Abstract
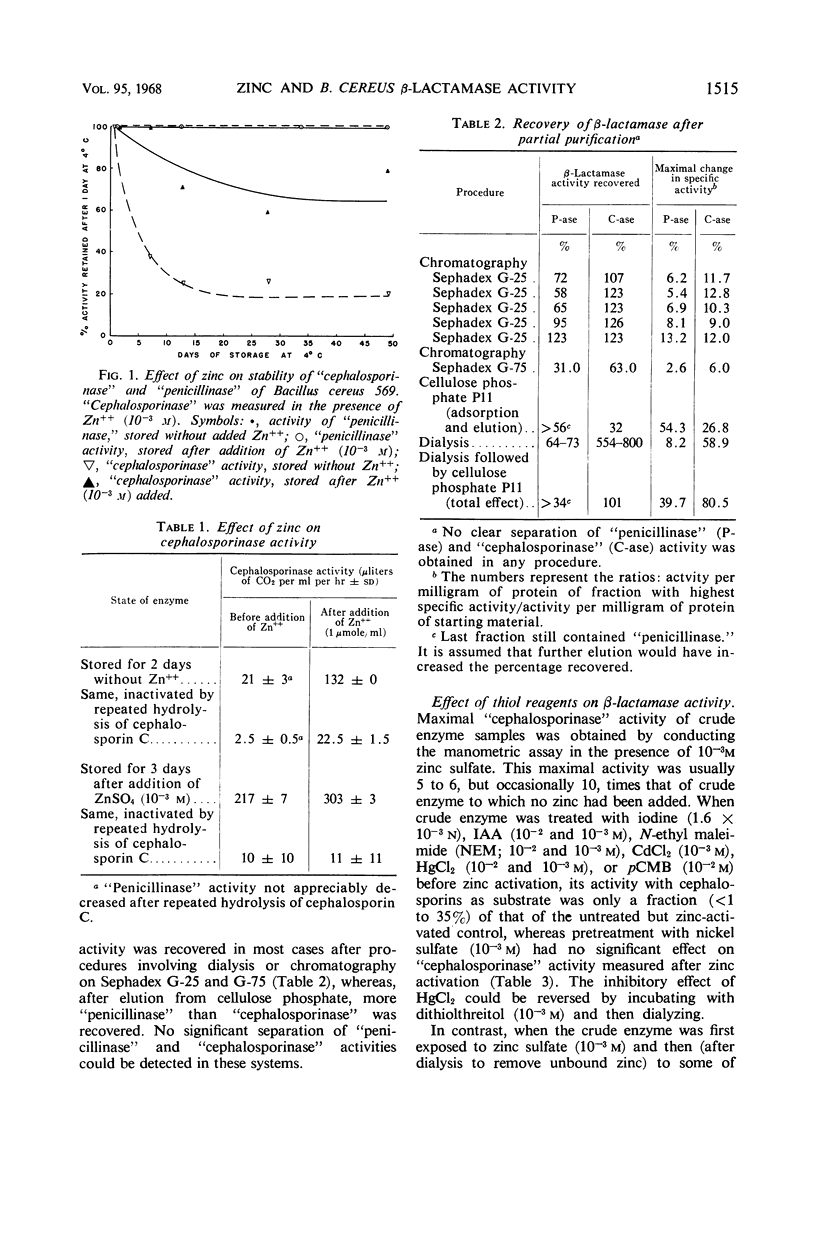
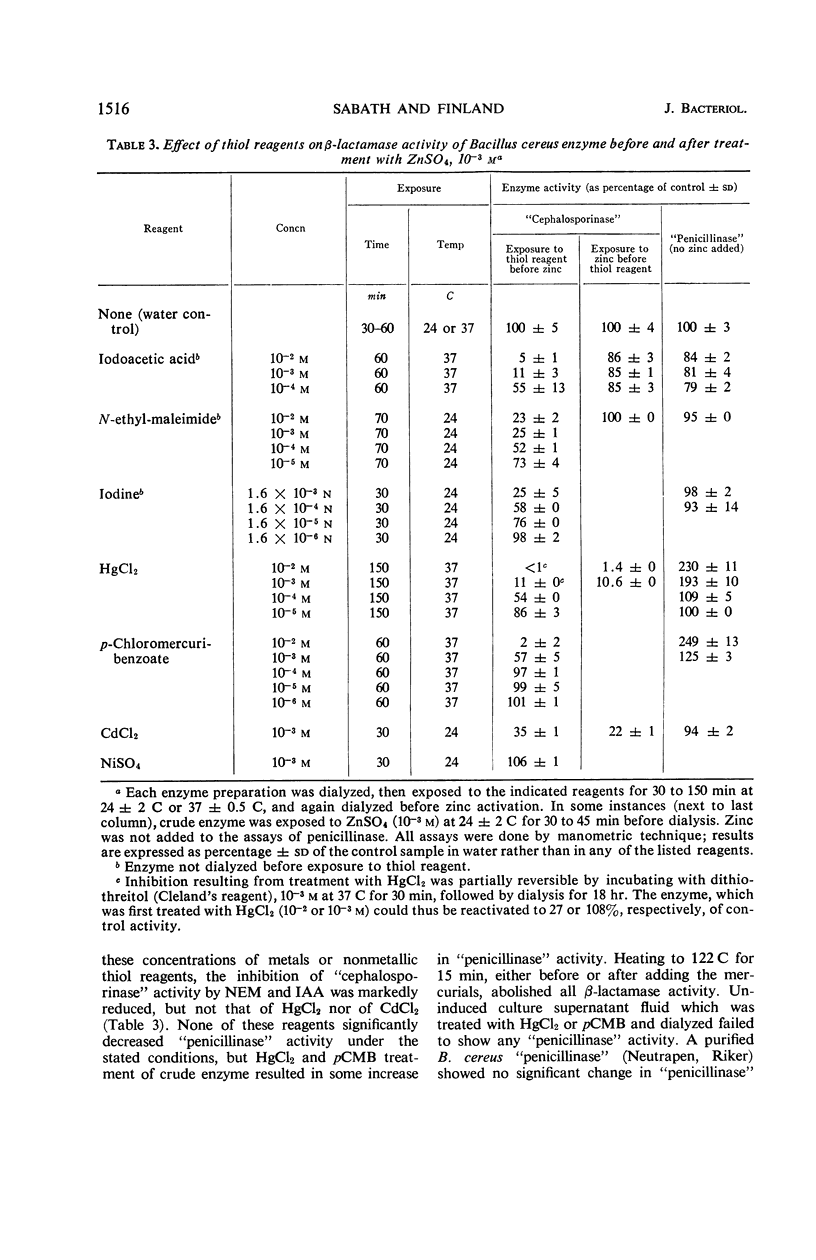
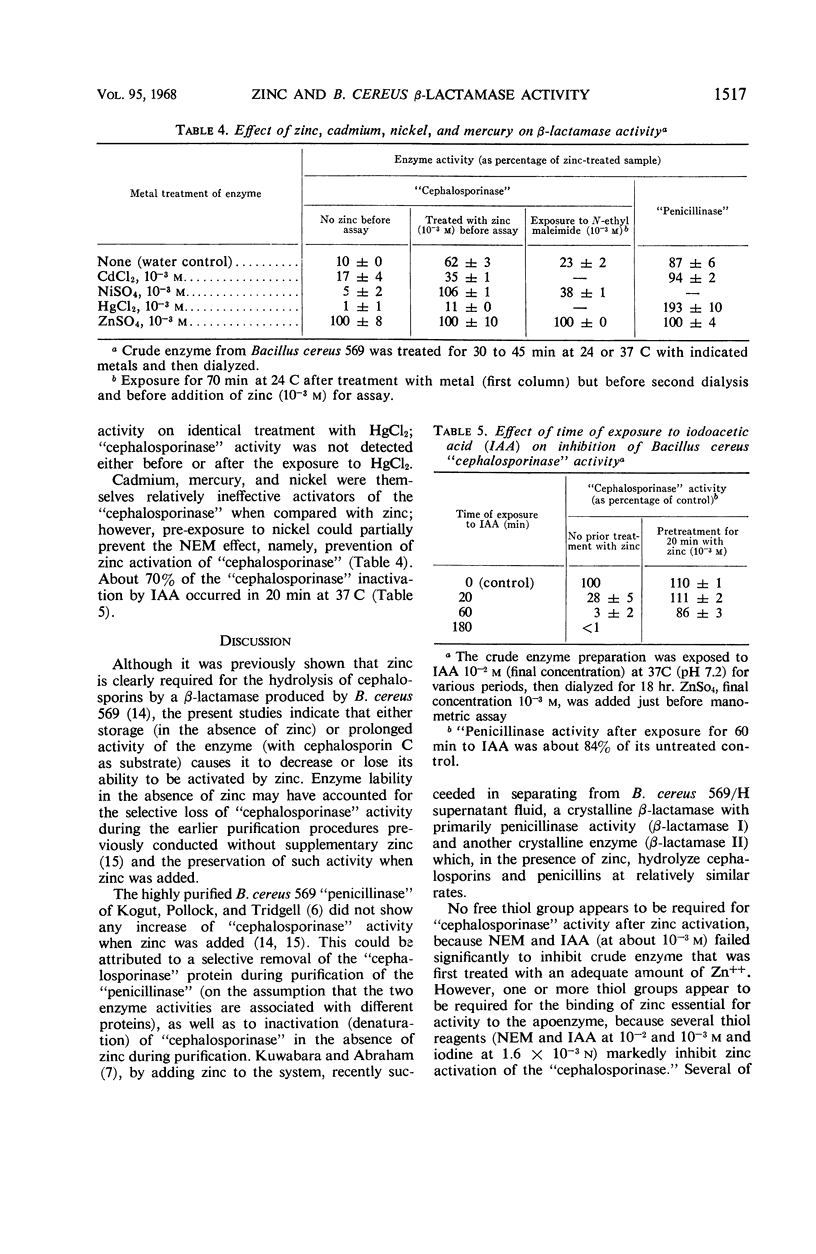
Zinc, which is required for the hydrolysis of cephalosporins by a crude enzyme from Bacillus cereus 569, also increased the stability of this activity during storage. A loss in activity of the zinc-activated enzyme which occurred on prolonged hydrolysis of cephalosporin C was not restored by further addition of zinc. The thiol reagents N-ethyl maleimide (NEM), iodoacetic acid (IAA), CdCl2, and p-chloromercuribenzoate, all at 10−3m, and iodine at 1.6 × 10−3n prevent zinc activation of the “cephalosporinase” activity. However, NEM and IAA have minimal or no demonstrable inhibitory effect if the enzyme is first treated with zinc. This suggests that zinc is linked to the apoenzyme by a thiol group. Activation by zinc is only partially prevented by NEM if the crude enzyme is pretreated with nickel, which alone causes negligible activation of the apoenzyme. The order of affinities of these metals for the apparent thiol group is thus Hg++, Cd++ > Zn++ > Ni++. The “cephalosporinase” inhibition by Hg++ was reversible with dithiothreitol. These metals and thiol