Abstract
Three covalent attachments anchor heterotrimeric G proteins to cellular membranes: the α subunits are myristoylated and/or palmitoylated, whereas the γ chain is prenylated. Despite the essential role of these modifications in membrane attachment, it is not clear how they cooperate to specify G protein localization at the plasma membrane, where the G protein relays signals from cell surface receptors to intracellular effector molecules. To explore this question, we studied the effects of mutations that prevent myristoylation and/or palmitoylation of an epitope-labeled α subunit, αz. Wild-type αz (αz-WT) localizes specifically at the plasma membrane. A mutant that incorporates only myristate is mistargeted to intracellular membranes, in addition to the plasma membrane, but transduces hormonal signals as well as does αz-WT. Removal of the myristoylation site produced a mutant αz that is located in the cytosol, is not efficiently palmitoylated, and does not relay the hormonal signal. Coexpression of βγ with this myristoylation defective mutant transfers it to the plasma membrane, promotes its palmitoylation, and enables it to transmit hormonal signals. Pulse-chase experiments show that the palmitate attached to this myristoylation-defective mutant turns over much more rapidly than does palmitate on αz-WT, and that the rate of turnover is further accelerated by receptor activation. In contrast, receptor activation does not increase the slow rate of palmitate turnover on αz-WT. Together these results suggest that myristate and βγ promote stable association with membranes not only by providing hydrophobicity, but also by stabilizing attachment of palmitate. Moreover, palmitoylation confers on αz specific localization at the plasma membrane.
INTRODUCTION
Heterotrimeric G proteins, composed of α, β, and γ polypeptides, relay signals from cell surface receptors to intracellular effector enzymes and ion channels (Neer, 1995). Ligand-bound receptor catalyzes exchange of GTP for GDP on the α subunit, followed by dissociation of αGTP from the dimeric βγ subunit. Each of these subunits can regulate effector molecules. Hydrolysis of bound GTP turns off signaling by the α subunit and allows αGDP to reassociate with and inactivate free βγ. Lacking transmembrane segments, the G protein trimer must nonetheless localize at the inner face of the plasma membrane to transduce signals received from receptors for extracellular stimuli.
Here, we report experiments designed to elucidate the mechanisms that target G protein α subunits to the plasma membrane. Our experiments extend work in many laboratories that have established the role of covalently attached lipids in promoting the association of both α and βγ subunits with membranes (reviewed in Casey, 1995; Wedegaertner et al., 1995; Resh, 1996; Bhatnagar and Gordon, 1997; Mumby, 1997). Mutational removal of sites of lipid attachment greatly reduces membrane avidity of G protein subunits (Jones et al., 1990; Mumby et al., 1990, 1994; Muntz et al., 1992; Wedegaertner et al., 1993; Degtyarev et al., 1994; Hallak et al., 1994; McCallum et al., 1995; Wilson and Bourne, 1995). Isoprenyl groups attached to γ chains allow βγ subunits to associate with membranes. Members of the αi family (αi1, αi2, αi3, αz, and αo) are myristoylated on the N-terminal glycine residue and palmitoylated on the adjacent cysteine (Wedegaertner et al., 1995). Several other α subunits are palmitoylated near their N termini, but are not myristoylated (Wedegaertner et al., 1995).
Myristate and palmitate differ in hydrophobicity and in the relative stability of their covalent links to proteins. Although myristate is linked to proteins by a stable amide bond, myristoylated proteins associate reversibly with membranes and dissociate from them rather easily (Peitzsch and McLaughlin, 1993; Bigay et al., 1994; Boman and Kahn, 1995; McLaughlin and Aderem, 1995), at least in part because this 14-carbon fatty acid provides insufficient hydrophobicity to anchor a protein tightly to membranes (Peitzsch and McLaughlin, 1993). The greater hydrophobicity of palmitate’s 16 carbons promotes tighter association with membranes (Shahinian and Silvius, 1995), but its bond to cysteine can be cleaved by cellular thioesterases (Camp and Hofmann, 1993; Mumby, 1997), providing a potential handle for regulating association of a protein with membranes (Milligan et al., 1995; Wedegaertner et al., 1995; Mumby, 1997). Activated αs, for example, is more rapidly depalmitoylated (Degtyarev et al., 1993; Mumby, et al., 1994; Wedegaertner and Bourne, 1994) and can translocate to the cytosol (Wedegaertner et al., 1996).
In addition to providing hydrophobicity, lipid modifications increase the affinities of G protein subunits for each other and for effector molecules (Wedegaertner et al., 1995). Myristate on αi proteins (Jones et al., 1990; Linder et al., 1991) and palmitate on αs (Iiri et al., 1996) increase the affinity for binding βγ and nonmyristoylated αi fails to inhibit adenylyl cyclase (Taussig et al., 1993). Similarly, prenylation of γ increases the affinity of βγ for α (Iñiguez-Lluhi et al., 1992) and is necessary for regulation of adenylyl cyclase (Iñiguez-Lluhi et al., 1992) and phospholipase C (Dietrich et al., 1994) by βγ.
How do G proteins target themselves to one cell membrane rather than another? Ras proteins and kinases in the Src family provide insight into the requirements for specific membrane targeting. These proteins combine two membrane-binding signals, either two lipids or one lipid plus a cluster of basic residues, to effect stable target membrane binding (Hancock et al., 1990; Resh, 1994, 1996). To address the role of dual lipid modifications as signals for plasma membrane localization of G protein α subunits, we studied a member of the αi subfamily, αz, in which mutations removed sites for attachment of myristate, palmitate, or both. Our results support a model in which palmitate provides plasma membrane selectivity, whereas myristate and βγ promote and stabilize both palmitoylation and membrane attachment.
MATERIALS AND METHODS
Materials
Glu-Glu (EE) monoclonal antibody was obtained from Onyx Pharmaceuticals (Richmond, CA), monoclonal antibody against hemagglutinin (HA) was obtained from Berkeley Antibody Co. (Berkeley, CA), and polyclonal antibodies against γ2 were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit anti-mannosidase II was kindly provided by Kelley Moremen (University of Georgia, Athens, GA). Pertussis toxin (PTX) was obtained from List Biologicals (Campbell, CA), quinpirole was obtained from Research Biochemicals (Natick, MA), and streptolysin O (SLO) was obtained from Murex Diagnostics (Norcross, GA). Fluorescein isothiocyanate (FITC) and Texas Red-conjugated secondary antibodies were obtained from Jackson Immunoresearch (West Grove, PA) and Texas Red-phalloidin and unlabeled phalloidin were obtained from Molecular Probes (Eugene, OR). All isotopes were from DuPont-New England Nuclear (Wilmington, DE).
Expression Vectors
cDNAs in pcDNA1 encoding αz-WT, αz-G2A, αz-C3A, and αz-G2AC3A, all with the EE epitope tag, were as described (Wilson and Bourne, 1995). To generate stable cell lines the αz-coding sequences were subcloned into pcDNA3 as EcoRI fragments. pHA-mitogen-activated protein kinase (MAPK) DNA was a gift from J. Pouysségur (CNRS, Nice, France), γ2 and β1 DNAs were gifts from Janet Robishaw, and D2 receptor DNA was a gift from Olivier Civelli.
Cell Culture and Transfection
CHO-K1 cells were propagated in minimal essential medium α (MEMα) with 10% fetal bovine serum. DNA was transiently transfected using the adenovirus method of Forsayeth and Garcia (1994). Plasmid DNAs were added to a transfection mix consisting of serum-free MEMα, 80 μg/ml DEAE-dextran, and a 1:20 dilution of adenovirus stock. This solution was added to subconfluent cells on dishes. Following a 1.5-h incubation cells were shocked with 10% DMSO in phosphate-buffered saline (PBS) and refed with normal serum-containing medium. The following DNA amounts/106 cells were used: 1 μg D2R, 0.5 μg αz, 0.2 μg β1, and 0.2 μg γ2.
