Abstract
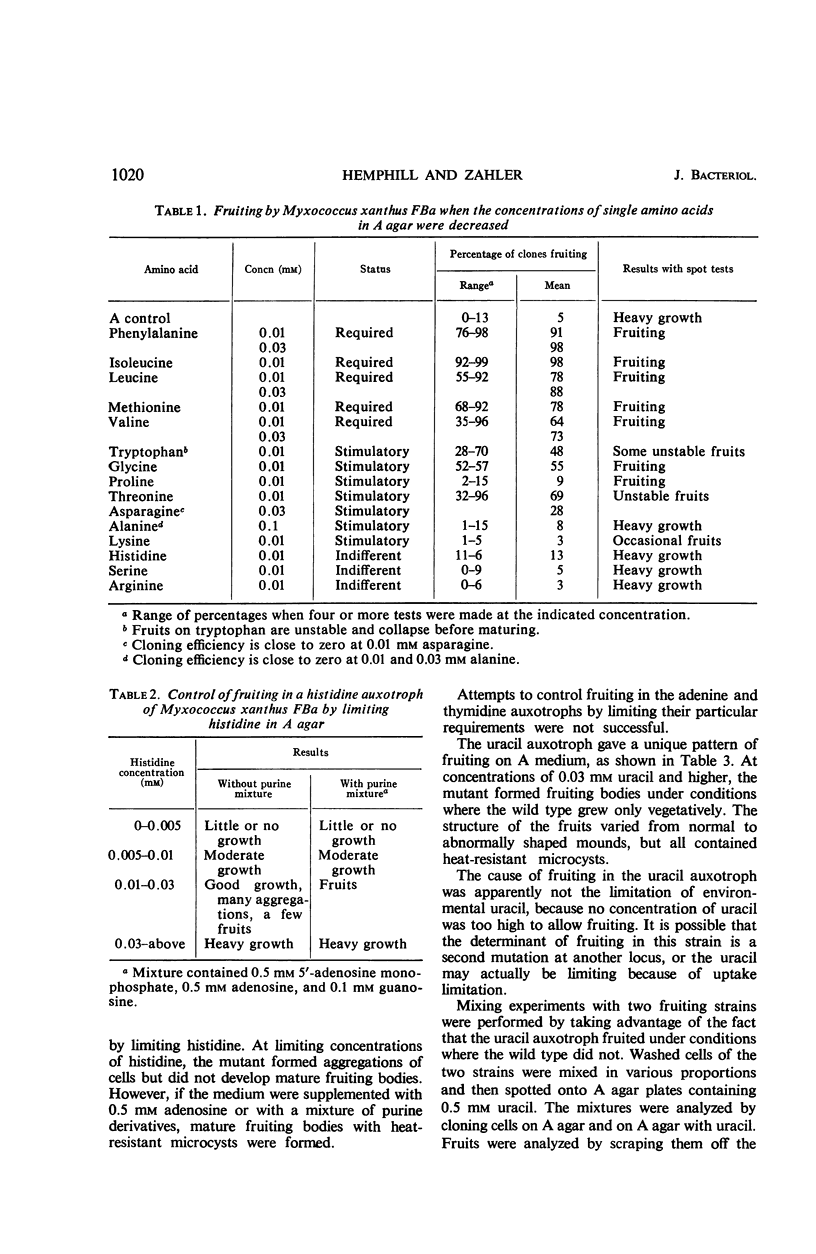
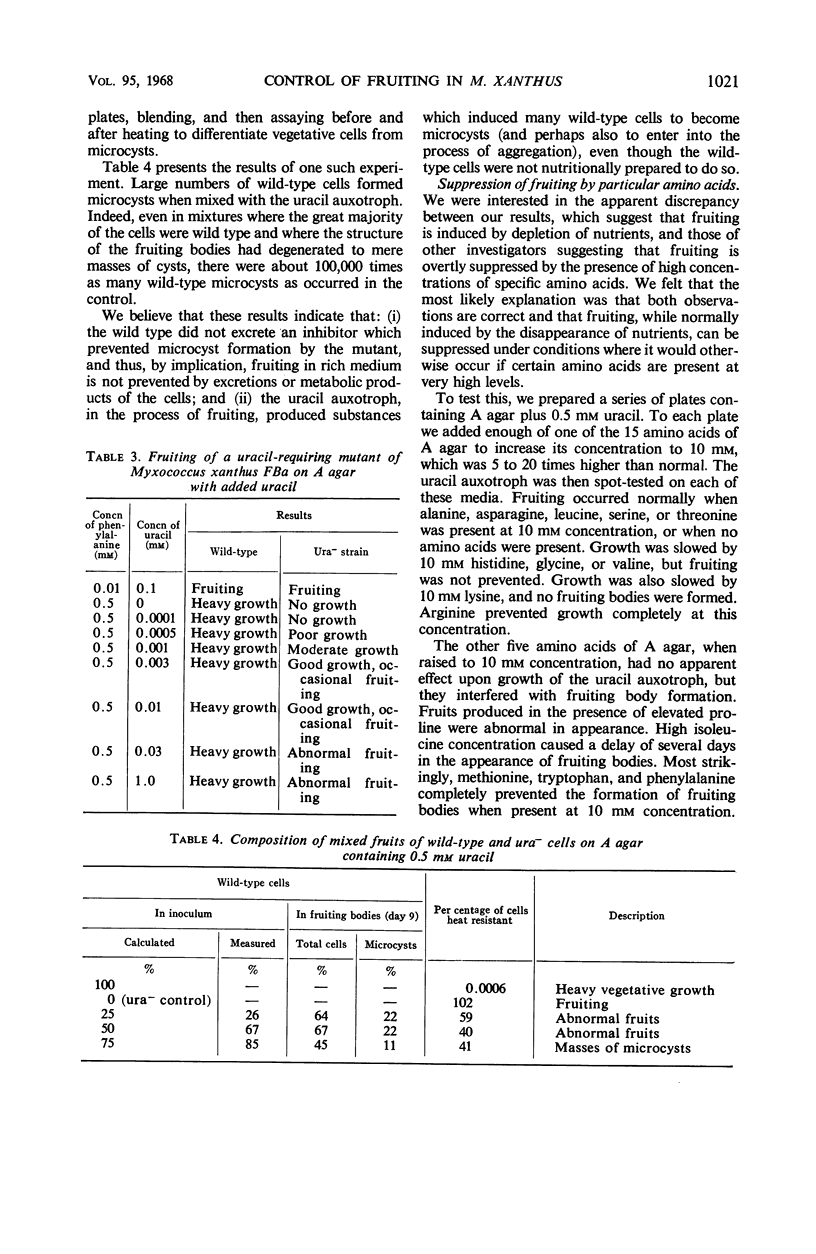
A defined agar medium (A agar) containing 15 amino acids in concentrations between 0.5 and 2 mm was developed for studying the fruiting cycle of Myxococcus xanthus FBa. Cells grew only vegetatively in this medium unless the initial concentration of one of nine required or stimulatory amino acids was lowered about 50-fold. In the latter circumstance, fruiting bodies developed after several days of vegetative growth. The conclusion was that fruiting occurred when any amino acid required for normal growth became limiting in the environment. High concentrations (10 mm) of phenylalanine, tryptophan, or methionine prevented fruiting without affecting growth. Mutants requiring arginine, thymidine, or adenine could not be induced to fruit by limiting their unique requirement although they responded to the same deprivations which brought about fruiting of the wild type. A histidine auxotroph formed fruiting bodies when histidine was lowered to growth-limiting concentrations, provided that the medium was supplemented with purines. A uracil auxotroph was isolated that, perhaps secondarily, had lost some of the mechanisms which control the formation of fruiting bodies; if uracil was present, it formed fruits even when no amino acid was limiting. No concentration of uracil was sufficient to prevent fruiting. Fruiting bodies were formed when mixtures of the uracil auxotroph and wild-type cells were inoculated on A agar plus uracil, even when 75% of the cells were wild type. Microcysts of both strains were present in the fruiting bodies.