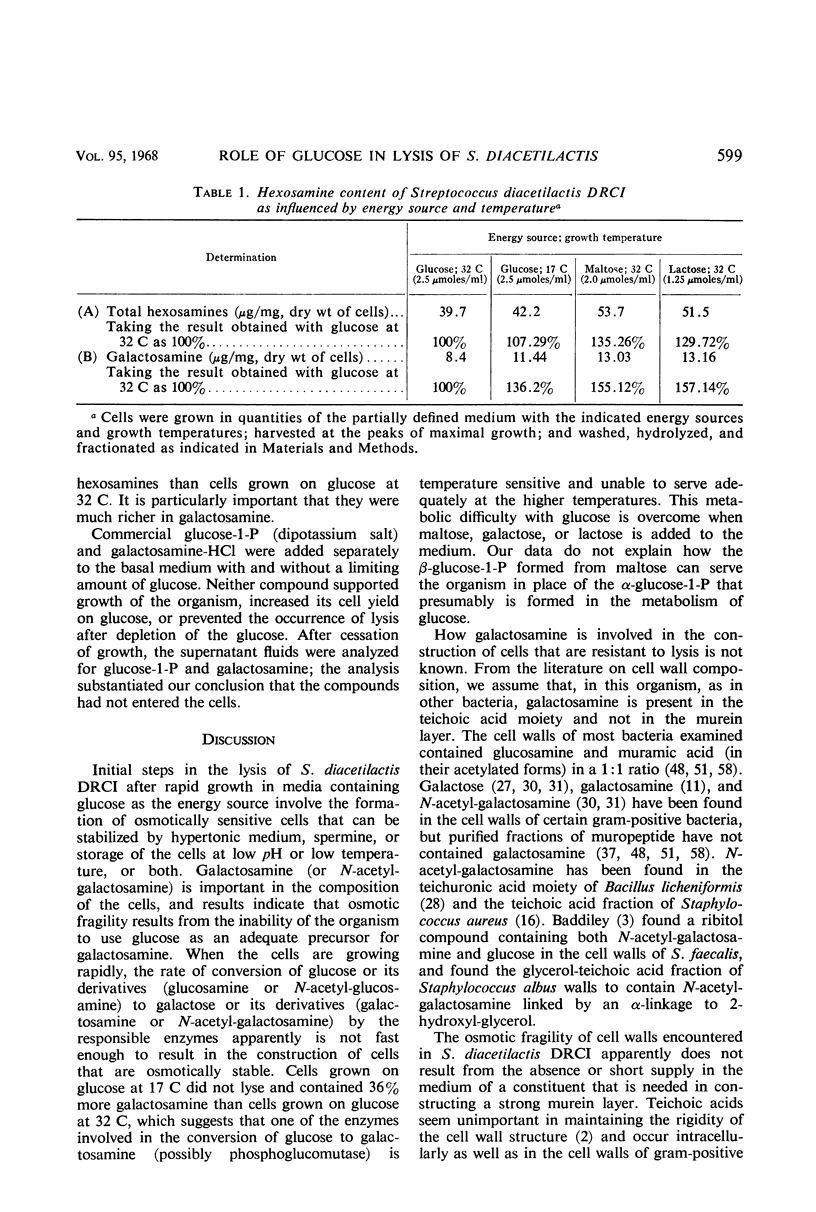
Cells of Streptococcus diacetilactis DRCI grown at 32 C in media containing glucose as the energy source were osmotically fragile and began to lyse immediately after growth was stopped (by the action of chloramphenicol or the exhaustion of glucose), unless they were then stabilized by hypertonic medium or spermine or by storage at low pH or low temperature, or both. In media containing excess glucose, with growth limited by exhaustion of some nutrient other than the energy source, the appearance of lysis was masked by the occurrence of a balance between lysis and synthesis. The osmotic fragility apparently resulted from inability of the organism to use glucose as an adequate precursor of galactosamine, and conditions of temperature and pH that promoted rapid growth on glucose were particularly conducive to the formation of cells that lysed readily. Growing the organism in media containing galactose, lactose, maltose, or glucose (at 17 C) as energy source resulted in the formation of cells that were resistant to lysis and richer in galactosamine than unstable cells formed on glucose at 32 C. The results indicate that the organism phosphorolyzes maltose to glucose plus β-glucose-1-phosphate, and suggest that it can use the β-glucose-1-phosphate in place of α-glucose-1-phosphate in the formation of cell materials.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARCHIBALD A. R., ARMSTRONG J. J., BADDILEY J., HAY J. B. Teichoic acids and the structure of bacterial walls. Nature. 1961 Aug 5;191:570–572. doi: 10.1038/191570a0. [DOI] [PubMed] [Google Scholar]
- Allison D. J., Smith Q. T. Application of a rapid and reliable method for determination of hexosamine in the skin of estrogen-treated rats. Anal Biochem. 1965 Dec;13(3):510–517. doi: 10.1016/0003-2697(65)90345-3. [DOI] [PubMed] [Google Scholar]
- BADDILEY J., DAVISON A. L. The occurrence and location of teichoic acids in lactobacilli. J Gen Microbiol. 1961 Feb;24:295–299. doi: 10.1099/00221287-24-2-295. [DOI] [PubMed] [Google Scholar]
- BAUMAN N., DAVIS B. D. Selection of auxotrophic bacterial mutants through diaminopimelic acid or thymine deprival. Science. 1957 Jul 26;126(3265):170–170. doi: 10.1126/science.126.3265.170. [DOI] [PubMed] [Google Scholar]
- BOAS N. F. Method for the determination of hexosamines in tissues. J Biol Chem. 1953 Oct;204(2):553–563. [PubMed] [Google Scholar]
- CHATTERJEE A. N., PARK J. T. BIOSYNTHESIS OF CELL WALL MUCOPEPTIDE BY A PARTICULATE FRACTION FROM STAPHYLOCOCCUS AUREUS. Proc Natl Acad Sci U S A. 1964 Jan;51:9–16. doi: 10.1073/pnas.51.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUMMINS C. S., HARRIS H. The chemical composition of the cell wall in some gram-positive bacteria and its possible value as a taxonomic character. J Gen Microbiol. 1956 Jul;14(3):583–600. doi: 10.1099/00221287-14-3-583. [DOI] [PubMed] [Google Scholar]