Stable cell lines were generated by transfecting Chinese hamster ovary (CHO)-K1 cells with pcDNA3 constructs. Two days later cells were reseeded at dilutions of 1:100 and 1:500 and transferred into medium containing G418 at 600 μg/ml to select for stable transformants. Serial dilution of the stable pool was used to obtain cell lines derived from single cells. Stable cell lines were maintained in G418-containing medium.
Measurement of MAPK Activity
Cells were transfected in 6-well plates at 1.5 × 106 cells/well, transferred to serum-free medium after 24 h, and assayed after 48 h. Cells were treated with PTX (100 ng/ml) for 4 h before agonist stimulation. Treated cells were washed once with cold PBS and lysed in buffer containing 1.0% Triton X-100. Insoluble material was removed by microcentrifugation, 5 μg of 12CA5 antibody were added to the supernatant, and samples were incubated at 4°C for 1 h. Protein A agarose (Life Technologies, Gaithersburg, MD) was added and samples were tumbled for 1 h. Immunoprecipitates were washed twice with lysis buffer and twice with kinase buffer as described (Faure and Bourne, 1995). MAPK activity was measured using myelin basic protein as substrate in an in vitro kinase reaction. Samples were run on 14% gels and dried, and radioactivity was quantified with a PhosphorImager (Molecular Dynamics, Sunnyvale, CA).
Cell Fractionation
Four 100-mm plates of CHO-K1 cells were trypsinized and spun down 48 h after transfection. Pellets were resuspended in 1 ml of lysis buffer (40 mM Tris, pH 8.0, 1 mM EDTA, 2 mM MgCl2, 2 mM β-mercaptoethanol) plus protease inhibitors (1 mM phenylmethyl sulfonyl fluoride, 1 μg/ml pepstatin, 2 μg/ml leupeptin, 4 μg/ml aprotinin) and passed 15 times through a 27-gauge needle. Following a low-speed spin to remove unlysed cells and nuclei, supernatant fractions were separated into soluble and particulate fractions by centrifugation for 30 min at 150,000 × g.
Palmitate Labeling and Turnover
One hundred-millimeter plates of CHO-K1 cells were incubated for 2 h with MEMα containing 5 mM sodium pyruvate and 0.75 mCi/ml [3H]palmitate. Cells were washed with PBS and incubated for 1 h in 1 ml of lysis buffer (40 mM Tris, pH 8.0, 1 mM EGTA, 2 mM MgCl2, 1% cholate) plus protease inhibitors. Following microcentrifugation at 14,000 rpm, αz was immunoprecipitated from the supernatant fraction with the EE monoclonal antibody (mAb) as described below. Samples were run on 10% gels. To determine hydroxylamine sensitivity, duplicate, fixed gels were treated for 12 h either with 1 M hydroxylamine (pH 7.0) or with 1 M Tris (pH 7.0). Gels were processed for fluorography with Amplify (Amersham, Arlington Heights, IL).
For measurement of palmitate turnover, cells were washed twice with chase medium (MEMα plus 5 mM sodium pyruvate plus 100 μM palmitic acid) after labeling. Cells were then treated with chase medium with or without 10 μM quinpirole and incubated at 37°C. Depalmitoylation was terminated by washing cells with ice-cold PBS.
Immunofluorescence Localization
CHO-K1 cells were grown on glass coverslips and fixed with 4% formaldehyde in PBS. For immunofluorescence, all incubations (except the final wash) were in PBS plus 5% nonfat milk plus 1% Triton X-100. Fixed cells were blocked for 30 min in this buffer prior to a 1-h incubation with primary antibody (anti-EE mAb, 20 μg/ml; rabbit anti-mannosidase II, 1:1000; rabbit anti-γ2, 2 μg/ml). After three 10-min washes, cells were incubated with the appropriate secondary antibody (donkey anti-mouse FITC conjugate and donkey anti-rabbit Texas Red conjugate) at 1:100 dilution for 30 min. The final three washes were in PBS plus 1% Triton X-100 and coverslips were mounted on glass slides with ProLong Antifade (Molecular Probes, Eugene, OR). For actin staining, Texas Red-phalloidin was included in the secondary antibody incubation.
Immunofluorescence microscopy was performed with a confocal laser scanning microscope (MRC-1000, Bio-Rad Labs, Hercules, CA) using FITC and Texas Red filters. Images were processed with Adobe Photoshop.
For SLO permeabilization, cells on coverslips were incubated for 10 min at room temperature with 1 U/ml SLO in 10 mM MES (pH 6.1), 140 mM KCl, 3 mM MgCl2, 2 mM EGTA, 0.3 M sucrose, and 1 μg/ml phalloidin (cytoskeletal stabilizer). Cells were fixed immediately after treatment.
Western Blotting and Immunoprecipitation
For Western blotting, proteins were transferred to polyvinylidene difluoride membrane (Bio-Rad Labs) and probed with EE mAb at 3 μg/ml. Secondary antibodies with conjugated horseradish peroxidase were used at 1:10,000 dilution and bands were visualized by ECL (DuPont-New England Nuclear).
For immunoprecipitation, Triton X-100 and SDS were added to cholate extracts of cells, to final concentrations of 1% and 0.5%, respectively. EE mAb (3 μg) was added and samples were tumbled for 1 h at 4°C. Protein G-Sepharose beads (Pharmacia, Pistcataway, NJ) were added and samples were tumbled for 1 h. Pelleted beads were washed three times with lysis buffer plus 1% Triton X-100 and 0.5% SDS. Beads were resuspended in SDS-polyacrylamide sample buffer.
RESULTS
Most experiments were performed with transiently transfected CHO-K1 cells expressing epitope-tagged αz subunits (Wilson and Bourne, 1995), including αz-WT (epitope tagged only), αz-G2A (myristoylation site mutated to alanine), αz-C3A (palmitoylation site mutated to alanine), and αz-G2AC3A (alanine substituted at both sites). The epitope tag did not disrupt signaling function or membrane attachment of αz-WT (Wilson and Bourne, 1995). With αz we have shown (Wilson and Bourne, 1995), as have other investigators with several αi family members (Mumby et al., 1990, 1994; Degtyarev et al., 1994; Hallak et al., 1994), that mutating the lipid modification sites abolishes incorporation of the corresponding lipid, and that in intact cells association of the G2A or the C3A mutant with membranes is completely lacking or impaired, respectively.
Membrane Attachment and Function
To assess signaling properties by the αz mutants, we assayed a positive signal, activation of the MAPK cascade. Plasmids encoding the four αz proteins were separately and transiently transfected into CHO-K1 cells along with plasmids encoding the D2 dopamine receptor (D2R), which activates αz, and epitope-tagged MAPK (HA-MAPK) to allow assay of MAPK activity after immunoprecipitation. PTX treatment inhibits D2R-mediated activation of MAPK in cells not transfected with αz (Figure 1), presumably because the toxin inactivates all αi family members except αz; thus, D2R-mediated signals in PTX-treated cells reflect activation of αz. In cells expressing αz-WT, quinpirole, a D2R agonist, stimulated HA-MAPK activity in a PTX-insensitive manner (Figure 1). In cells expressing αz-G2A or αz-G2AC3A, quinpirole did not activate HA-MAPK, whereas HA-MAPK activation in cells expressing αz-C3A was comparable to that mediated by αz-WT (Figure 1). Abilities of the αz mutants to mediate agonist activation of MAPK paralleled our previous observations of their abilities to regulate a different effector pathway, inhibition of adenylyl cyclase (Wilson and Bourne, 1995), with a subtle difference: αz-C3A inhibited adenylyl cyclase even in the absence of agonist stimulation, to a much greater degree than αz-WT (Wilson and Bourne, 1995), whereas this mutant caused activation of MAPK activity only in response to agonist (Figure 1). We do not know the mechanism responsible for this apparent discrepancy, which does not affect inferences we draw from the data presented below.
Figure 1.
