Abstract
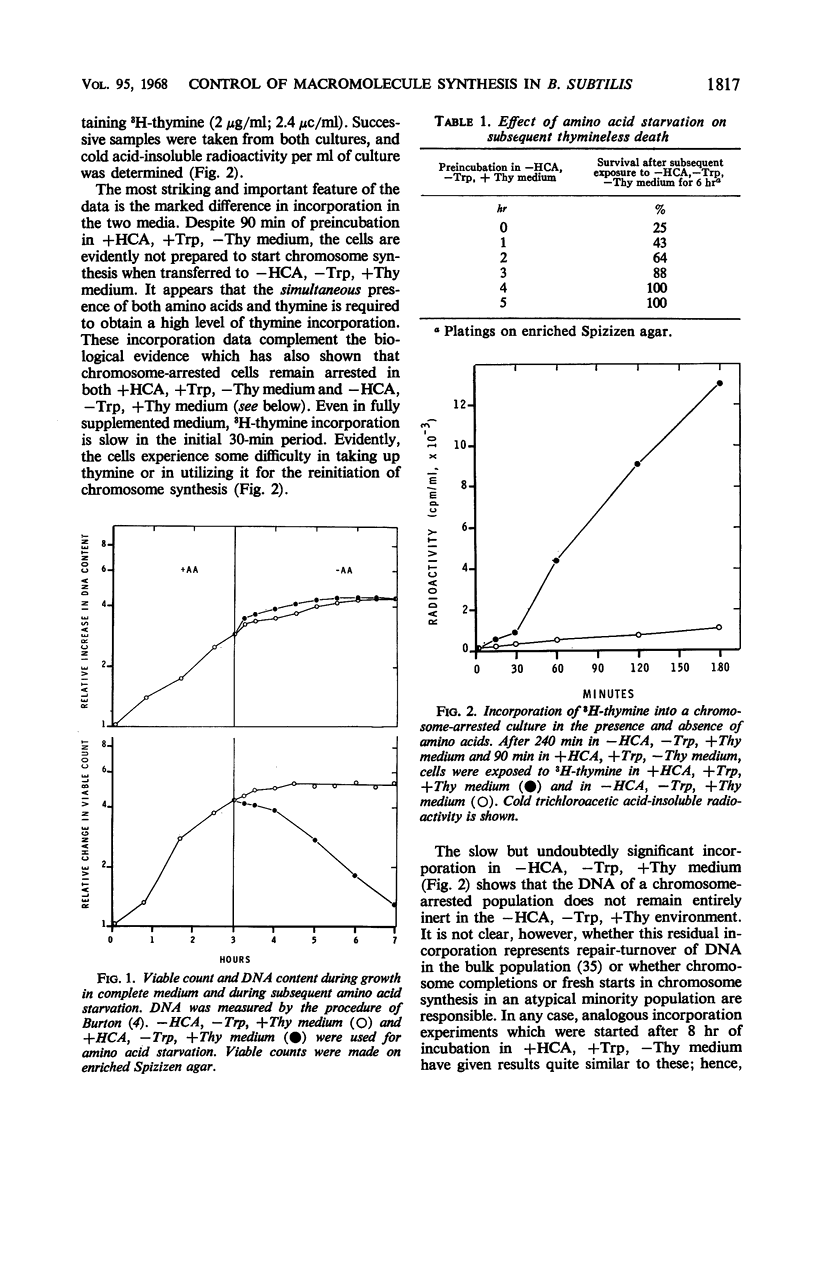
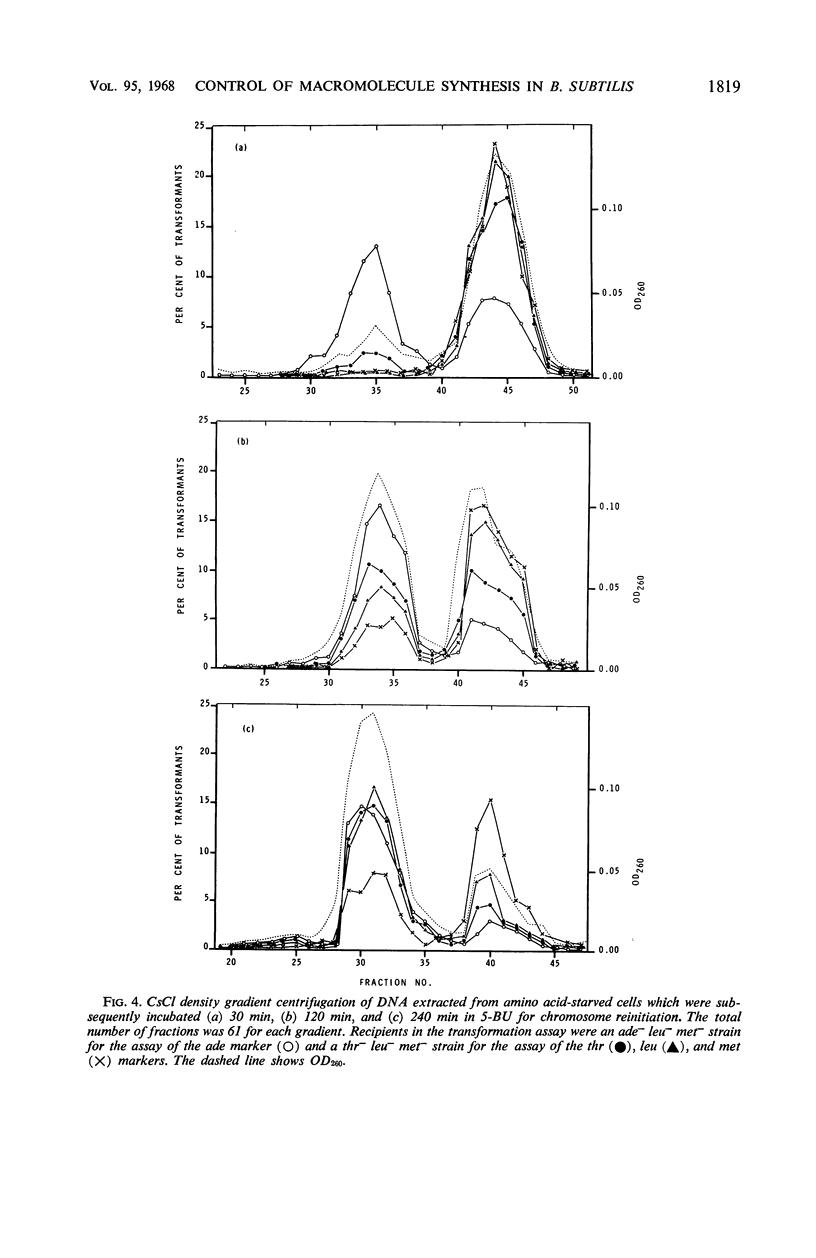
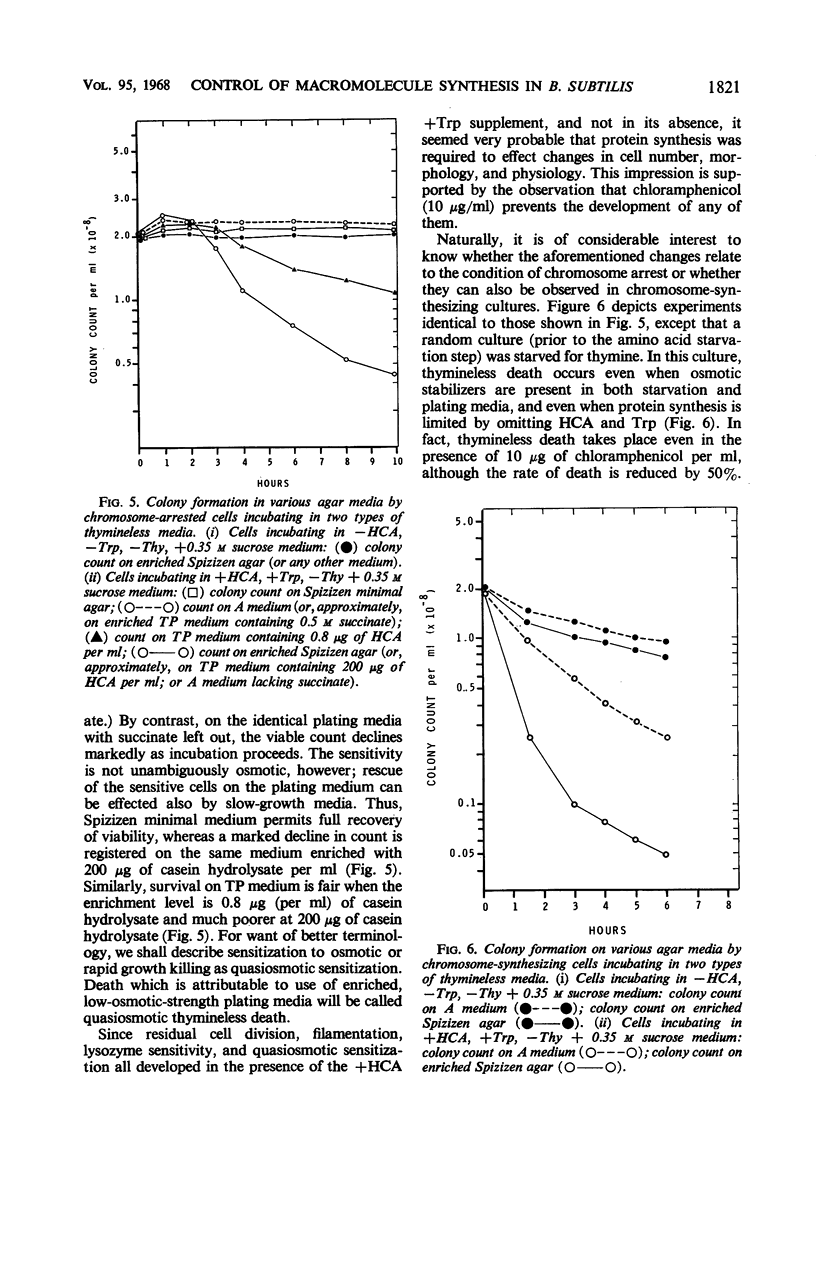
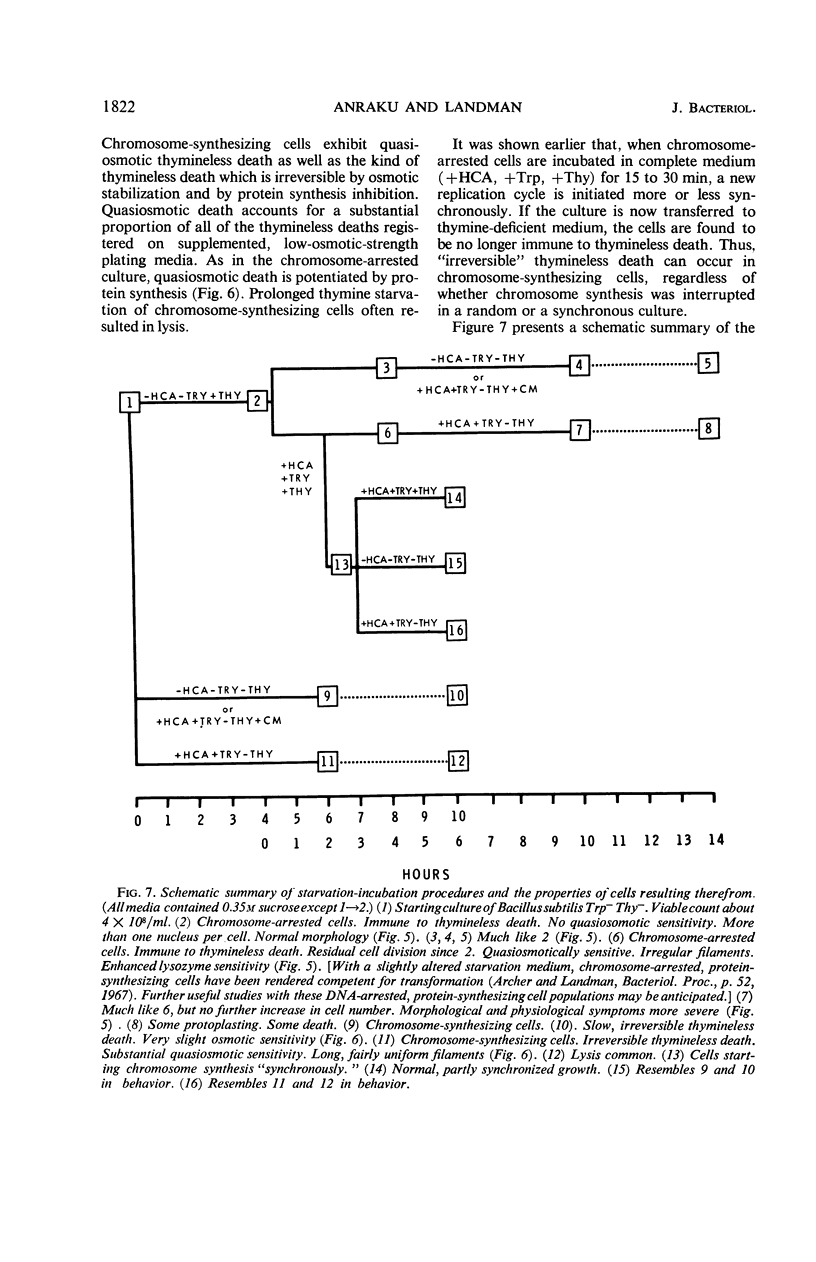
Studies of Maaløe, Lark, and others with amino acid- and thymine-starved cultures revealed successive steps in the biosynthesis of Escherichia coli chromosomes. In this study, the corresponding mechanisms in Bacillus subtilis 168 were examined. Using a strain requiring both thymine and tryptophan, we found that, 3 hr after the start of amino acid starvation, when the deoxyribonucleic acid (DNA) content of the culture had increased 40 to 50%, DNA synthesis ceased. After 4 to 5 hr, 100% of the cells were immune to thymineless death; their chromosomes had presumably been completed. Immune cultures slowly incorporated 3H-thymine. Thymine incorporation increased 20-fold 30 min after readdition of amino acids, indicating reinitiation of chromosome synthesis. Simultaneous presence of amino acids and thymine was required for reinitiation. If 5-bromouracil (5-BU) was added instead of thymine, newly replicated DNA segments could be separated by centrifugation in CsCl. Analysis of the CsCl fractions by a transformation assay showed that the order in which the markers were synthesized was ade-16, thr-5, leu-8, metB5. Less than half the chromosomes started resynthesis synchronously in 5-BU. Nevertheless, chromosome alignment in the amino acid-starved culture is probably very good: marker