Abstract
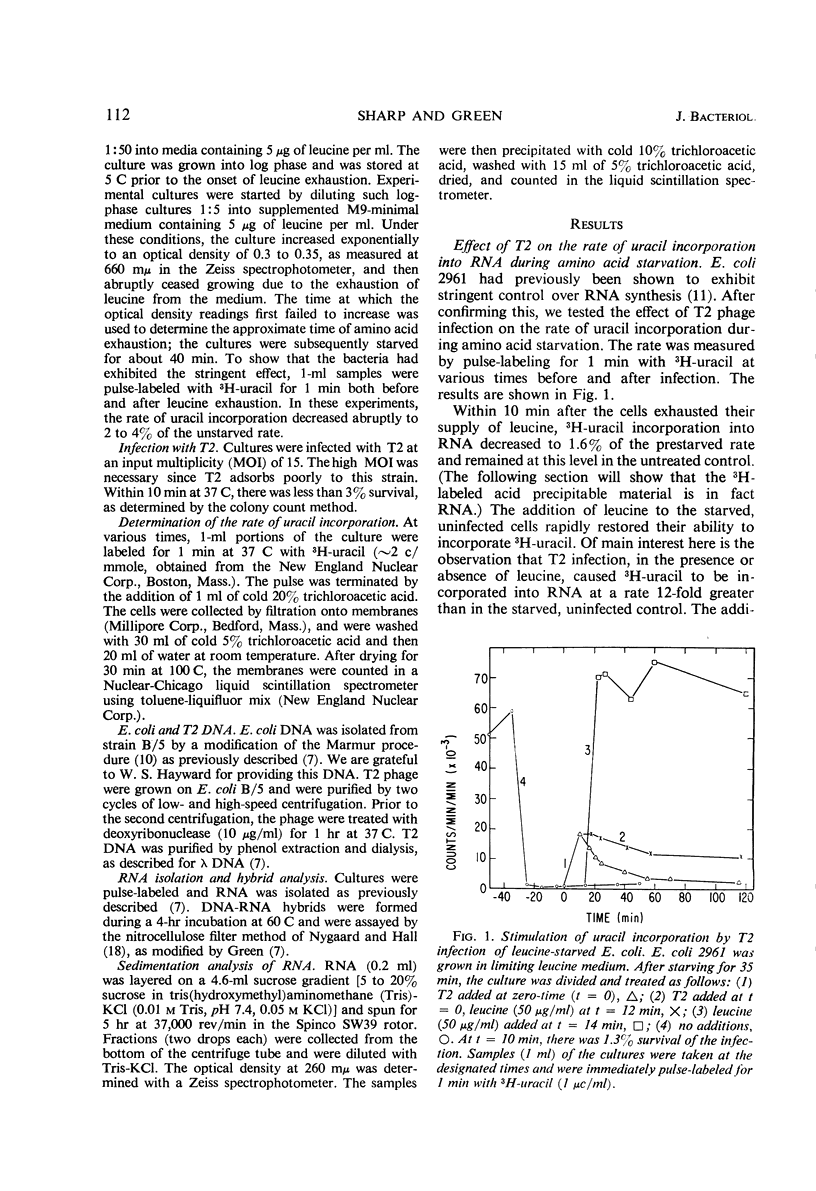
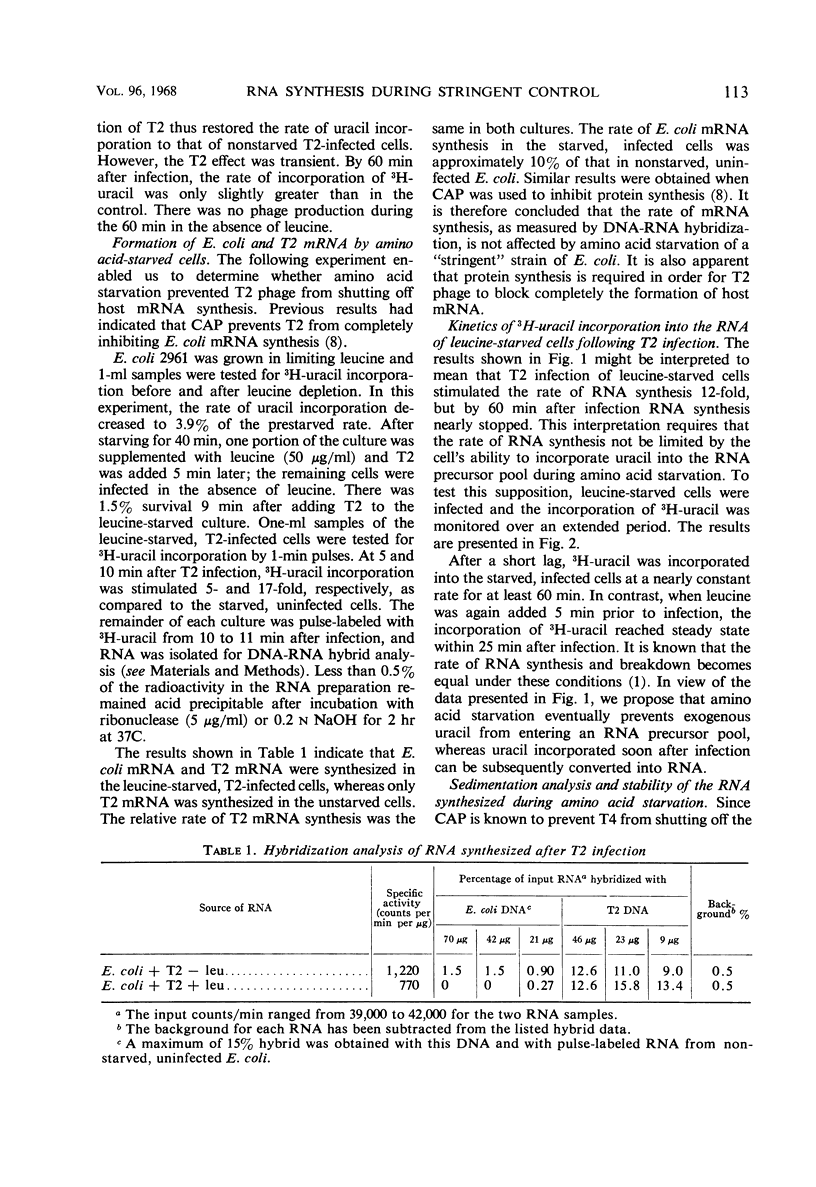
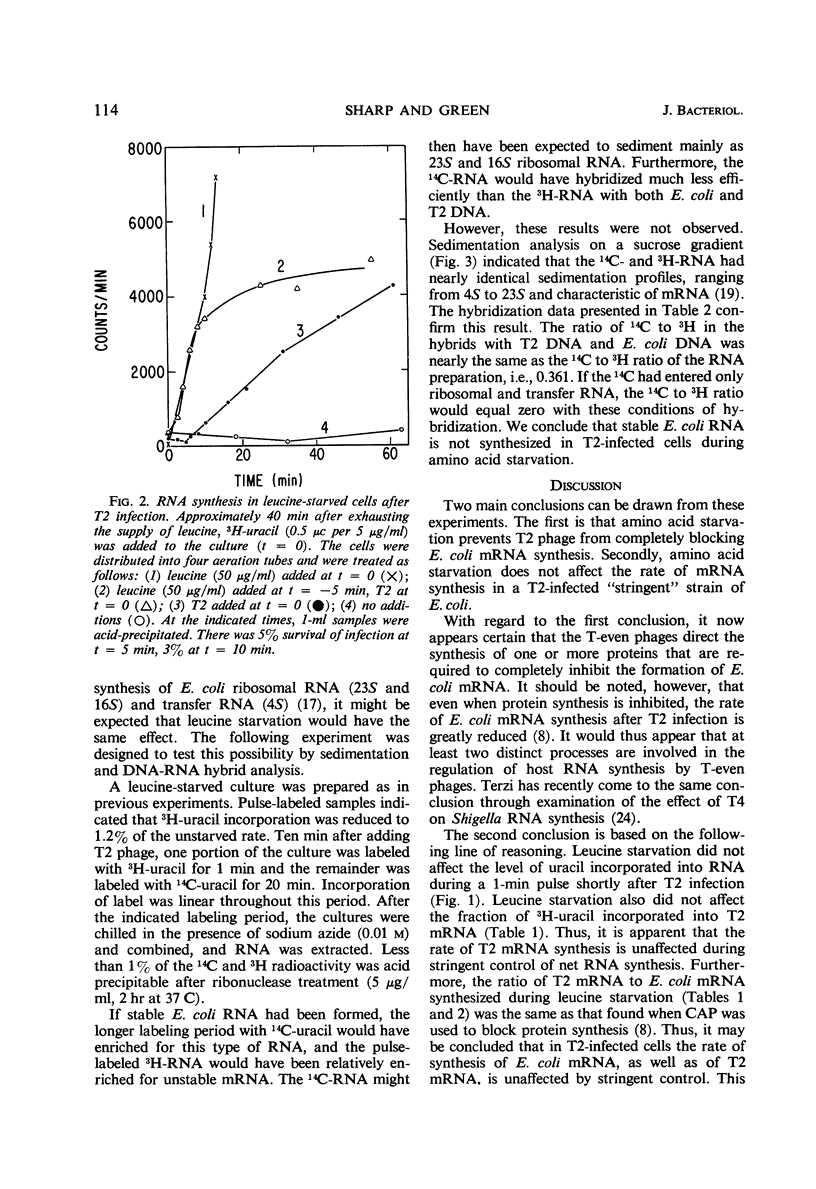
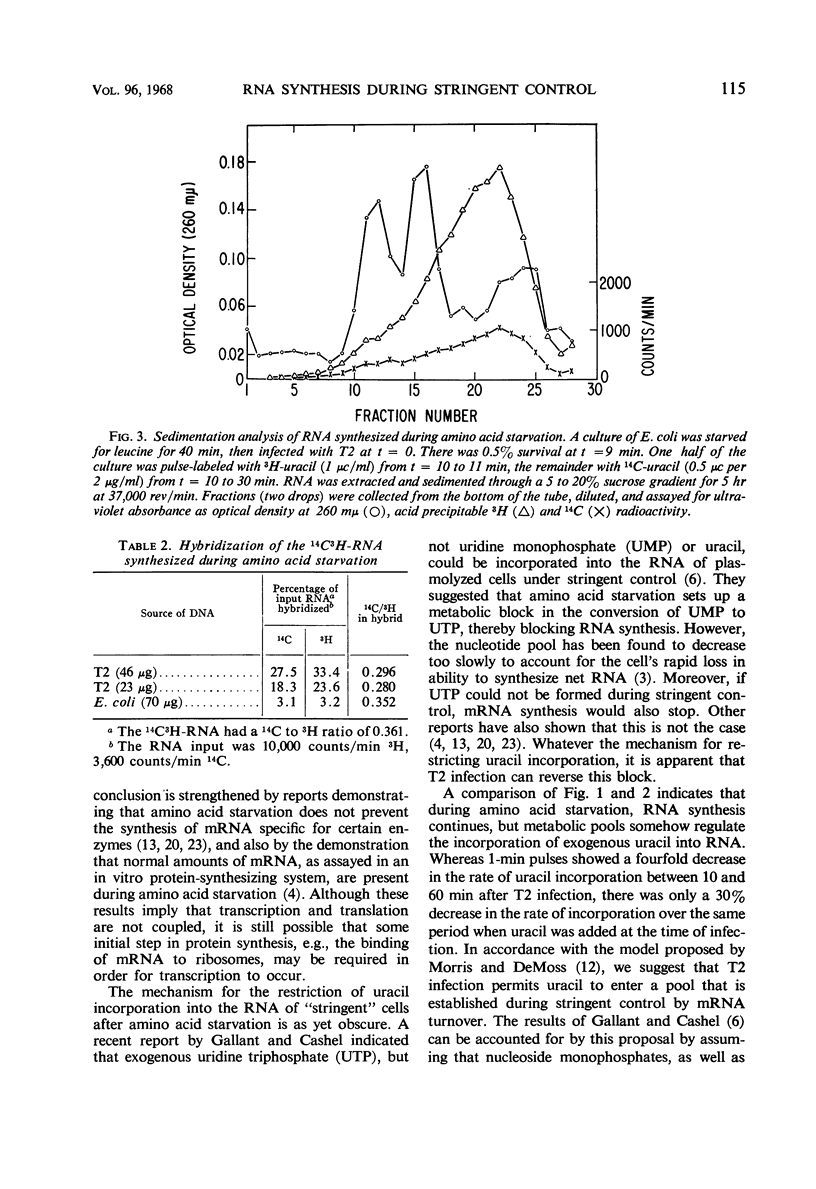
The leucine auxotroph Escherichia coli 2961 exhibited stringent control of net ribonucleic acid (RNA) synthesis during amino acid starvation. After leucine was exhausted from the medium, the rate of uracil incorporation into RNA rapidly decreased to 2 to 4% of the prestarvation value. Infection of the starved cells with T2 phage stimulated uracil incorporation to a level equivalent to that of unstarved, T2-infected cells. The RNA synthesized during leucine starvation of the T2-infected cells consisted of T2 and E. coli messenger RNA, but stable ribosomal RNA (23S and 16S) did not appear to be synthesized. It is concluded that one or more T2-specific proteins are required to shut off host messenger RNA synthesis. Furthermore, transcription of E. coli and T2 deoxyribonucleic acid is not necessarily coupled to the translation of messenger RNA during stringent control of net RNA synthesis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASTRACHAN L., VOLKIN E. Properties