Activation of HA-MAPK by αz and mutants. cDNAs encoding D2R and either αz-WT, αz-G2A, αz-C3A, αz-G2AC3A, or vector were transfected into CHO-K1 cells. Cells were serum starved for 24 h. After 4 h of pretreatment with or without PTX, cells were treated with serum-free medium (basal) or 10 μM quinpirole for 7 min. HA-MAPK activity was measured as described in MATERIALS AND METHODS. Bars, mean ± 2 SE of triplicate determinations. Similar results were obtained in two additional experiments.
The same αz mutants that fail to signal (Figure 1) also fail to associate with membranes (Figure 2A). Separation of CHO-K1 cell homogenates into particulate (P100) and soluble (S100) fractions shows that αz-WT is associated exclusively with the particulate fraction and that αz-C3A partitions into both fractions, whereas αz-G2A and αz-G2AC3A are almost entirely soluble (Figure 2A).
Figure 2.
Particulate versus soluble distribution of αz mutants with or without βγ. CHO-K1 cells were transiently transfected with αz mutants alone (A) or transfected with αz mutants plus β1 and γ2 (B). Crude particulate (P) and soluble (S) fractions were prepared as described in MATERIALS AND METHODS. Equivalent proportions of each fraction were analyzed by SDS-PAGE and Western blotting with the EE mAb.
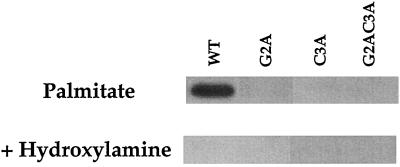
Despite an intact palmitoylation site, αz-G2A does not incorporate palmitate. Metabolic labeling with [3H]palmitate shows that αz-WT is palmitoylated, while none of the mutants, including αz-G2A, incorporate palmitate (Figure 3). These results confirm previous reports (Degtyarev et al., 1994; Hallak et al., 1994; Mumby et al., 1994; Wilson and Bourne, 1995) that G2A mutants of αi family members incorporate neither myristate nor palmitate.
Figure 3.
Incorporation of palmitate by αz mutants. CHO-K1 cells, transiently transfected with αz mutants, were incubated with 0.75 mCi/ml [3H]palmitic acid for 2 h. αz was immunoprecipitated from total cell extracts with the EE mAb followed by SDS-PAGE. Identical gels were treated with either 1 M Tris (pH 7.0) or 1 M hydroxylamine (pH 7.0) for 12 h. Gels were then processed for fluorography (19-d exposure).
Immunofluorescence Microscopy Reveals Distinct Patterns
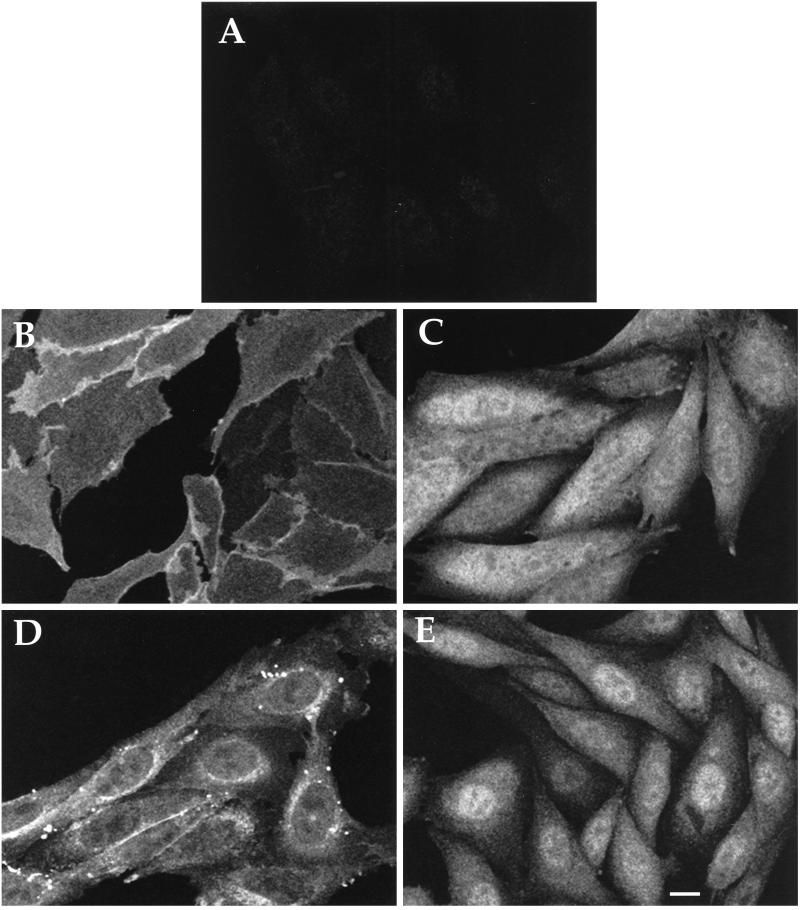
We examined the subcellular localization of both stably and transiently expressed αz mutants by indirect immunofluorescence. The two kinds of expression produced identical staining patterns, thus ruling out artifacts that might result from short-term overexpression. The epitope tag allowed specific detection of the mutants with the EE antibody; very low background staining is seen in untransfected cells (Figure 4A).
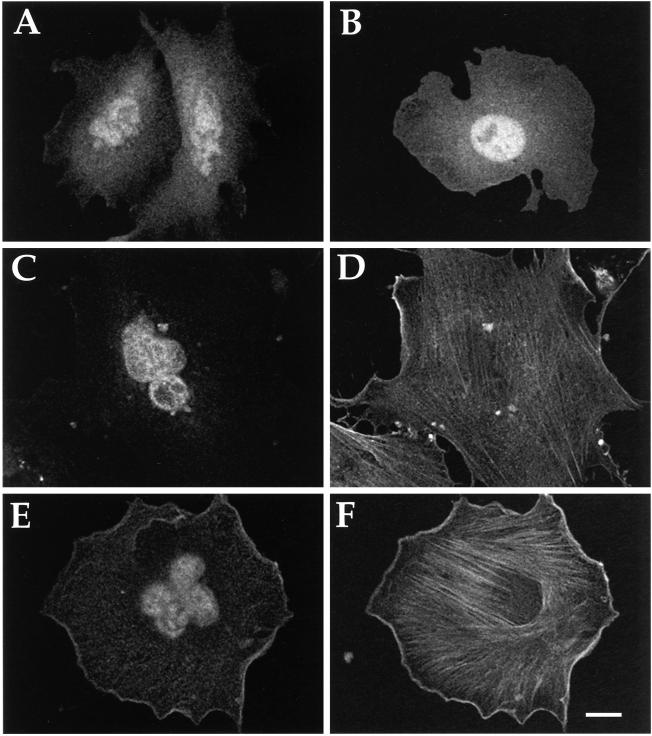
Figure 4.
Subcellular localization of WT and mutant αz in stably transfected cells. CHO-K1 cells were stably transfected with pcDNA3 vector alone (A), αz-WT (B), αz-G2A (C), αz-C3A (D), or αz-G2AC3A (E). Cells were plated onto glass coverslips and fixed with 4% formaldehyde in PBS. Permeabilized cells were incubated with EE mAb followed by incubation with FITC-conjugated antimouse antibody. Images were obtained by confocal laser scanning microscopy. Bar, 10 μm
Immunofluorescence revealed that αz without palmitate is partially mistargeted to intracellular membranes, whereas αz mutants without myristate or without sites for either myristate or palmitate do not associate with membranes or with any other structure in the cytoplasm. αz-WT shows characteristic plasma membrane localization, with uniform staining across the cell surface that is enhanced between adjacent cells (Figure 4B). A plasma membrane protein, Na+-K+-ATPase, shows the same pattern (our unpublished results). αz-C3A, which incorporates myristate but not palmitate, shows a more complex distribution pattern. Unlike αz-WT, αz-C3A associates with internal structures clustered around the nucleus (Figure 4D). This partial mistargeting of αz-C3A significantly diminishes but does not abolish its association with the plasma membrane (Figure 4D). The perinuclear and plasma membrane staining pattern is seen with both transiently and stably transfected αz-C3A (our unpublished results). αz-G2A (Figure 4C) and αz-G2AC3A (Figure 4E) show no plasma membrane staining; instead, these mutants are diffusely distributed throughout the cytosol and nucleus.
