Abstract
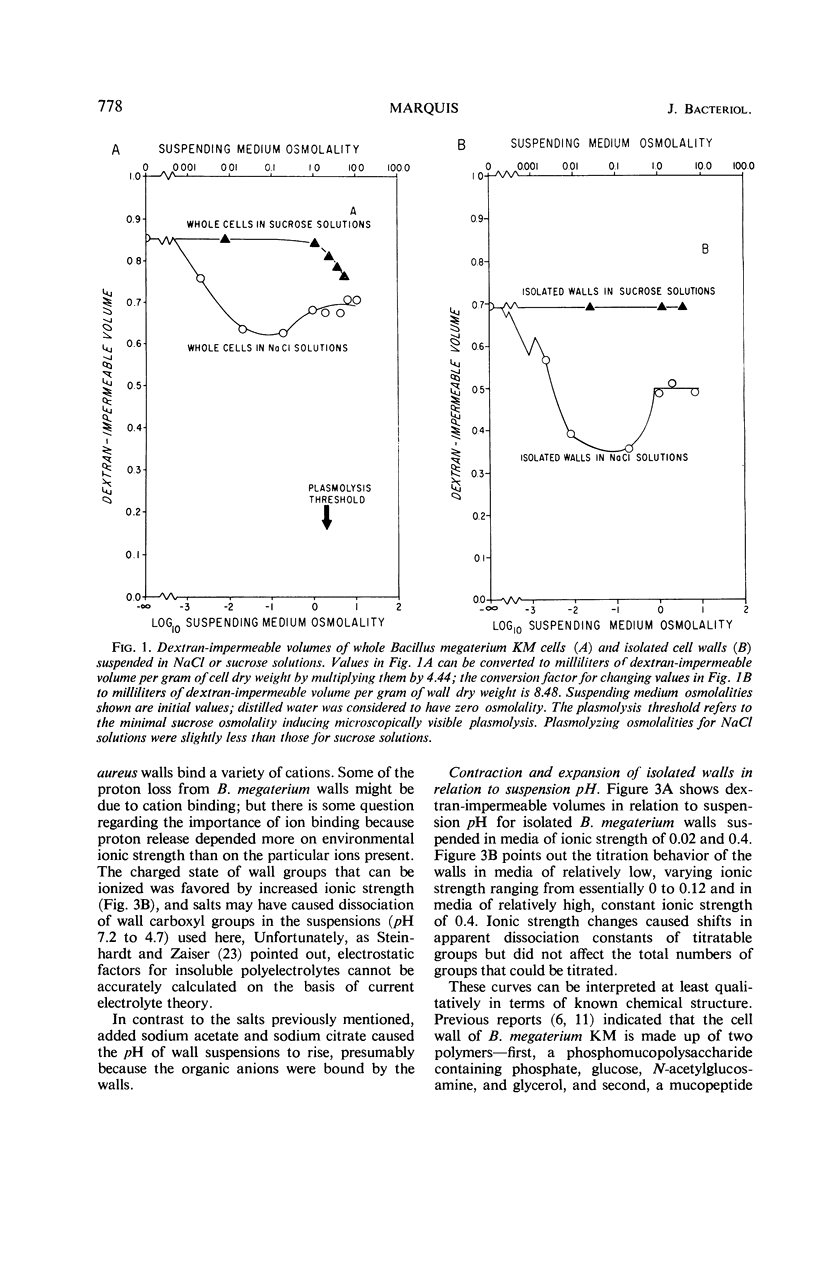
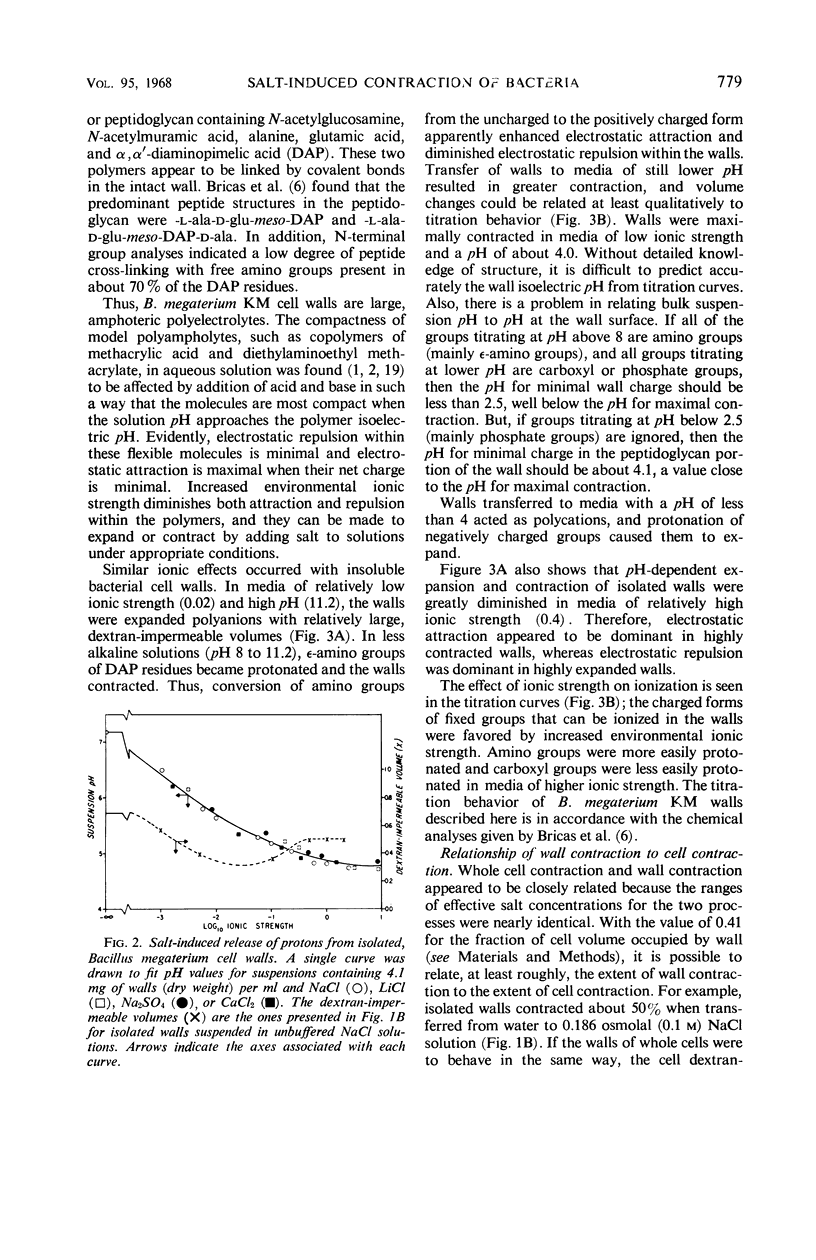
Intact Bacillus megaterium cells were found to contract as much as 26% in terms of dextran-impermeable volume when transferred from water to unbuffered, non-plasmolyzing NaCl solutions. This shrinkage appeared to be primarily due to electrostatic wall contraction rather than to any osmotic response of the cells. A variety of salts (but not sucrose) added to water suspensions of isolated cell walls caused protons to be released from the walls with resultant lowering of suspension pH and contraction of the structures. In effect, B. megaterium walls behaved as flexible, amphoteric polyelectrolytes, and their compactness in aqueous suspensions was affected by changes in environmental ionic strength and pH. Isolated walls were most compact in low ionic strength media with a pH of about 4, a value close to the apparent isoelectric pH of wall peptidoglycan. Electrostatic attractions appeared to play a major role in determining the compactness of highly contracted walls, and the walls responded to increased environmental ionic strength by expanding. In contrast, electrostatic repulsions were dominant in highly expanded walls, and increased environmental ionic strength induced wall contraction. Walls of whole bacteria also shrank when the cells were plasmolyzed. This second type of contraction seemed to result from relief of wall tension during plasmolysis, and it could be induced with nonionic solutes. Thus, cell wall tone in B. megaterium appeared to be set both by mechanical tension and by electrostatic interactions among wall ions.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVI-DOR Y., KUCZYNSKI M., SCHATZBERG G., MAGER J. Turbidity changes in bacterial suspensions: kinetics and relation to metabolic state. J Gen Microbiol. 1956 Feb;14(1):76–83. doi: 10.1099/00221287-14-1-76. [DOI] [PubMed] [Google Scholar]