of ribonucleic acid turnover in T2-infected Escherichia coli. Biochim Biophys Acta. 1958 Sep;29(3):536–544. doi: 10.1016/0006-3002(58)90010-6. [DOI] [PubMed] [Google Scholar]
- BYRNE R., LEVIN J. G., BLADEN H. A., NIRENBERG M. W. THE IN VITRO FORMATION OF A DNA-RIBOSOME COMPLEX. Proc Natl Acad Sci U S A. 1964 Jul;52:140–148. doi: 10.1073/pnas.52.1.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlin G., Neuhard J. Regulation of nucleoside triphosphate pools in Escherichia coli. J Mol Biol. 1967 Mar 14;24(2):225–230. doi: 10.1016/0022-2836(67)90328-2. [DOI] [PubMed] [Google Scholar]
- Friesen J. D. Control of messenger RNA synthesis and decay in Escherichia coli. J Mol Biol. 1966 Oct;20(3):559–573. doi: 10.1016/0022-2836(66)90011-8. [DOI] [PubMed] [Google Scholar]
- Gallant J., Cashel M. On the mechanism of amino acid control of ribonucleic acid biosynthesis. J Mol Biol. 1967 May 14;25(3):545–553. doi: 10.1016/0022-2836(67)90205-7. [DOI] [PubMed] [Google Scholar]
- Green M. H. Inactivation of the prophage lambda repressor without induction. J Mol Biol. 1966 Mar;16(1):134–148. doi: 10.1016/s0022-2836(66)80268-1. [DOI] [PubMed] [Google Scholar]