Immunofluorescence of cells treated with SLO confirmed that αz-G2A is cytosolic, whereas αz-WT and αz-C3A are membrane associated. SLO, a bacterial cytolysin, specifically perforates the plasma membrane, allowing cytoplasmic proteins to escape but leaving intracellular membranes intact (Miller and Moore, 1991). SLO treatment abolishes the apparent cytosolic distribution of αz-G2A, leaving only the nuclear staining (Figure 5, C and E). αz-G2AC3A shows an identical pattern after SLO treatment (our unpublished results). In contrast, SLO treatment does not affect the distribution of αz-WT (Figure 5A) or of αz-C3A (Figure 5G). The latter result (Figure 5G) confirms that the staining pattern seen with αz-C3A represents αz associated not only with internal cellular membranes but also with the plasma membrane (in addition to the internal staining, note enhanced staining between adjacent cells). Staining of actin filaments (Texas Red-palloidin) (Figure 5, B, D, and H) and cis-Golgi (mannosidase II antibody; Figure 5F) shows that cellular structures other than the nucleus are intact after SLO treatment.
Figure 5.
Immunofluorescence pattern of αz following SLO treatment. CHO-K1 cell lines stably expressing αz-WT (A and B), αz-G2A (C–F), or αz-C3A (G and H) were treated with SLO as described in MATERIALS AND METHODS and fixed. αz was detected by incubation with EE mAb followed by FITC-conjugated antimouse antibody (A, C, E, and G). The same cells were stained with Texas Red-conjugated phalloidin to visualize actin (B, D, and H) or with rabbit anti-mannosidase II followed by Texas Red-conjugated antirabbit antibodies to visualize Golgi (F). Images were obtained by confocal laser scanning microscopy. Bar, 10 μm
Effects of Overexpressing βγ
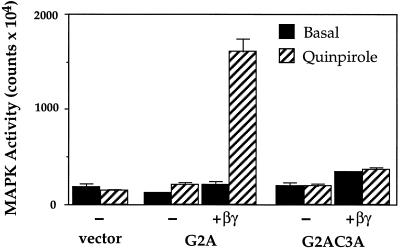
What role does βγ play in targeting of αz and anchoring it to membranes? Overexpression of βγ with αz-G2A and αz-G2AC3A revealed that βγ facilitates palmitoylation, membrane association, and receptor-mediated signaling of αz. As shown (Figure 1), αz-G2A and αz-G2AC3A fail to mediate MAPK stimulation following activation of the D2R. In contrast, coexpression of βγ with these mutants rescues the ability of αz-G2A to signal (Figure 6). Quinpirole stimulates HA-MAPK activity only when αz-G2A is coexpressed with βγ. αz-G2AC3A fails to signal regardless of whether βγ is coexpressed (Figure 6).
Figure 6.
Effect of coexpressing βγ on activation of HA-MAPK by αz-G2A and αz-G2AC3A. D2R was transiently transfected into CHO-K1 cells with vector, αz-G2A, αz-G2A plus β1 and γ2, αz-G2AC3A, or αz-G2AC3A plus β1 and γ2. Cells were serum starved for 24 h and pretreated with PTX for 4 h before stimulating with serum-free medium (basal) or 10 μM quinpirole for 7 min. HA-MAPK activity was assayed as described in MATERIALS AND METHODS. Bars, mean ± 2 SE of triplicate determinations. Similar results were obtained in two separate experiments.
To determine whether coexpression of βγ affects the ability of αz to associate with membranes, cells expressing each of the αz proteins with or without βγ were separated into S100 and P100 fractions. βγ coexpression increases the amount of αz associated with the particulate fraction (Figure 2). This effect is especially marked in the case of αz-G2A, which is entirely cytosolic when expressed alone but associates significantly with the particulate fraction when βγ is coexpressed; this shift to the particulate fraction parallels the effect of βγ in restoring to αz-G2A the ability to transmit hormonal signals (Figure 6). αz-G2AC3A, which fails to signal even in the presence of coexpressed βγ (Figure 6), also shifts to the P100 fraction in cells coexpressing βγ, but consistently to a lower extent than does αz-G2A (Figure 2).
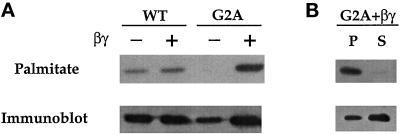
Does membrane association promoted by βγ affect the palmitoylation state of αz-G2A? Coexpression of βγ greatly increases palmitoylation of αz-G2A, but palmitate labeling of αz-WT is unchanged by excess βγ (Figure 7A). All of the [3H]palmitate on αz-G2A is found in the particulate fraction (Figure 7B), in keeping with the idea that palmitate plays a role in membrane association.
Figure 7.
Effect of βγ on palmitate incorporation by αz-G2A and the cellular distribution of palmitoylated αz-G2A. (A) αz-WT and αz-G2A were separately transfected into CHO-K1 cells without or with β1 and γ2. Palmitate incorporation was determined as described in the legend of Figure 3. A third identical gel was analyzed by Western blotting with the EE mAb. (B) CHO-K1 cells transfected with αz-G2A, β1 and γ2 were labeled with [3H]palmitate for 2 h. Following radiolabeling, particulate (P) and soluble (S) extracts were prepared. After immunoprecipitation with the EE mAb, proteins were resolved by SDS-PAGE and visualized by fluorography (21-d exposure); an identical gel was analyzed by Western blotting with the EE mAb.
βγ Targets αz to the Plasma Membrane
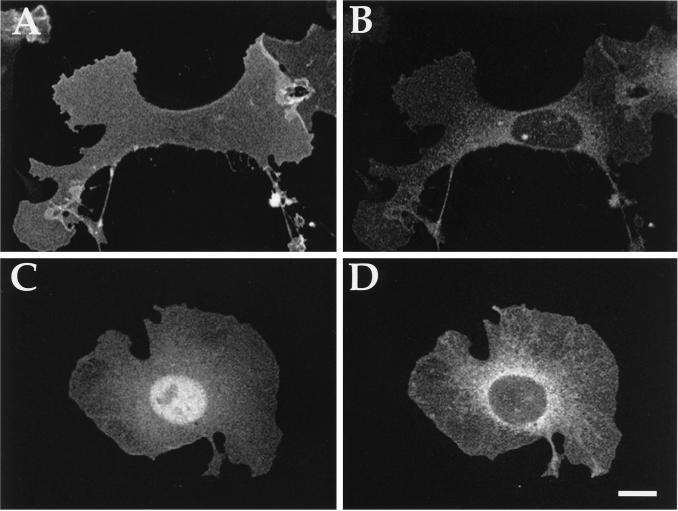
Immunofluorescence microscopy of cells transiently coexpressing αz mutants and βγ revealed that βγ targets αz to the plasma membrane. αz-G2A shows two different staining patterns, depending on whether βγ is coexpressed. As observed in stable cell lines (Figure 4C), αz-G2A alone localizes diffusely throughout the cytosol and nucleus with the intensity of fluorescent signal diminishing toward the edges where the cell is thinnest (Figure 8A). In cells coexpressing βγ, however, αz-G2A is localized over the entire cell surface, extending uniformly to the cell edges, as well as in the nucleus (Figure 8B). The nuclear staining probably reflects solubility of a fraction of αz-G2A, as shown by subcellular fractionation (Figure 2A). To confirm association of αz-G2A with the plasma membrane, cells were treated with SLO. Although SLO treatment abolishes cytosolic staining of αz-G2A expressed without exogenous βγ (Figure 8C), αz-G2A coexpressed with βγ remains associated with the plasma membrane, and no staining of intracellular membranes is observed (Figure 8E). αz-G2AC3A coexpressed with βγ shows a pattern similar to that seen with αz-G2A, but with apparently less robust staining at the plasma membrane (our unpublished results).
Figure 8.