- BERNHEIM F. Factors which affect the size of the organisms and the optical density of suspensions of Pseudomonas aeruginosa and Escherichia coli. J Gen Microbiol. 1963 Jan;30:53–58. doi: 10.1099/00221287-30-1-53. [DOI] [PubMed] [Google Scholar]
- BLACK S. H., GERHARDT P. Permeability of bacterial spores. IV. Water content, uptake, and distribution. J Bacteriol. 1962 May;83:960–967. doi: 10.1128/jb.83.5.960-967.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bricas E., Ghuysen J. M., Dezélée P. The cell wall peptidoglycan of Bacillus megaterium KM. I. Studies on the stereochemistry of alpha, alpha'-diaminopimelic acid. Biochemistry. 1967 Aug;6(8):2598–2607. doi: 10.1021/bi00860a043. [DOI] [PubMed] [Google Scholar]
- Cutinelli C., Galdiero F. Ion-binding properties of the cell wall of Staphylococcus aureus. J Bacteriol. 1967 Jun;93(6):2022–2023. doi: 10.1128/jb.93.6.2022-2023.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham N. N., Noller E. C., Burger M. W., Best G. K. Time-lapse cinematography of vancomycin--treated microbial cells. Can J Microbiol. 1967 Apr;13(4):417–421. doi: 10.1139/m67-054. [DOI] [PubMed] [Google Scholar]
- GERHARDT P., JUDGE J. A. POROSITY OF ISOLATED CELL WALLS OF SACCHAROMYCES CEREVISIAE AND BACILLUS MEGATERIUM. J Bacteriol. 1964 Apr;87:945–951. doi: 10.1128/jb.87.4.945-951.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GHUYSEN J. M. ALKALINE DEGRADATION OF THE PHOSPHOMUCOPOLYSACCHARIDE FROM CELL WALLS OF BACILLUS MEGATERIUM KM. Biochim Biophys Acta. 1964 Mar 2;83:132–134. doi: 10.1016/0926-6526(64)90063-1. [DOI] [PubMed] [Google Scholar]
- HENNEMAN D. H., UMBREIT W. W. FACTORS WHICH MODIFY THE EFFECT OF SODIUM AND POTASSIUM ON BACTERIAL CELL MEMBRANES. J Bacteriol. 1964 Jun;87:1266–1273. doi: 10.1128/jb.87.6.1266-1273.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGER J., KUCZYNSKI M., SCHATZBERG G., AVI-DOR Y. Turbidity changes in bacterial suspensions in relation to osmotic pressure. J Gen Microbiol. 1956 Feb;14(1):69–75. doi: 10.1099/00221287-14-1-69. [DOI] [PubMed] [Google Scholar]
