Abstract
d-Galacturonic acid 1-phosphate was found to be one of the products formed during hydrolysis of the cell wall lipopolysaccharide of Xanthomonas campestris in 0.01 n acetic acid at pH 3.3. The molecule was shown to consist of equimolar amounts of d-galacturonic acid and phosphate. Resistance to borohydride reduction before, but not after, treatment with Escherichia coli alkaline phosphatase indicated that the phosphate group is attached to carbon-1 of the galacturonic acid. The presence of an additional phosphate group in the heteropolysaccharide of the cell wall lipopolysaccharide was also demonstrated. This phosphate group was considerably more resistant to acid hydrolysis than was the phosphate associated with the galacturonic acid. It is suggested that the more resistant phosphate is attached to either mannose or glucose, or to both.
Full text
PDF

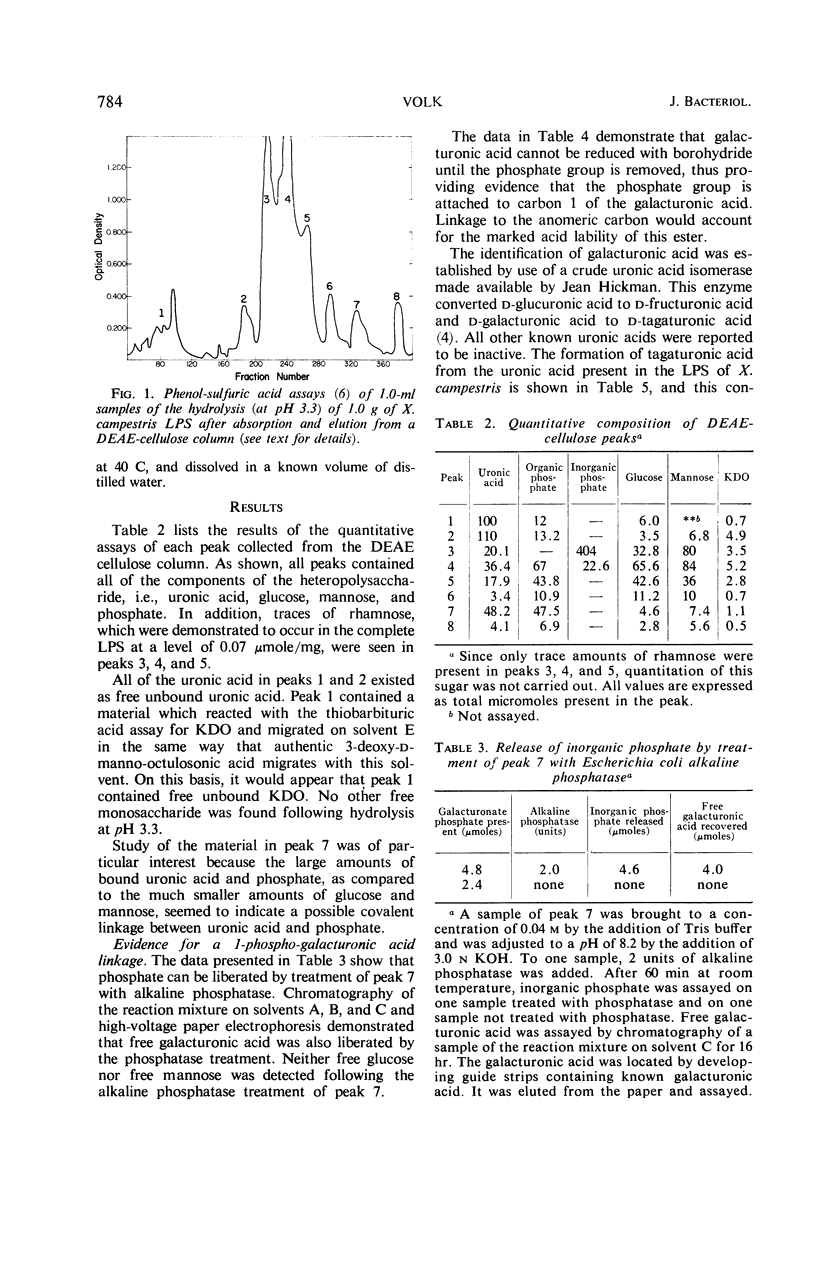
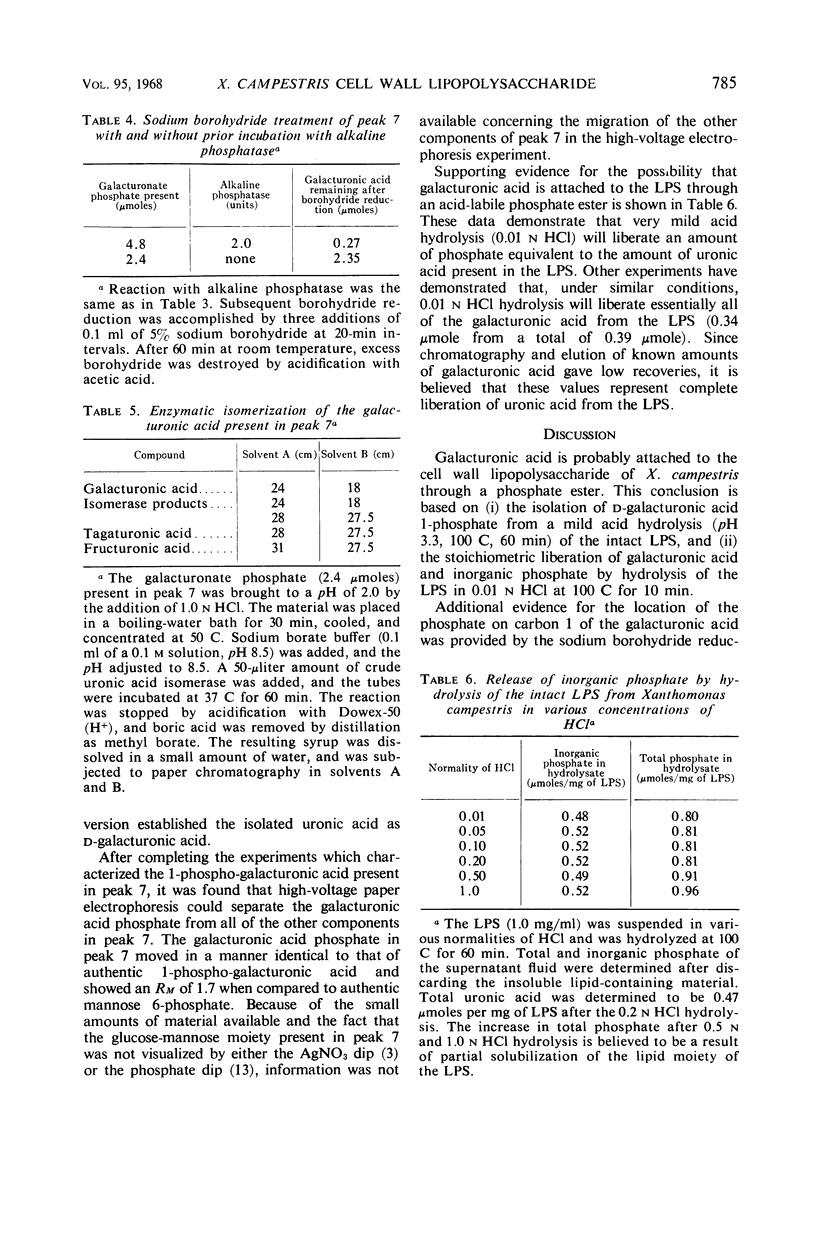

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- AMINOFF D. Methods for the quantitative estimation of N-acetylneuraminic acid and their application to hydrolysates of sialomucoids. Biochem J. 1961 Nov;81:384–392. doi: 10.1042/bj0810384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASHWELL G., WAHBA A. J., HICKMAN J. Uronic acid metabolism in bacteria. I. Purification and properties of uronic acid isomerase in Escherichia coli. J Biol Chem. 1960 Jun;235:1559–1565. [PubMed] [Google Scholar]
- CHAPPELL J. B., GREVILLE G. D. Effect of silver ions on mitochondrial adenosine triphosphatase. Nature. 1954 Nov 13;174(4437):930–931. doi: 10.1038/174930b0. [DOI] [PubMed] [Google Scholar]
- GREGORY J. D. The effect of borate on the carbazole reaction. Arch Biochem Biophys. 1960 Aug;89:157–159. doi: 10.1016/0003-9861(60)90036-9. [DOI] [PubMed] [Google Scholar]