frequency analysis of its DNA gives the same normalized frequencies as DNA from “perfectly” aligned spores. Full viability is maintained in the chromosome-arrested culture for 10 hr in thymine-free medium in the absence or presence of amino acids. In the latter condition, protein synthesis proceeds, and the cells filament and become more lysozyme-sensitive. Such cells must be incubated and plated on hypertonic or on slow-growth media; otherwise, they undergo “quasiosmotic” thymineless death. This death is thus apparently not directly attributable to any damage of chromosomal DNA. Further, weakening of the teichoic acid portion of the cell wall is not involved, since 32P incorporation into teichoic acid is normal. Chloramphenicol prevents quasiosmotic thymineless death and also inhibits 32P incorporation into teichoic acid. Chromosome-synthesizing cultures suffer thymineless death of two types: quasiosmotic death, and death insusceptible to osmotic rescue.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anagnostopoulos C., Spizizen J. REQUIREMENTS FOR TRANSFORMATION IN BACILLUS SUBTILIS. J Bacteriol. 1961 May;81(5):741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billen D., Hewitt R. Influence of starvation for methionine and other amino acids on subsequent bacterial deoxyribonucleic acid replication. J Bacteriol. 1966 Sep;92(3):609–617. doi: 10.1128/jb.92.3.609-617.