- MARQUIS R. E., GERHARDT P. RESPIRATION-COUPLED AND PASSIVE UPTAKE OF ALPHA-AMINOISOBUTYRIC ACID, A METABOLICALLY INERT TRANSPORT ANALOGUE, BY BACILLUS MEGATERIUM. J Biol Chem. 1964 Oct;239:3361–3371. [PubMed] [Google Scholar]
- Marquis R. E. Osmotic sensitivity of bacterial protoplasts and the response of their limiting membrane to stretching. Arch Biochem Biophys. 1967 Feb;118(2):323–331. doi: 10.1016/0003-9861(67)90356-6. [DOI] [PubMed] [Google Scholar]
- Morris D. L. Quantitative Determination of Carbohydrates With Dreywood's Anthrone Reagent. Science. 1948 Mar 5;107(2775):254–255. doi: 10.1126/science.107.2775.254. [DOI] [PubMed] [Google Scholar]
- STEINHARDT J., ZAISER E. M. Hydrogen ion equilibria in native and denatured proteins. Adv Protein Chem. 1955;10:151–226. doi: 10.1016/s0065-3233(08)60105-9. [DOI] [PubMed] [Google Scholar]
- VENNES J. W., GERHARDT P. Antigenic analysis of cell structures isolated from Bacillus megaterium. J Bacteriol. 1959 May;77(5):581–592. doi: 10.1128/jb.77.5.581-592.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIBULL C. Bacterial protoplasts. Annu Rev Microbiol. 1958;12:1–26. doi: 10.1146/annurev.mi.12.100158.000245. [DOI] [PubMed] [Google Scholar]
- WEIBULL C. Osmotic properties of protoplasts of Bacillus megaterium. Exp Cell Res. 1955 Oct;9(2):294–304. doi: 10.1016/0014-4827(55)90102-6. [DOI] [PubMed] [Google Scholar]
- WEIBULL C. The localisation of a permeability barrier in the cells of Bacillus megatherium. Exp Cell Res. 1955 Aug;9(1):139–147. doi: 10.1016/0014-4827(55)90168-3. [DOI] [PubMed] [Google Scholar]
- WHITFIELD J. F., MURRAY R. G. The effects of the ionic environment on the chromatin structures of bacteria. Can J Microbiol. 1956 May;2(3):245–260. doi: 10.1139/m56-029. [DOI] [PubMed] [Google Scholar]