- DIENA B. B., WALLACE R., GREENBERG L. THE PRODUCTION AND PROPERTIES OF SALMONELLA TYPHI SPHEROPLASTS. Can J Microbiol. 1964 Aug;10:543–549. doi: 10.1139/m64-068. [DOI] [PubMed] [Google Scholar]
- Davie J. M., Brock T. D. Effect of teichoic acid on resistance to the membrane-lytic agent of Streptococcus zymogenes. J Bacteriol. 1966 Dec;92(6):1623–1631. doi: 10.1128/jb.92.6.1623-1631.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECKER R. E., LOCKHART W. R. Specific effect of limiting nutrient on physiological events during culture growth. J Bacteriol. 1961 Oct;82:511–516. doi: 10.1128/jb.82.4.511-516.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellwood D. C., Kelemen M. V., Baddiley J. The glycerol teichoic acid from the walls of Staphylococcus albus N.T.C.C. 7944. Biochem J. 1963 Feb;86(2):213–225. doi: 10.1042/bj0860213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FITTING C., DOUDOROFF M. Phosphorolysis of maltose by enzyme preparations from Neisseria meningitidis. J Biol Chem. 1952 Nov;199(1):153–163. [PubMed] [Google Scholar]
- FUKASAWA T., NIKAIDO H. Formation of protoplasts' in mutant strains of Salmonella induced by galactose. Nature. 1959 Apr 18;183(4668):1131–1132. doi: 10.1038/1831131a0. [DOI] [PubMed] [Google Scholar]
- FUKASAWA T., NIKAIDO H. Galactose-sensitive mutants of Salmonella. II. Bacteriolysis induced by galactose. Biochim Biophys Acta. 1961 Apr 15;48:470–483. doi: 10.1016/0006-3002(61)90045-2. [DOI] [PubMed] [Google Scholar]
- GERHARDT P., MAC GREGOR D. R., MARR A. G., OLSEN C. B., WILSON J. B. The metabolism of brucellae: the role of cellular permeability. J Bacteriol. 1953 May;65(5):581–586. doi: 10.1128/jb.65.5.581-586.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HADJIPETROU L. P., STOUTHAMER A. H. AUTOLYSIS OF BACILLUS SUBTILIS BY GLUCOSE DEPLETION. Antonie Van Leeuwenhoek. 1963;29:256–260. doi: 10.1007/BF02046066. [DOI] [PubMed] [Google Scholar]
- HARVEY R. J., COLLINS E. B. ROLES OF CITRATE AND ACETOIN IN THE METABOLISM OF STREPTOCOCCUS DIACETILACTIS. J Bacteriol. 1963 Dec;86:1301–1307. doi: 10.1128/jb.86.6.1301-1307.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAUKENES G., ELLWOOD D. C., BADDILEY J., OEDING P. Serological cross-reactivity between polysaccharide A and teichoic acid of Staphylococcus aureus. Biochim Biophys Acta. 1961 Oct 28;53:425–426. doi: 10.1016/0006-3002(61)90461-9. [DOI] [PubMed] [Google Scholar]
- HAY J. B., WICKEN A. J., BADDILEY J. The location of intracellular teichoic acids. Biochim Biophys Acta. 1963 Apr 2;71:188–190. doi: 10.1016/0006-3002(63)90999-5. [DOI] [PubMed] [Google Scholar]