Effect of βγ on the subcellular localization of αz-G2A. CHO-K1 cells transiently transfected with αz-G2A (A, C, and D) or αz-G2A plus β1 and γ2 (B, E, and F) were fixed after no treatment (A and B) or after treatment with SLO (C–F). αz-G2A was detected by incubation with the EE mAb followed by incubation with FITC-conjugated antimouse antibody (A–C and E). Texas Red-conjugated phalloidin-stained actin filaments (D and F). Bar, 10 μm
Costaining for α and γ revealed that αz and βγ localize in distinct but partially overlapping locations. Cells expressing either αz-WT plus βγ or αz-G2A plus βγ were stained for αz, using the anti-EE mAb, and for γ, using a γ2 specific polyclonal antibody. Although the αz subunits associate predominantly with the plasma membrane (Figure 9, A and C), γ subunits localize to extensive perinuclear structures in the cytoplasm in addition to the plasma membrane (Figure 9, B and D). Other observations support the inference that βγ subunits stably localize in at least two cellular locations: First, polyclonal antisera show identical staining patterns for endogenous γ and β (our unpublished results). In addition, cells coexpressing recombinant γ2 and a tagged β1 subunit show overlapping staining, seen on the plasma membrane and intracellular membranes (our unpublished results).
Figure 9.
Subcellular localization of αz and βγ. CHO-K1 cells transiently transfected with β1 and γ2 plus αz-WT (A and B) or αz-G2A (C and D) were fixed in 4% formaldehyde. αz was detected by incubation with EE mAb followed by FITC-conjugated antimouse antibody (A and C). γ2 was detected by incubation with polyclonal antiserum against γ2 followed by Texas Red antirabbit antibody (B and D). Bar, 10 μm.
Myristate Stabilizes Palmitate on αz
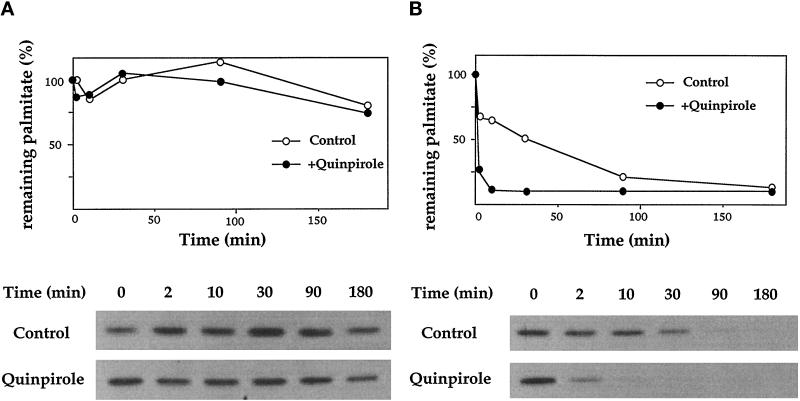
Is the palmitoylation state of αz dynamically regulated, as reported (Wedegaertner and Bourne, 1994) in the case of αs, which is rapidly depalmitoylated upon activation? Does the adjacent myristate on αz affect palmitate turnover? We measured the rate of depalmitoylation of αz-WT, with or without D2R stimulation, by metabolic labeling with [3H]palmitate followed by a chase with excess unlabeled palmitate. Unlike previous observations (Wedegaertner and Bourne, 1994) with αs, depalmitoylation of αz-WT is quite slow and is not increased by treatment with agonist (Figure 10A). To address whether stability of the palmitate linkage depends upon myristate, we coexpressed βγ with αz-G2A to induce palmitoylation and membrane association. Compared with αz-WT, αz-G2A loses its palmitate label much more rapidly; agonist treatment greatly accelerates this rate (Figure 10B). Taken together, these results suggest that myristate stabilizes the palmitate on αz-WT; removing myristate produces an αz that behaves like αs.
Figure 10.
Turnover of palmitate on αz-WT and αz-G2A plus βγ. Cells were transfected with either D2R and αz-WT (A) or D2R, αz-G2A, β1, and γ2 (B). Cells were incubated with 0.75 mCi/ml [3H]palmitate for 2 h. After radiolabeling cells were incubated in chase medium in the presence or absence of 10 μM quinpirole. At indicated times, cells (100-mm plate) were harvested and αz was immunoprecipitated from the total cell extract. Depalmitoylation was determined by densitometry of the fluorographs (A, 12-d exposure; B, 9-d exposure).
DISCUSSION
In lieu of amino acid sequences that traverse the membrane bilayer, many proteins use covalently attached lipids as anchors for mooring to cellular membranes. Accumulating evidence indicates that the attached lipid may provide not only a hydrophobic anchor but also a signal that specifies localization of the protein to a distinct membrane compartment in the cell. In many cases, two attached lipids cooperate to trap the protein in a membrane compartment. As outlined by several investigators (Cadwallader et al., 1994; Shahinian and Silvius, 1995; Resh, 1996), the two-lipid bilayer-trapping mechanism involves three key elements: 1) Together lipids A and B moor a protein much more securely to the membrane bilayer than does either lipid alone; thus, attachment of lipid B to a protein that is already modified by lipid A can trap the protein on the bilayer. 2) A membrane compartment becomes the target for localization of a protein when the enzyme for attaching lipid B is itself restricted to that compartment. 3) Lipid A both promotes attachment of lipid B and reinforces tight association with the membrane.
Our experiments suggest that for αz palmitate functions as lipid B, providing plasma membrane specificity, whereas myristate, lipid A, promotes palmitoylation and stable membrane association. As an extension of the two-signal hypothesis, we show that the signal provided by lipid A can be provided by an interacting protein, in that βγ can substitute for myristate to allow palmitoylation-dependent trapping of αz on the plasma membrane. We propose that βγ similarly provides signal A for α subunits that are palmitoylated but not myristoylated, such as αs, αq, α12, and α13.
Palmitate Can Serve as Lipid B
Two observations support the inference that palmitate is the signal that specifies plasma membrane localization of αz: 1) αz-C3A, which incorporates myristate but not palmitate, partially mislocalizes to intracellular membranes (Figure 4D). 2) When coexpressed with βγ, αz-G2A is palmitoylated and associates specifically with the plasma membrane, as indicated by immunofluorescence staining in SLO-treated cells (Figure 8E); palmitoylation and the consequent association of this mutant with the plasma membrane restore its ability to transduce hormonal signals.
Palmitate also provides the specific signal for association of Ras proteins and Src kinases with the plasma membrane (Hancock et al., 1990; Cadwallader et al., 1994; Schroeder et al., 1996; van’t Hof and Resh, 1997; Wolven et al., 1997; Zlatkine et al., 1997). In the case of H-ras, a prenyl group at the carboxyl terminus normally serves as lipid A, and attachment of palmitate as lipid B is required for targeting to the plasma membrane (Hancock et al., 1990; Cadwallader et al., 1994). Removal of the palmitoylation site on H-ras, combined with the addition of an N-terminal myristate, causes the protein to be mistargeted to numerous membranes (Cadwallader et al., 1994); palmitoylation in combination with either myristate or a prenyl group, however, causes H-ras to localize at the plasma membrane (Cadwallader et al., 1994). Similarly, synthetic cell-permeant myristoylated peptides are rapidly palmitoylated and localize at the plasma membrane when added to cultured cells, but only if the peptide contains a palmitoylation site resembling the sites in proteins (G protein α subunits and Src kinases) that are modified by palmitate as well as myristate (Schroeder et al., 1996). Fyn kinase, which is both myristoylated and palmitoylated, targets rapidly and efficiently to the plasma membrane following synthesis (van’t Hof and Resh, 1997). Fyn mutants that are only myristoylated, however, move slowly to membranes (van’t Hof and Resh, 1997) and mislocalize to intracellular membranes (Wolven et al., 1997).