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. S., Barner H. D. STUDIES ON UNBALANCED GROWTH IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1954 Oct;40(10):885–893. doi: 10.1073/pnas.40.10.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings D. J., Mondale L. Thymineless death in Escherichia coli: strain specificity. J Bacteriol. 1967 Jun;93(6):1917–1924. doi: 10.1128/jb.93.6.1917-1924.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donachie W. D., Hobbs D. G. Recovery from "thymineless death" in Escherichia coli 15T. Biochem Biophys Res Commun. 1967 Oct 26;29(2):172–177. doi: 10.1016/0006-291x(67)90582-7. [DOI] [PubMed] [Google Scholar]
- FARMER J. L., ROTHMAN F. TRANSFORMABLE THYMINE-REQUIRING MUTANT OF BACILLUS SUBTILS. J Bacteriol. 1965 Jan;89:262–263. doi: 10.1128/jb.89.1.262-263.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANAWALT P. C. Involvement of synthesis of RNA in thymineless death. Nature. 1963 Apr 20;198:286–286. doi: 10.1038/198286a0. [DOI] [PubMed] [Google Scholar]
- HANAWALT P. C., MAALOE O., CUMMINGS D. J., SCHAECHTER M. The normal DNA replication cycle. II. J Mol Biol. 1961 Apr;3:156–165. doi: 10.1016/s0022-2836(61)80042-9. [DOI] [PubMed] [Google Scholar]
- HERSHEY A. D. Nucleic acid economy in bacteria infected with bacteriophage T2. J Gen Physiol. 1953 Sep;37(1):1–23. doi: 10.1085/jgp.37.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORN D., WEISSBACH A. Thymineless induction in Escherichia coli K12 (lambda). Biochim Biophys Acta. 1962 Nov 26;61:775–790. doi: 10.1016/0926-6550(62)90060-9. [DOI] [PubMed] [Google Scholar]