- HOLDSWORTH E. S. The nature of the cell wall of corynebacterium diphtheriae. Isolation of an oligosaccharide. Biochim Biophys Acta. 1952;9(1):19–28. doi: 10.1016/0006-3002(52)90115-7. [DOI] [PubMed] [Google Scholar]
- Hoffman H., Valdina J., Frank M. E. Effects of high incubation temperature upon the cell wall of Escherichia coli. J Bacteriol. 1966 Apr;91(4):1635–1637. doi: 10.1128/jb.91.4.1635-1637.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANCZURA E., PERKINS H. R., ROGERS H. J. Teichuronic acid: a mucopolysaccharide present in wall preparations from vegetative cells of Bacillus subtilis. Biochem J. 1961 Jul;80:82–93. doi: 10.1042/bj0800082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KNOX K. W., BRANDSEN J. The isolation of components from the cell wall of Lactobacillus casei. Biochem J. 1962 Oct;85:15–23. doi: 10.1042/bj0850015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KNOX K. W., HALL E. A. THE ISOLATION OF OLIGOSACCHARIDES FROM THE CELL-WALL POLYSACCHARIDE OF LACTOBACILLUS CASEI, SEROLOGICAL GROUP C. Biochem J. 1965 Mar;94:525–533. doi: 10.1042/bj0940525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lark C., Schichtel R. COMPARISON OF SPHEROPLAST INDUCTION IN ALCALIGENES FAECALIS BY THREE DIFFERENT AGENTS. J Bacteriol. 1962 Dec;84(6):1241–1244. doi: 10.1128/jb.84.6.1241-1244.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEADOW P., HOARE D. S., WORK E. Interrelationships between lysine and alpha epsilon-diaminopimelic acid and their derivatives and analogues in mutants of Escherichia coli. Biochem J. 1957 Jun;66(2):270–282. doi: 10.1042/bj0660270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITCHELL P., MOYLE J. Autolytic release and osmotic properties of protoplasts from Staphylococcus aureus. J Gen Microbiol. 1957 Feb;16(1):184–194. doi: 10.1099/00221287-16-1-184. [DOI] [PubMed] [Google Scholar]
- MONOD J., TORRIANI A. M. De l'amylomaltase d'Escherichia coli. Ann Inst Pasteur (Paris) 1950 Jan;78(1):65–77. [PubMed] [Google Scholar]
- Maculla E. S., Cowles P. B. The Use of Glycine in the Disruption of Bacterial Cells. Science. 1948 Apr 9;107(2780):376–377. doi: 10.1126/science.107.2780.376. [DOI] [PubMed] [Google Scholar]
- Martin H. H. Biochemistry of bacterial cell walls. Annu Rev Biochem. 1966;35:457–484. doi: 10.1146/annurev.bi.35.070166.002325. [DOI] [PubMed] [Google Scholar]
- McQUILLEN K. Bacterial protoplasts: effects of diaminopimelic acid deprival and penicillin addition compared in Escherichia coli. Biochim Biophys Acta. 1958 Feb;27(2):410–411. doi: 10.1016/0006-3002(58)90351-2. [DOI] [PubMed] [Google Scholar]
- McQUILLEN K. Lysis resulting from metabolic disturbance. J Gen Microbiol. 1958 Apr;18(2):498–512. doi: 10.1099/00221287-18-2-498. [DOI] [PubMed] [Google Scholar]