It is likely that palmitate is attached to αz, Fyn kinase, and H-ras by enzymes that are themselves restricted to the plasma membrane. Indeed, a recently described palmitoyltransferase (Dunphy et al., 1996), partially purified on the basis of its ability to palmitoylate G protein α subunits, is enriched in plasma membrane fractions. Our observations with αz mutants are consistent with the idea that palmitoylation occurs at the plasma membrane. Thus, the G2A mutant of αz, as previously reported for αi (Degtyarev et al., 1994), is soluble and fails to incorporate palmitate, but coexpression with βγ partially restores association with membranes and palmitoylation. The inference that palmitoylation of αz-G2A occurs specifically at the plasma membrane accords with immunolocalization of the mutant and its ability to transduce hormonal signals when coexpressed with βγ. Moreover, βγ is found in internal membranes as well as the plasma membrane (Figure 9, B and D), but delivers αz-G2A to the plasma membrane only, as would be the case if βγ provides the nonspecific membrane association signal (replacing lipid A, myristate), whereas lipid B, palmitate, provides the specific signal. Similarly, two different lipids can serve as lipid A for H-ras (Cadwallader et al., 1994): Mutations that prevent prenylation of H-ras inhibit its palmitoylation (Hancock et al., 1989), but a myristoylation site at the N terminus can substitute for the prenyl group in permitting modification by palmitate (Cadwallader et al., 1994).
Signal A Promotes Palmitoylation
An essential feature of the two-lipid bilayer-trapping mechanism is that the nonspecific signal, lipid A, promotes and stabilizes attachment of lipid B. How does myristate—or its substitute, coexpressed βγ—facilitate palmitoylation and stable trapping of αz and other G protein α subunits at the plasma membrane? Available evidence suggests that myristate and βγ not only promote the forward palmitoylation reaction but also stabilize the link between palmitate and the α subunit. Myristate and βγ could promote palmitoylation indirectly by delivering α subunits to membranes and increasing the concentration of protein substrate accessible to a membrane-bound palmitoyltransferase. More direct roles for βγ and myristate are suggested by the observations that: 1) A palmitoyltransferase enriched in plasma membrane fractions prefers as a substrate an α subunit complexed to βγ in comparison to the free α subunit (Dunphy et al., 1996). 2) A second palmitoyltransferase activity, recently characterized (Berthiaume and Resh, 1995), attaches palmitate only to myristoylated proteins, including G protein α subunits.
Other observations indicate that myristate and βγ also inhibit depalmitoylation of αz. Thus, αz-WT is depalmitoylated much more slowly than is αz-G2A, which is modified by palmitate but not myristate, in cells coexpressing βγ (Figure 10). Moreover, receptor activation increases the rate of turnover of the palmitate attached to αs (Wedegaertner and Bourne, 1994) and of the palmitate attached to αz-G2A in cells that coexpress βγ (Figure 10). Because activation of the α subunit causes it to dissociate from βγ, these effects of receptor activation on palmitate turnover suggest that release of βγ increases the susceptibility of α to attack by cellular thioesterases. Indeed, pure βγ inhibits depalmitoylation of pure αs by an esterase in vitro (Iiri et al., 1996). Three-dimensional structures of G protein trimers (Wall et al., 1995; Lambright et al., 1996) suggest a possible explanation for these effects of βγ and myristate. The extreme N terminus of the α subunit, to which both myristate and palmitate are attached, is located in close proximity to the βγ dimer and to its prenylation site at the C terminus of the γ polypeptide. The proximity of two additional modifying lipids may limit the accessibility of palmitate to esterases, thus stabilizing its attachment to the protein.
Our observations with αz are consistent with the bilayer-trapping mechanism for specific membrane localization as proposed by other investigators (Shahinian and Silvius, 1995; Resh, 1996). After myristate is attached (cotranslationally, in the cytoplasm), αz can associate, weakly and nonspecifically, with any cellular membrane. Transient binding of myristoylated αz to the plasma membrane and association with βγ make αz a potential substrate for posttranslational palmitoylation; attachment of palmitate traps the protein at the plasma membrane (Shahinian and Silvius, 1995; Schroeder et al., 1996) rather than at other membranes, presumably because the Gα-specific transferase that catalyzes palmitoylation is itself located at the plasma membrane (Dunphy et al., 1996). Ultimately, plasma membrane association of αz is maintained through strong but nonspecific hydrophobicity provided by both myristate and palmitate. A similar pathway has been described for Fyn kinase (van’t Hof and Resh, 1997); synthesis and myristoylation of Fyn in the cytoplasm is followed by rapid palmitoylation and association with the plasma membrane (van’t Hof and Resh, 1997).
Several groups have reported localization of G protein α subunits and several other signaling proteins to specialized regions of the plasma membrane, including caveolae and/or detergent-insoluble complexes (Sargiacomo et al., 1993; Mineo et al., 1996). In many cases, localization to these microdomains appears to depend on palmitoylation (Shenoy-Scaria et al., 1994; García-Cardeña et al., 1996 and references in Resh, 1996; Mumby, 1997). Palmitoylation-dependent targeting to a microdomain of the plasma membrane may explain the ability of coexpressed βγ to restore signaling function to αz-G2A but not to αz-G2AC3A. Although βγ delivers some fraction of both proteins to membranes, only αz-G2A is palmitoylated. Although our experiments did not address this issue, perhaps palmitate targets αz-G2A to plasma membrane microdomains where signaling can take place.
Other G protein α Subunits
Although αz and other members of the αi family are both myristoylated and palmitoylated, the N termini of other α subunits, αs, αq, α11, α12, and α13, are palmitoylated but lack sites for myristoylation. In the absence of myristate, a source of hydrophobicity and a stabilizer of palmitoylation, how does palmitate become attached to these other α subunits? The mere presence of a palmitoylation site does not suffice for membrane attachment. For example, an αs/Fyn kinase chimera, in which residues from the N terminus of αs (including its palmitoylation site) replace the N-terminal myristoylation and palmitoylation sites of Fyn, is not palmitoylated and does not associate with membranes (van’t Hof and Resh, 1997). If a palmitoylation site alone is not enough, how does an α subunit lacking myristate avail itself of palmitate and localize at the plasma membrane?
We propose that the βγ subunit performs the missing functions of lipid A for such an α subunit. In this proposed mechanism, exactly as in the case of αz-G2A, association of βγ with nonmyristoylated α subunits promotes their palmitoylation and stable association with the plasma membrane; the necessary hydrophobicity is supplied by the prenyl group of βγ, whereas association of βγ with the N terminus of the α subunit helps to stabilize attached palmitate.
The presence or absence of myristate on an α subunit may determine its ability to dissociate from the plasma membrane upon hormonal activation. Activation of αs, a nonmyristoylated protein, accelerates its depalmitoylation (Mumby et al., 1994; Wedegaertner and Bourne, 1994) and causes its translocation from the plasma membrane into cytosol (Wedegaertner et al., 1996). GTP-induced dissociation of αs from βγ probably accounts for both of these effects, as suggested previously (Wedegaertner and Bourne, 1994; Iiri et al., 1996; Wedegaertner, et al., 1996). Rapid depalmitoylation and membrane dissociation are not general phenomena for G protein α subunits, as shown by the very slow rate of palmitate turnover on αz-WT, with or without agonist stimulation (Figure 10A). The presence of myristate may ensure that activated αz remains palmitoylated and bound to the plasma membrane even when bound GTP reduces its affinity for βγ. Indeed, neither receptor activation nor mutational activation caused detectable translocation of αz-WT into the cytosol (our unpublished results).