- LANDMAN O. E., HALLE S. ENZYMICALLY AND PHYSICALLY INDUCED INHERITANCE CHANGES IN BACILLUS SUBTILIS. J Mol Biol. 1963 Dec;7:721–738. doi: 10.1016/s0022-2836(63)80119-9. [DOI] [PubMed] [Google Scholar]
- LARK C., LARK K. G. EVIDENCE FOR TWO DISTINCT ASPECTS OF THE MECHANISM REGULATING CHROMOSOME REPLICATION IN ESCHERICHIA COLI. J Mol Biol. 1964 Oct;10:120–136. doi: 10.1016/s0022-2836(64)80032-2. [DOI] [PubMed] [Google Scholar]
- LARK K. G., REPKO T., HOFFMAN E. J. THE EFFECT OF AMINO ACID DEPRIVATION ON SUBSEQUENT DEOXYRIBONUCLEIC ACID REPLICATION. Biochim Biophys Acta. 1963 Sep 17;76:9–24. [PubMed] [Google Scholar]
- Lark K. G. Regulation of chromosome replication and segregation in bacteria. Bacteriol Rev. 1966 Mar;30(1):3–32. doi: 10.1128/br.30.1.3-32.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAALOE O., HANAWALT P. C. Thymine deficiency and the normal DNA replication cycle. I. J Mol Biol. 1961 Apr;3:144–155. doi: 10.1016/s0022-2836(61)80041-7. [DOI] [PubMed] [Google Scholar]
- MANDEL M., ROWLEY D. B. CONFIGURATION AND BASE COMPOSITION OF DEOXYRIBONUCLEIC ACID FROM SPORES OF BACILLUS SUBTILIS VAR. NIGER. J Bacteriol. 1963 Jun;85:1445–1446. doi: 10.1128/jb.85.6.1445-1446.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MENNIGMANN H. D., SZYBALSKI W. Molecular mechanism of thymine-less death. Biochem Biophys Res Commun. 1962 Nov 27;9:398–404. doi: 10.1016/0006-291x(62)90023-2. [DOI] [PubMed] [Google Scholar]