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DWORKIN M., GIBSON S. M. A SYSTEM FOR STUDYING MICROBIAL MORPHOGENESIS: RAPID FORMATION OF MICROCYSTS IN MYXOCOCCUS XANTHUS. Science. 1964 Oct 9;146(3641):243–244. doi: 10.1126/science.146.3641.243. [DOI] [PubMed] [Google Scholar]
- DWORKIN M. NUTRITIONAL REGU.ATION OF MORPHOGENESIS IN MYXOCOCCUS XANTHUS. J Bacteriol. 1963 Jul;86:67–72. doi: 10.1128/jb.86.1.67-72.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DWORKIN M. Nutritional requirements for vegetative growth of Myxococcus xanthus. J Bacteriol. 1962 Aug;84:250–257. doi: 10.1128/jb.84.2.250-257.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin M. Biology of the myxobacteria. Annu Rev Microbiol. 1966;20:75–106. doi: 10.1146/annurev.mi.20.100166.000451. [DOI] [PubMed] [Google Scholar]
- Hemphill H. E., Zahler S. A. Nutrition of Myxococcus xanthus FBa and some of its auxotrophic mutants. J Bacteriol. 1968 Mar;95(3):1011–1017. doi: 10.1128/jb.95.3.1011-1017.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEADBETTER E. R. CONTROL OF GROWTH AND MORPHOGENESIS IN SOME MYXOCOCCUS SPECIES. Nature. 1963 Dec 14;200:1127–1128. doi: 10.1038/2001127a0. [DOI] [PubMed] [Google Scholar]
- MCCURDY H. D., Jr GROWTH AND FRUITING BODY FORMATION OF CHONDROMYCES CROCATUS IN PURE CULTURE. Can J Microbiol. 1964 Dec;10:935–936. doi: 10.1139/m64-126. [DOI] [PubMed] [Google Scholar]
- Neidhardt F. C. Roles of amino acid activating enzymes in cellular physiology. Bacteriol Rev. 1966 Dec;30(4):701–719. doi: 10.1128/br.30.4.701-719.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]