How do G protein βγ subunits localize at the plasma membrane? In terms of the two-signal bilayer-trapping mechanism, the prenyl group on the γ polypeptide probably serves as lipid A for βγ, as does the C terminal farnesyl modification for H-ras (Cadwallader et al., 1994). Lipid A provides a nonspecific hydrophobic signal for attachment to membranes, but what about lipid B? For βγ, an obvious candidate for this role is the G protein α subunit; in this case, palmitate attached to the α subunit would play the role of lipid B, targeting βγ to the plasma membrane via its association with the α subunit. Thus, the affinities of α and βγ for associating with one another would allow each protein to participate in targeting the other to the plasma membrane—βγ by supplying lipid A (a prenyl group) and α by supplying lipid B, palmitate. In keeping with the idea that α helps to retain βγ at the plasma membrane, genetic deletion of the α subunit in S. cerevisiae shifts βγ to cytosol and internal membranes of the yeast cell, markedly reducing its concentration at the plasma membrane (Hirschman et al., 1997). In this scenario, α and βγ subunits reciprocally help to tether one another to the lipid bilayer, whereas palmitate on the α subunit provides specificity of membrane association. Although speculative, this reciprocity could provide an effective way to maintain α:βγ stoichiometry at the site where signals are transduced.
ACKNOWLEDGMENTS
We thank Keith Mostov, Phil Wedegaertner, and members of the Bourne laboratory for useful discussions and/or critical reading of the manuscript. This work was supported in part by grants from the National Institutes of Health (to H.R.B) and the Cancer Research Fund of the Damon Runyon-Walter Winchell Foundation Fellowship, DRG-1379, and the Human Frontier Science Program (to C.S.F.).
Footnotes
Abbreviations used: D2R, D2-dopamine receptor; EE, glu-glu; FITC, fluorescein isothiocyanate; mAb, monoclonal antibody; MAPK, mitogen-activated protein kinase; PTX, pertussis toxin; SLO, streptolysin O.
Our inferences about subcellular targeting of normal and mutant αz assume that observed patterns of immunofluorescence are not distorted by expression of the proteins in amounts large enough to cause mistargeting (“overflow”) to the wrong compartment. Three considerations suggest that this assumption is valid in our experiments: 1) We observed mistargeting of mutant αz proteins, but not αz-WT, to cytoplasm, nucleus, and internal membranes (Figures 4 and 5). Immunoblots show, however, that these mutant proteins were expressed in amounts similar or identical to αz-WT (Figure 2), making overflow mistargeting of the mutants very unlikely. 2) After transient expression, staining patterns are uniform in all cells examined, despite great variation in relative staining intensities of individual cells. 3) Staining patterns are identical for αz constructs expressed stably or transiently; for example, compare Figure 4B with Figure 9A.
The αz-C3A immunofluorescence associated with these internal structure(s) partially overlaps with (but is not limited to) the immunofluorescence patterns seen with antibodies to β-COP, mannosidase II, and TGN38, and it redistributes following brefeldin A treatment (our unpublished results). We infer that αz-C3A associates with membranes of the Golgi network and probably with other internal membranes as well.
The function of lipid A could also be supplied in other ways, including a previously undetected lipid modification (Kleuss and Gilman, 1997) or a sequence of amino acids that provides hydrophobicity or attachment to phospholipids.
REFERENCES
- Berthiaume L, Resh MD. Biochemical characterization of a palmitoyl acyltransferase activity that palmitoylates myristoylated proteins. J Biol Chem. 1995;270:22399–22405. doi: 10.1074/jbc.270.38.22399. [DOI] [PubMed] [Google Scholar]
- Bhatnagar RS, Gordon JI. Understanding covalent modifications of proteins by lipids: where cell biology and biophysics mingle. Trends Cell Biol. 1997;7:14–20. doi: 10.1016/S0962-8924(97)10044-7. [DOI] [PubMed] [Google Scholar]
- Bigay J, Faurobert E, Franco M, Chabre M. Roles of lipid modifications of transducin subunits in their GDP-dependent association and membrane binding. Biochemistry. 1994;33:14081–14090. doi: 10.1021/bi00251a017. [DOI] [PubMed] [Google Scholar]
- Boman AL, Kahn RA. Arf proteins: the membrane traffic police? Trends Biochem Sci. 1995;20:147–150. doi: 10.1016/s0968-0004(00)88991-4. [DOI] [PubMed] [Google Scholar]
- Cadwallader KA, Paterson H, MacDonald SG, Hancock JF. N-terminally myristoylated ras proteins require palmitoylation or a polybasic domain for plasma membrane localization. Mol Cell Biol. 1994;14:4722–4730. doi: 10.1128/mcb.14.7.4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camp LA, Hofmann SL. Purification and properties of a palmitoyl-protein thioesterase that cleaves palmitate from H-ras. J Biol Chem. 1993;268:22566–22574. [PubMed] [Google Scholar]
- Casey PJ. Protein lipidation in cell signaling. Science. 1995;268:221–225. doi: 10.1126/science.7716512. [DOI] [PubMed] [Google Scholar]
- Degtyarev MY, Spiegel AM, Jones TL. Palmitoylation of a G protein αi subunit requires membrane localization not myristoylation. J Biol Chem. 1994;269:30898–30903. [PubMed] [Google Scholar]
- Degtyarev MY, Spiegel AM, Jones TLZ. Increased palmitoylation of the Gs protein α subunit after activation by the β-adrenergic receptor or cholera toxin. J Biol Chem. 1993;268:23769–23772. [PubMed] [Google Scholar]
- Dietrich A, Meister M, Brazil D, Camps M, Gierschik P. Stimulation of phospholipase C-β2 by recombinant guanine-nucleotide-binding protein βγ dimers produced in a baculovirus/insect cell expression system. Requirement of γ-subunit isoprenylation for stimulation of phospholipase C. Eur J Biochem. 1994;219:171–178. doi: 10.1111/j.1432-1033.1994.tb19927.x. [DOI] [PubMed] [Google Scholar]
- Dunphy JT, Greentree WK, Manahan CL, Linder ME. G-protein palmitoyltransferase activity is enriched in plasma membranes. J Biol Chem. 1996;271:7154–7159. doi: 10.1074/jbc.271.12.7154. [DOI] [PubMed] [Google Scholar]
- Faure M, Bourne HR. Differential effects of cAMP on the MAP kinase cascade: evidence for a cAMP-insensitive step that can bypass raf-1. Mol Biol Cell. 1995;6:1025–1035. doi: 10.1091/mbc.6.8.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsayeth JR, Garcia PD. Adenovirus-mediated transfection of cultured cells. Biotechniques. 1994;17:354–359. [PubMed] [Google Scholar]
- García-Cardeña G, Oh P, Liu J, Schnitzer JE, Sessa WC. Targeting of nitric oxide synthase to endothelial cell caveolae via palmitoylation: implications for nitric oxide signaling. Proc Natl Acad Sci USA. 1996;93:6448–6453. doi: 10.1073/pnas.93.13.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallak H, Brass LF, Manning DR. Failure to myristoylate the α subunit of Gz is correlated with an inhibition of palmitoylation and membrane attachment, but has no affect on phosphorylation by protein kinase C. J Biol Chem. 1994;269:4571–4576. [PubMed] [Google Scholar]
- Hancock JF, Magee AI, Childs JE, Marshall CJ. All ras proteins are polyisoprenylated but only some are palmitoylated. Cell. 1989;57:1167–1177. doi: 10.1016/0092-8674(89)90054-8. [DOI] [PubMed] [Google Scholar]
- Hancock JF, Paterson H, Marshall CJ. A polybasic domain or palmitoylation is required in addition to the CAAX motif to localize p21ras to the plasma membrane. Cell. 1990;63:133–139. doi: 10.1016/0092-8674(90)90294-o. [DOI] [PubMed] [Google Scholar]