- Mennigmann H. D. Induction in Escherichia coli 15 of the colicinogenic factor by thymine-less death. Biochem Biophys Res Commun. 1964 Jul 1;16(4):373–378. doi: 10.1016/0006-291x(64)90043-9. [DOI] [PubMed] [Google Scholar]
- OISHI M., YOSHIKAWA H., SUEOKA N. SYNCHRONOUS AND DICHOTOMOUS REPLICATIONS OF THE BACILLUS SUBTILIS CHROMOSOME DURING SPORE GERMINATION. Nature. 1964 Dec 12;204:1069–1073. doi: 10.1038/2041069a0. [DOI] [PubMed] [Google Scholar]
- Oishi M., Oishi A., Sueoka N. Location of genetic loci of soluble RNA on Bacillus subtilis chromosome. Proc Natl Acad Sci U S A. 1966 May;55(5):1095–1103. doi: 10.1073/pnas.55.5.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritikin W. B., Romig W. R. Death of Bacillus subtilis Auxotrophs Due to Deprivation of Thymine, Tryptophan, or Uracil. J Bacteriol. 1966 Aug;92(2):291–296. doi: 10.1128/jb.92.2.291-296.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfe R. On the mechanism of thymineless death in Bacillus subtilis. Proc Natl Acad Sci U S A. 1967 Jan;57(1):114–121. doi: 10.1073/pnas.57.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAITO H., MIURA K. I. PREPARATION OF TRANSFORMING DEOXYRIBONUCLEIC ACID BY PHENOL TREATMENT. Biochim Biophys Acta. 1963 Aug 20;72:619–629. [PubMed] [Google Scholar]
