Abstract
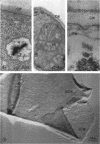
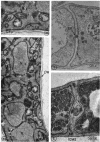
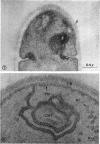
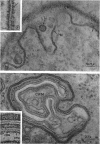
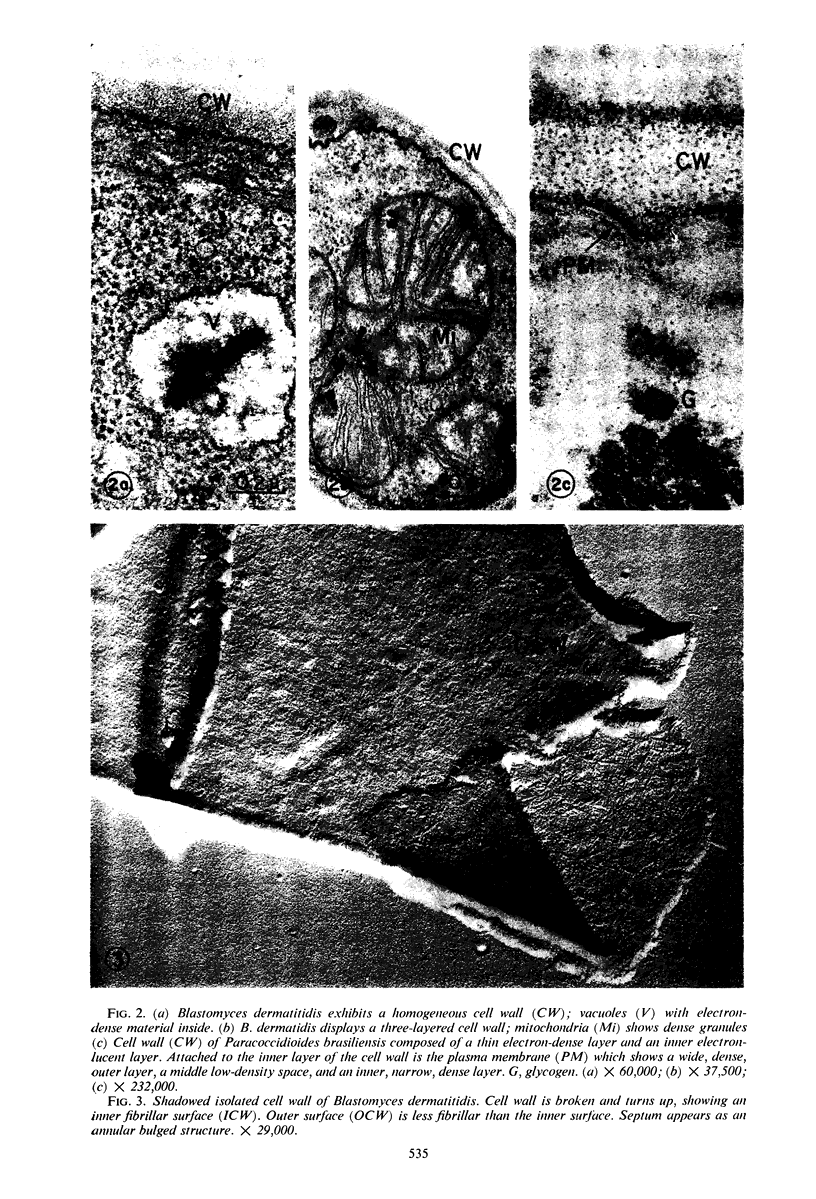