- Ghalambor M. A., Levine E. M., Heath E. C. The biosynthesis of cell wall lipopolysaccharide in Escherichia coli. 3. The isolation and characterization of 3-deoxyoctulosonic acid. J Biol Chem. 1966 Jul 10;241(13):3207–3215. [PubMed] [Google Scholar]
- NAIDE Y., NIKAIDO H., MAEKELAE P. H., WILKINSON R. G., STOCKER B. A. SEMIROUGH STRAINS OF SALMONELLA. Proc Natl Acad Sci U S A. 1965 Jan;53:147–153. doi: 10.1073/pnas.53.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSBORN M. J., ROSEN S. M., ROTHFIELD L., HORECKER B. L. Biosynthesis of bacterial lipopolysaccharide. I. Enzymatic incorporation of galactose in a mutant strain of Salmonella. Proc Natl Acad Sci U S A. 1962 Oct 15;48:1831–1838. doi: 10.1073/pnas.48.10.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSBORN M. J. STUDIES ON THE GRAM-NEGATIVE CELL WALL. I. EVIDENCE FOR THE ROLE OF 2-KETO- 3-DEOXYOCTONATE IN THE LIPOPOLYSACCHARIDE OF SALMONELLA TYPHIMURIUM. Proc Natl Acad Sci U S A. 1963 Sep;50:499–506. doi: 10.1073/pnas.50.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBBINS P. W., WRIGHT A., BELLOWS J. L. ENZYMATIC SYNTHESIS OF THE SALMONELLA O-ANTIGEN. Proc Natl Acad Sci U S A. 1964 Nov;52:1302–1309. doi: 10.1073/pnas.52.5.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUNECKLES V. C., KROTKOV G. The separation of phosphate esters and other metabolites by ionophoresis and chromatography on paper. Arch Biochem Biophys. 1957 Aug;70(2):442–453. doi: 10.1016/0003-9861(57)90132-7. [DOI] [PubMed] [Google Scholar]
- Volk W. A. Cell wall lipopolysaccharides from xanthomonas species. J Bacteriol. 1966 Jan;91(1):39–42. doi: 10.1128/jb.91.1.39-42.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]