- Wachsman J. T., Kemp S., Kogg L. Thymineless death in Bacillus megaterium. J Bacteriol. 1964 May;87(5):1079–1086. doi: 10.1128/jb.87.5.1079-1086.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M. The radiation sensitivity of Escherichia coli B: a hypothesis relating filament formation and prophage induction. Proc Natl Acad Sci U S A. 1967 May;57(5):1275–1279. doi: 10.1073/pnas.57.5.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOSHIKAWA H., O'SULLIVAN A., SUEOKA N. SEQUENTIAL REPLICATION OF THE BACILLUS SUBTILIS CHROMOSOME. 3. REGULATION OF INITIATION. Proc Natl Acad Sci U S A. 1964 Oct;52:973–980. doi: 10.1073/pnas.52.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOSHIKAWA H., SUEOKA N. Sequential replication of Bacillus subtilis chromosome. I. Comparison of marker frequencies in exponential and stationary growth phases. Proc Natl Acad Sci U S A. 1963 Apr;49:559–566. doi: 10.1073/pnas.49.4.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOSHIKAWA H., SUEOKA N. Sequential replication of the Bacillus subtilis chromosome. II. Isotopic transfer experiments. Proc Natl Acad Sci U S A. 1963 Jun;49:806–813. doi: 10.1073/pnas.49.6.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa H. The initiation of DNA replication in Bacillus subtilis. Proc Natl Acad Sci U S A. 1967 Jul;58(1):312–319. doi: 10.1073/pnas.58.1.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young F. E., Jackson A. P. Extent and significance of contamination of DNA by teichoic acid in Bacillus subtilis. Biochem Biophys Res Commun. 1966 May 25;23(4):490–495. doi: 10.1016/0006-291x(66)90755-8. [DOI] [PubMed] [Google Scholar]