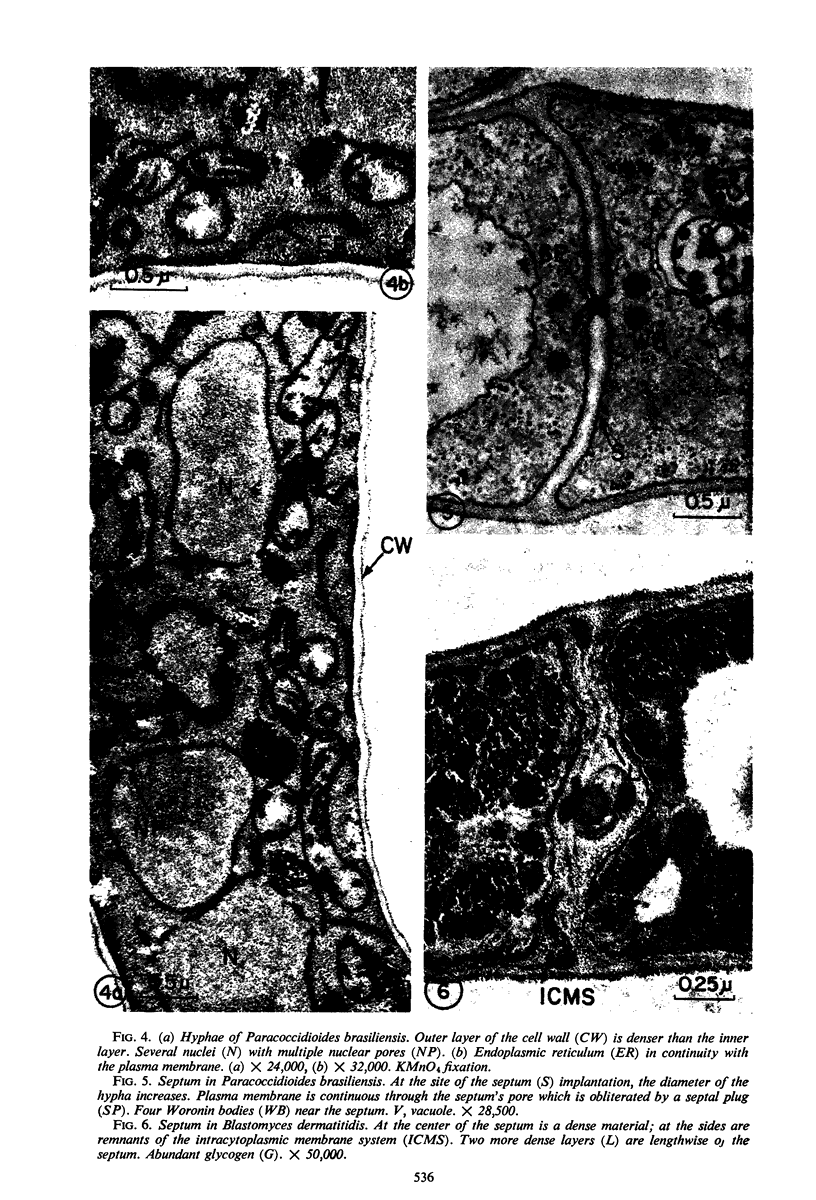
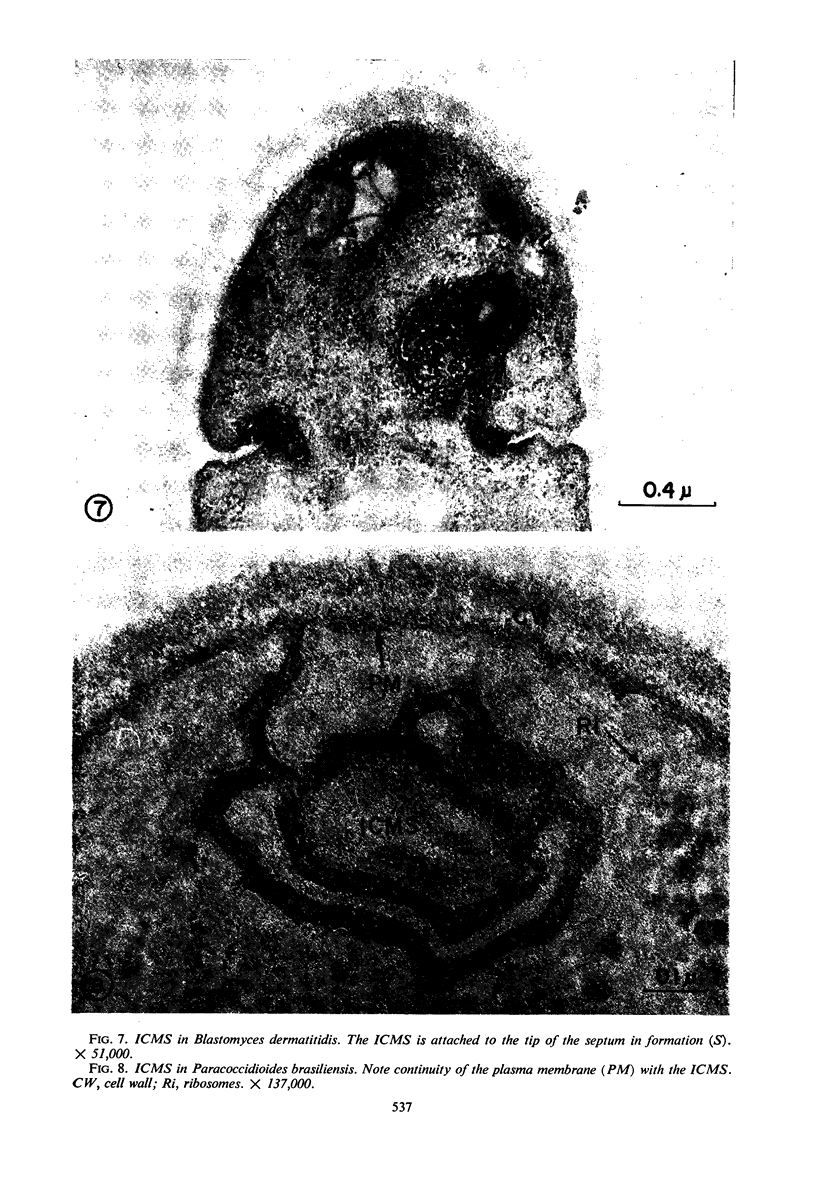