reagents do not decrease the ability of the crude enzyme to hydrolyze benzylpenicillin, which is consistent with the report that purified “penicillinase” from B. cereus contains no cysteine residue. This suggests that the β-lactamases of B. cereus that hydrolyze penicillin and cephalosporins differ from each other by at least one amino acid (cysteine).
Full text
PDF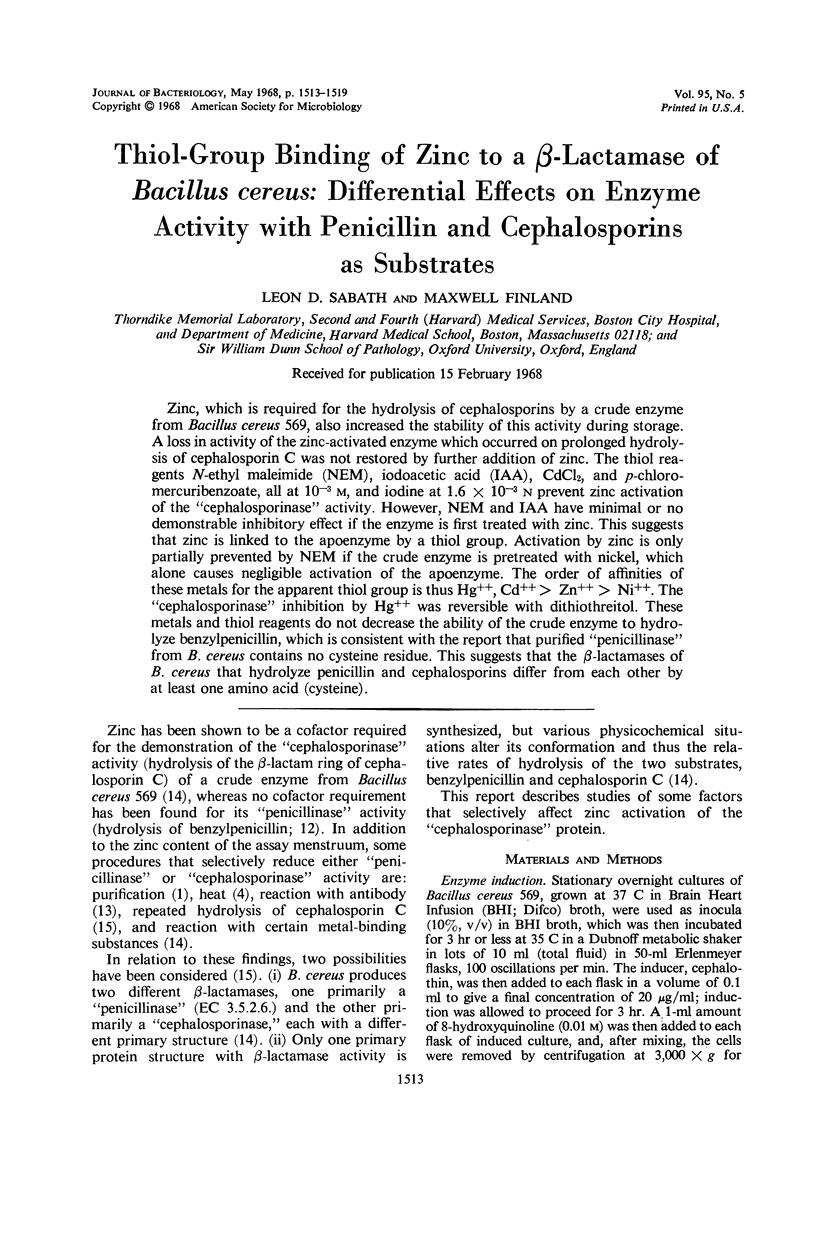



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAHAM E. P., NEWTON G. G. A comparison of the action of penicillinase on benzylpenicillin and cephalosporin N and the competitive inhibition of penicillinase by cephalosporin C. Biochem J. 1956 Aug;63(4):628–634. doi: 10.1042/bj0630628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CITRI N., GARBER N., KALKSTEIN A. THE INTERACTION OF PENICILLINASE WITH PENICILLINS. 3. COMPARISON OF EXOPENICILLINASE PREPARATIONS OF VARIOUS ORIGINS. Biochim Biophys Acta. 1964 Dec 23;92:572–581. doi: 10.1016/0926-6569(64)90017-3. [DOI] [PubMed] [Google Scholar]
- CROMPTON B., JAGO M., CRAWFORD K., NEWTON G. G., ABRAHAM E. P. Behaviour of some derivatives of 7-aminocephalosporanic acid and 6-aminopenicillanic acidas substrates, inhibitors and inducers