Abstract
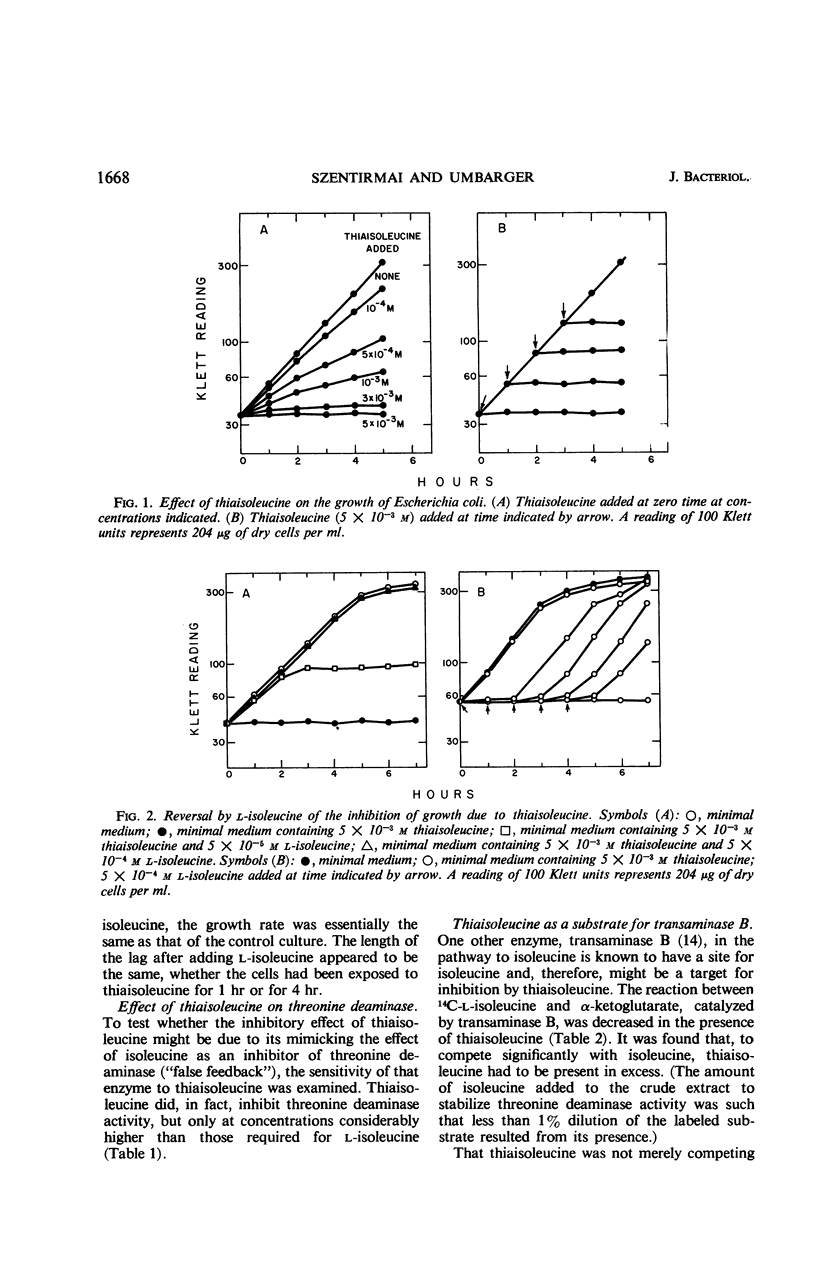
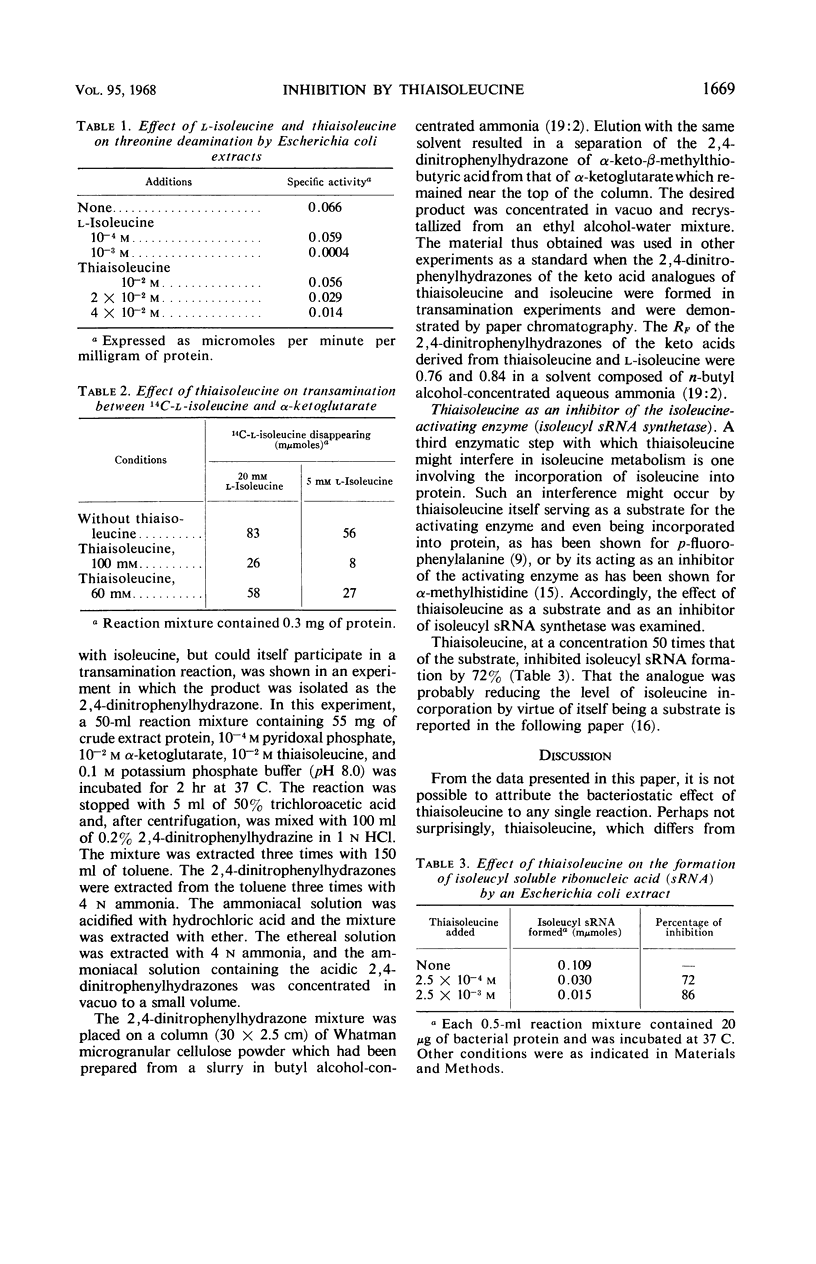
Thiaisoleucine (2-amino-3-methylthiobutyrate) completely inhibited the growth of strain K-12 of Escherichia coli at a concentration of 5 × 10−3m. The inhibition was antagonized by growth-factor amounts of l-isoleucine. Thiaisoleucine inhibited the deamination of threonine and the transfer of 14C-isoleucine to soluble ribonucleic acid and underwent transamination with α-ketoglutarate as the amino acceptor. In each case, the analogue appeared to be less effective than isoleucine as either an inhibitor or substrate.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COHEN G. N., MUNIER R. Incorporation d'analogues structuraux d'aminoacides dans les protéines bactériennes. Biochim Biophys Acta. 1956 Sep;21(3):592–593. doi: 10.1016/0006-3002(56)90207-4. [DOI] [PubMed] [Google Scholar]
- HIRSCH M. L. Analyse de l'inhibition de la croissance de mutants auxotrophes d'Escherichia coli exigeant l'homosérine, par l'acide alpha-amino-delta-hydroxyvalérianique. Ann Inst Pasteur (Paris) 1957 Aug;93(2):199–209. [PubMed] [Google Scholar]
- HOLLEY R. W., APGAR J., DOCTOR B. P., FARROW J., MARINI M. A., MERRILL S. H. A simplified procedure for the preparation of tyrosine and valine-acceptor fractions of yeast "soluble ribonucleic acid". J Biol Chem. 1961 Jan;236:200–202. [PubMed] [Google Scholar]
- MCCORD T. J., HOWELL D. C., THARP D. L., DAVIS A. L. 2-AMINO-3-METHYLTHIOBUTYRIC ACID, AN ISOLEUCINE ANTAGONIST. J Med Chem. 1965 May;8:290–292. doi: 10.1021/jm00327a004. [DOI] [PubMed] [Google Scholar]
- MOYED H. S. False feedback inhibition: inhibition of tryptophan biosynthesis by 5-methyltryptophan. J Biol Chem. 1960 Apr;235:1098–1102. [PubMed] [Google Scholar]