- Meadow P. M., Anderson J. S., Strominger J. L. Enzymatic polymerization of UDP-acetylmuramyl.L-ala.D-glu.L-lys.D-ala.D-ala and UDP-acetylglucosamine by a particulate enzyme from Staphylococcus aureus and its inhibition by antibiotics. Biochem Biophys Res Commun. 1964;14:382–387. doi: 10.1016/s0006-291x(64)80014-0. [DOI] [PubMed] [Google Scholar]
- NIKAIDO H. Galactose-sensitive mutants of Salmonella. I. Metabolism of galactose. Biochim Biophys Acta. 1961 Apr 15;48:460–469. doi: 10.1016/0006-3002(61)90044-0. [DOI] [PubMed] [Google Scholar]
- PERKINS H. R. Chemical structure and biosynthesis of bacterial cell walls. Bacteriol Rev. 1963 Mar;27:18–55. doi: 10.1128/br.27.1.18-55.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RHULAND L. E. Role of alpha, epsilon-diaminopimelic acid in the cellular integrity of Escherichia coli. J Bacteriol. 1957 Jun;73(6):778–783. doi: 10.1128/jb.73.6.778-783.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALTON M. R. The lysis of micro-organisms by lysozyme and related enzymes. J Gen Microbiol. 1958 Apr;18(2):481–490. doi: 10.1099/00221287-18-2-481. [DOI] [PubMed] [Google Scholar]
- SHOCKMAN G. D., KOLB J. J., TOENNIES G. Relations between bacterial cell wall synthesis, growth phase, and autolysis. J Biol Chem. 1958 Feb;230(2):961–977. [PubMed] [Google Scholar]
- Sempere J. M., Gancedo C., Asensio C. Determination of galactosamine and N-acetylgalactosamine in the presence of other hexosamines with galactose oxidase. Anal Biochem. 1965 Sep;12(3):509–515. doi: 10.1016/0003-2697(65)90217-4. [DOI] [PubMed] [Google Scholar]
- Shockman G. D., Conover M. J., Kolb J. J., Phillips P. M., Riley L. S., Toennies G. LYSIS OF STREPTOCOCCUS FAECALIS. J Bacteriol. 1961 Jan;81(1):36–43. doi: 10.1128/jb.81.1.36-43.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speckman R. A., Collins E. B. Diacetyl biosynthesis in Streptococcus diacetilactis and Leuconostoc citrovorum. J Bacteriol. 1968 Jan;95(1):174–180. doi: 10.1128/jb.95.1.174-180.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TUBBS P. K. Effects of inhibitors on mitochondrial D-alpha-hydroxy acid dehydrogenase. Biochem J. 1962 Jan;82:36–42. doi: 10.1042/bj0820036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toennies G., Das D. N., Feng F. Pantothenate and coenzyme A in bacterial growth. J Bacteriol. 1966 Sep;92(3):707–713. doi: 10.1128/jb.92.3.707-713.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIDEL W., PELZER H. BAGSHAPED MACROMOLECULES--A NEW OUTLOOK ON BACTERIAL CELL WALLS. Adv Enzymol Relat Areas Mol Biol. 1964;26:193–232. doi: 10.1002/9780470122716.ch5. [DOI] [PubMed] [Google Scholar]
- Yarmolinsky M. B., Wiesmeyer H., Kalckar H. M., Jordan E. HEREDITARY DEFECTS IN GALACTOSE METABOLISM IN ESCHERICHIA COLI MUTANTS, II. GALACTOSE-INDUCED SENSITIVITY. Proc Natl Acad Sci U S A. 1959 Dec;45(12):1786–1791. doi: 10.1073/pnas.45.12.1786. [DOI] [PMC free article] [PubMed] [Google Scholar]