of penicillinases. Biochem J. 1962 Apr;83:52–63. doi: 10.1042/bj0830052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citri N., Pollock M. R. The biochemistry and function of beta-lactamase (penicillinase). Adv Enzymol Relat Areas Mol Biol. 1966;28:237–323. doi: 10.1002/9780470122730.ch4. [DOI] [PubMed] [Google Scholar]
- KOGUT M., POLLOCK M. R., TRIDGELL E. J. Purification of penicillin-induced penicillinase of Bacillus cereus NRRL 569: a comparison of its properties with those of a similarly purified penicillinase produced spontaneously by a constitutive mutant strain. Biochem J. 1956 Mar;62(3):391–401. doi: 10.1042/bj0620391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwabara S., Abraham E. P. Some properties of two extracellular beta-lactamases from Bacillus cereus 569/H. Biochem J. 1967 Jun;103(3):27C–30C. doi: 10.1042/bj1030027c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- PERRET C. J. Iodometric assay of penicillinase. Nature. 1954 Nov 27;174(4439):1012–1013. doi: 10.1038/1741012a0. [DOI] [PubMed] [Google Scholar]
- POLLOCK M. R. Penicillinase adaptation in Bacillus cereus; an analysis of three phases in the response of logarithmically growing cultures to induction of penicillinase formation by penicillin. Br J Exp Pathol. 1952 Dec;33(6):587–600. [PMC free article] [PubMed] [Google Scholar]
- POLLOCK M. R. STIMULATING AND INHIBITING ANTIBODIES FOR BACTERIAL PENICILLINASE. Immunology. 1964 Nov;7:707–723. [PMC free article] [PubMed] [Google Scholar]
- Sabath L. D., Abraham E. P. Cephalosporinase and penicillinase activity of Bacillus cereus. Antimicrob Agents Chemother (Bethesda) 1965;5:392–397. [PubMed] [Google Scholar]
- Sabath L. D., Abraham E. P. Zinc as a cofactor for cephalosporinase from Bacillus cereus 569. Biochem J. 1966 Jan;98(1):11C–13C. doi: 10.1042/bj0980011c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabath L. D., Jago M., Abraham E. P. Cephalosporinase and penicillinase activities of a beta-lactamase from Pseudomonas pyocyanea. Biochem J. 1965 Sep;96(3):739–752. doi: 10.1042/bj0960739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpless N. E., Flavin M. The reactions of amines and amino acids with maleimides. Structure of the reaction products deduced from infrared and nuclear magnetic resonance spectroscopy. Biochemistry. 1966 Sep;5(9):2963–2971. doi: 10.1021/bi00873a028. [DOI] [PubMed] [Google Scholar]