- NISHIMURA S., NOVELLI G. D. AMINO ACID ACCEPTOR ACTIVITY OF ENZYMICALLY ALTERED SOLUBLE RNA FROM ESCHERICHIA COLI. Biochim Biophys Acta. 1964 Apr 27;80:574–586. doi: 10.1016/0926-6550(64)90302-0. [DOI] [PubMed] [Google Scholar]
- Piperno J. R., Oxender D. L. Amino-acid-binding protein released from Escherichia coli by osmotic shock. J Biol Chem. 1966 Dec 10;241(23):5732–5734. [PubMed] [Google Scholar]
- RABINOVITZ M., McGRATH H. Protein synthesis by rabbit reticulocytes. II. Interruption of the pathway of hemoglobin synthesis by a valine analogue. J Biol Chem. 1959 Aug;234(8):2091–2095. [PubMed] [Google Scholar]
- RUDMAN D., MEISTER A. Transamination in Escherichia coli. J Biol Chem. 1953 Feb;200(2):591–604. [PubMed] [Google Scholar]
- Ravel J. M., White M. N., Shive W. Activation of tyrosine analogs in relation to enzyme repression. Biochem Biophys Res Commun. 1965 Jul 26;20(3):352–359. doi: 10.1016/0006-291x(65)90372-4. [DOI] [PubMed] [Google Scholar]
- SCHLESINGER S., MAGASANIK B. EFFECT OF ALPHA-METHYLHISTIDINE ON THE CONTROL OF HISTIDINE SYNTHESIS. J Mol Biol. 1964 Sep;9:670–682. doi: 10.1016/s0022-2836(64)80174-1. [DOI] [PubMed] [Google Scholar]
- Szentirmai A., Szentirmai M., Umbarger H. E. Isoleucine and valine metabolism of Escherichia coli. XV. Biochemical properties of mutants resistant to thiaisoleucine. J Bacteriol. 1968 May;95(5):1672–1679. doi: 10.1128/jb.95.5.1672-1679.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UMBARGER H. E., BROWN B. Isoleucine and valine metabolism in Escherichia coli. V. Antagonism between isoleucine and valine. J Bacteriol. 1955 Aug;70(2):241–248. doi: 10.1128/jb.70.2.241-248.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UMBARGER H. E., UMBARGER M. A., SIU P. M. BIOSYNTHESIS OF SERINE IN ESCHERICHIA COLI AND SALMONELLA TYPHIMURIUM. J Bacteriol. 1963 Jun;85:1431–1439. doi: 10.1128/jb.85.6.1431-1439.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]