- Hirschman JE, De Zutter GS, Simonds WF, Jenness DD. The Gβγ complex of the yeast pheromone response pathway. J Biol Chem. 1997;272:240–248. doi: 10.1074/jbc.272.1.240. [DOI] [PubMed] [Google Scholar]
- Iiri T, Backlund PS, Jones TLZ, Wedegaertner PB, Bourne HR. Reciprocal regulation of Gsα by palmitate and the βγ subunit. Proc Natl Acad Sci USA. 1996;93:14592–14597. doi: 10.1073/pnas.93.25.14592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iñiguez-Lluhi JA, Simon MI, Robishaw JD, Gilman AG. G protein βγ subunits synthesized in Sf9 cells. J Biol Chem. 1992;267:23409–23417. [PubMed] [Google Scholar]
- Jones TL, Simonds WF, Merendino JJ, Brann MR, Spiegel AM. Myristoylation of an inhibitory GTP-binding protein α subunit is essential for its membrane attachment. Proc Natl Acad Sci USA. 1990;87:568–572. doi: 10.1073/pnas.87.2.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleuss C, Gilman AG. Gsα contains an unidentified covalent modification that increases its affinity for adenylyl cyclase. Proc Natl Acad Sci USA. 1997;94:6116–6120. doi: 10.1073/pnas.94.12.6116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambright DG, Sondek J, Bohm A, Skiba NP, Hamm HE, Sigler PB. The 2.0 Å crystal structure of a heterotrimeric G protein. Nature. 1996;379:311–319. doi: 10.1038/379311a0. [DOI] [PubMed] [Google Scholar]
- Linder ME, Pang I-H, Duronio RJ, Gordon JI, Sternweis PC, Gilman AG. Lipid modifications of G protein subunits. Myristoylation of Goα increases its affinity for βγ. J Biol Chem. 1991;266:4654–4659. [PubMed] [Google Scholar]
- McCallum JF, Wise A, Grassie MA, Magee AI, Guzzi F, Parenti M, Milligan G. The role of palmitoylation of the guanine nucleotide binding protein G11α in defining interaction with the plasma membrane. Biochem J. 1995;310:1021–1027. doi: 10.1042/bj3101021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin S, Aderem A. The myristoyl-electrostatic switch: a modulator of reversible protein-membrane interactions. Trends Biochem Sci. 1995;20:272–276. doi: 10.1016/s0968-0004(00)89042-8. [DOI] [PubMed] [Google Scholar]
- Miller SG, Moore H-PH. Reconstitution of constitutive secretion using semi-intact cells: regulation by GTP but not calcium. J Cell Biol. 1991;112:39–54. doi: 10.1083/jcb.112.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan G, Parenti M, Magee AI. The dynamic role of palmitoylation in signal transduction. Trends Biochem Sci. 1995;20:181–186. doi: 10.1016/s0968-0004(00)89004-0. [DOI] [PubMed] [Google Scholar]
- Mineo C, James GL, Smart EJ, Anderson RGW. Localization of epidermal growth factor-stimulated Ras/Raf-1 interaction to caveolae membrane. J Biol Chem. 1996;271:11930–11935. doi: 10.1074/jbc.271.20.11930. [DOI] [PubMed] [Google Scholar]
- Mumby SM. Reversible palmitoylation of signaling proteins. Curr Opin Cell Biol. 1997;9:148–154. doi: 10.1016/s0955-0674(97)80056-7. [DOI] [PubMed] [Google Scholar]
- Mumby SM, Heukeroth RO, Gordon JI, Gilman AG. G-protein α-subunit expression, myristoylation, and membrane association in COS cells. Proc Natl Acad Sci USA. 1990;87:728–732. doi: 10.1073/pnas.87.2.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby SM, Kleuss C, Gilman AG. Receptor regulation of G-protein palmitoylation. Proc Natl Acad Sci USA. 1994;91:2800–2804. doi: 10.1073/pnas.91.7.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muntz KH, Sternweis PC, Gilman AG, Mumby SM. Influence of γ subunit prenylation on association of guanine nucleotide-binding regulatory proteins with membranes. Mol Biol Cell. 1992;3:49–61. doi: 10.1091/mbc.3.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neer EJ. Heterotrimeric G proteins: organizers of transmembrane signals. Cell. 1995;80:249–257. doi: 10.1016/0092-8674(95)90407-7. [DOI] [PubMed] [Google Scholar]
- Peitzsch RM, McLaughlin S. Binding of acylated peptides and fatty acids to phospholipid vesicles: pertinence to myristoylated proteins. Biochemistry. 1993;32:10436–10443. doi: 10.1021/bi00090a020. [DOI] [PubMed] [Google Scholar]
- Resh MD. Myristylation and palmitylation of src family members: the fats of the matter. Cell. 1994;76:411–413. doi: 10.1016/0092-8674(94)90104-x. [DOI] [PubMed] [Google Scholar]
- Resh MD. Regulation of cellular signalling by fatty acid acylation and prenylation of signal transduction proteins. Cell Signal. 1996;8:403–412. doi: 10.1016/s0898-6568(96)00088-5. [DOI] [PubMed] [Google Scholar]
- Sargiacomo M, Sudol M, Tang Z, Lisanti MP. Signal transducing molecules and glycosyl-phosphatidylinositol-linked proteins form a caveolin-rich insoluble complex in MDCK cells. J Cell Biol. 1993;122:789–807. doi: 10.1083/jcb.122.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder H, Leventis R, Shahinian S, Walton PA, Silvius JR. Lipid-modified, cysteinyl-containing peptides of diverse structures are efficiently s-acylated at the plasma membrane of mammalian cells. J Cell Biol. 1996;134:647–660. doi: 10.1083/jcb.134.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahinian S, Silvius JR. Doubly-lipid-modified protein sequence motifs exhibit long-lived anchorage to lipid bilayer membranes. Biochemistry. 1995;34:3813–3822. doi: 10.1021/bi00011a039. [DOI] [PubMed] [Google Scholar]
- Shenoy-Scaria AM, Dietzen DJ, Kwong J, Link DC, Lublin DM. Cysteine3 of src family protein tyrosine kinases determines palmitoylation and localization in caveolae. J Cell Biol. 1994;126:353–363. doi: 10.1083/jcb.126.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taussig R, Iñiguez-Lluhi JA, Gilman AG. Inhibition of adenylyl cyclase by Giα. Science. 1993;261:218–221. doi: 10.1126/science.8327893. [DOI] [PubMed] [Google Scholar]
- van’t Hof W, Resh MD. Rapid plasma membrane anchoring of newly synthesized p59fyn: selective requirement for NH2-terminal myristoylation and palmitoylation at cysteine-3. J Cell Biol. 1997;136:1023–1035. doi: 10.1083/jcb.136.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall MA, Coleman DE, Lee E, Iñiguez-Lluhi JA, Posner BA, Gilman AG, Sprang SR. The structure of the G protein heterotrimer Giα1β1γ2. Cell. 1995;80:1047–1058. doi: 10.1016/0092-8674(95)90220-1. [DOI] [PubMed] [Google Scholar]
- Wedegaertner PB, Bourne HR. Activation and depalmitoylation of Gsα. Cell. 1994;77:1063–1070. doi: 10.1016/0092-8674(94)90445-6. [DOI] [PubMed] [Google Scholar]
- Wedegaertner PB, Bourne HR, von Zastrow M. Activation-induced subcellular redistribution of Gsα. Mol Biol Cell. 1996;7:1225–1233. doi: 10.1091/mbc.7.8.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedegaertner PB, Chu DH, Wilson PT, Levis MJ, Bourne HR. Palmitoylation is required for signaling functions and membrane attachment of Gqα and Gsα. J Biol Chem. 1993;268:25001–25008. [PubMed] [Google Scholar]
- Wedegaertner PB, Wilson PT, Bourne HR. Lipid modifications of trimeric G proteins. J Biol Chem. 1995;270:503–506. doi: 10.1074/jbc.270.2.503. [DOI] [PubMed] [Google Scholar]
- Wilson PT, Bourne HR. Fatty acylation of αz. J Biol Chem. 1995;270:9667–9675. doi: 10.1074/jbc.270.16.9667. [DOI] [PubMed] [Google Scholar]
- Wolven A, Okamura H, Rosenblatt Y, Resh MD. Palmitoylation of p59fyn is reversible and sufficient for plasma membrane association. Mol Biol Cell. 1997;8:1159–1173. doi: 10.1091/mbc.8.6.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlatkine P, Mehul B, Magee AI. Retargeting of cytosolic proteins to the plasma membrane by the Lck protein tyrosine kinase dual acylation motif. J Cell Sci. 1997;110:673–679. doi: 10.1242/jcs.110.5.673. [DOI] [PubMed] [Google Scholar]