- Hayward W. S., Green M. H. Inhibition of Escherichia coli and bacteriophage lambda messenger RNA synthesis by T4. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1675–1678. doi: 10.1073/pnas.54.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KURLAND C. G., MAALOE O. Regulation of ribosomal and transfer RNA synthesis. J Mol Biol. 1962 Mar;4:193–210. doi: 10.1016/s0022-2836(62)80051-5. [DOI] [PubMed] [Google Scholar]
- Morris D. W., DeMoss J. A. Polysome transitions and the regulation of ribonucleic acid synthesis in Escherichia coli. Proc Natl Acad Sci U S A. 1966 Jul;56(1):262–268. doi: 10.1073/pnas.56.1.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris D. W., DeMoss J. A. Role of aminoacyl-transfer ribonucleic acid in the regulation of ribonucleic acid synthesis in Escherichia coli. J Bacteriol. 1965 Dec;90(6):1624–1631. doi: 10.1128/jb.90.6.1624-1631.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKADA D., MAGASANIK B. THE ROLES OF INDUCER AND CATABOLITE REPRESSOR IN THE SYNTHESIS OF BETA-GALACTOSIDASE BY ESCHERICHIA COLI. J Mol Biol. 1964 Jan;8:105–127. doi: 10.1016/s0022-2836(64)80153-4. [DOI] [PubMed] [Google Scholar]
- NOMURA M., OKAMOTO K., ASANO K. RNA metabolism in Escherichia coli infected with bacteriophage T4. Inhibition of host ribosomal and soluble RNA synthesis by phage and effect of chloromycetin. J Mol Biol. 1962 May;4:376–387. doi: 10.1016/s0022-2836(62)80018-7. [DOI] [PubMed] [Google Scholar]
- Neidhardt F. C. Roles of amino acid activating enzymes in cellular physiology. Bacteriol Rev. 1966 Dec;30(4):701–719. doi: 10.1128/br.30.4.701-719.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C. The regulation RNA synthesis in bacteria. Prog Nucleic Acid Res Mol Biol. 1964;3:145–181. doi: 10.1016/s0079-6603(08)60741-2. [DOI] [PubMed] [Google Scholar]
- SAGIK B. P., GREEN M. H., HAYASHI M., SPIEGELMAN S. Size distribution of "informational" RNA. Biophys J. 1962 Sep;2:409–421. doi: 10.1016/s0006-3495(62)86864-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEKIGUCHI M., COHEN S. S. THE SYNTHESIS OF MESSENGER RNA WITHOUT PROTEIN SYNTHESIS. II. SYNTHESIS OF PHAGE-INDUCED RNA AND SEQUENTIAL ENZYME PRODUCTION. J Mol Biol. 1964 May;8:638–659. doi: 10.1016/s0022-2836(64)80114-5. [DOI] [PubMed] [Google Scholar]
- STENT G. S., BRENNER S. A genetic locus for the regulation of ribonucleic acid synthesis. Proc Natl Acad Sci U S A. 1961 Dec 15;47:2005–2014. doi: 10.1073/pnas.47.12.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STENT G. S. THE OPERON: ON ITS THIRD ANNIVERSARY. MODULATION OF TRANSFER RNA SPECIES CAN PROVIDE A WORKABLE MODEL OF AN OPERATOR-LESS OPERON. Science. 1964 May 15;144(3620):816–820. doi: 10.1126/science.144.3620.816. [DOI] [PubMed] [Google Scholar]
- STERN J. L., SEKIGUCHI M., BARNER H. D., COHEN S. S. THE SYNTHESIS OF MESSENGER RNA WITHOUT PROTEIN SYNTHESIS. I. STUDIES WITH THYMINELESS STRAINS OF ESCHERICHIA COLI. J Mol Biol. 1964 May;8:629–637. doi: 10.1016/s0022-2836(64)80113-3. [DOI] [PubMed] [Google Scholar]
- Terzi M. Studies on the mechanism of bacteriophage T4 interference with host metabolism. J Mol Biol. 1967 Aug 28;28(1):37–44. doi: 10.1016/s0022-2836(67)80075-5. [DOI] [PubMed] [Google Scholar]
- VOLKIN E., ASTRACHAN L. Phosphorus incorporation in Escherichia coli ribo-nucleic acid after infection with bacteriophage T2. Virology. 1956 Apr;2(2):149–161. doi: 10.1016/0042-6822(56)90016-2. [DOI] [PubMed] [Google Scholar]