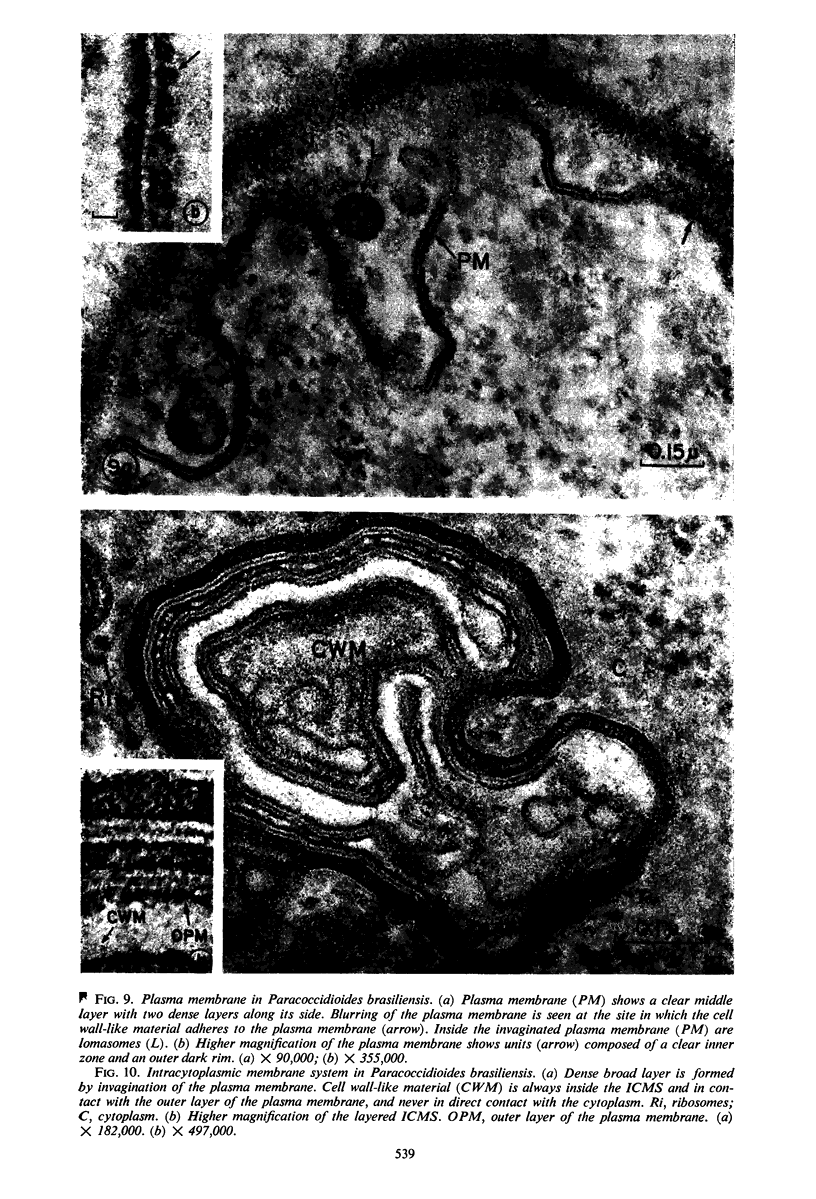
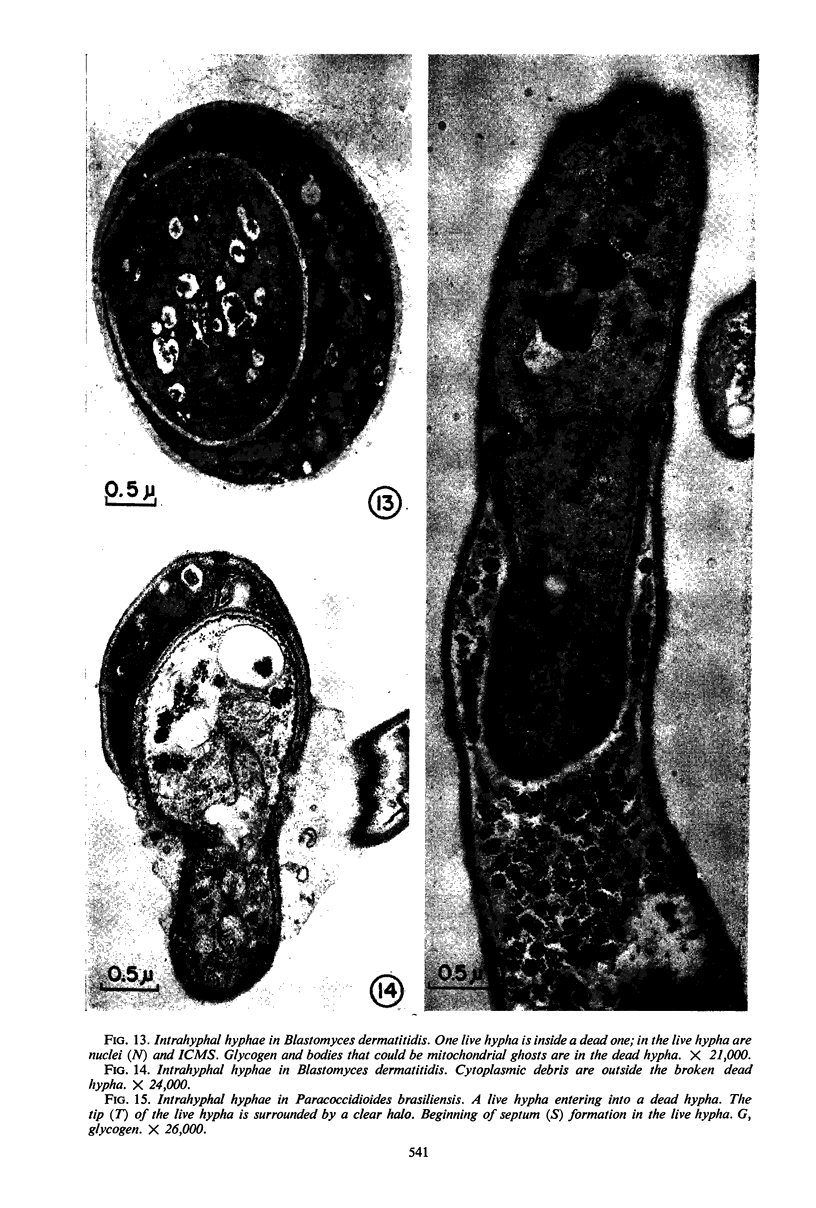
A comparative study of the mycelial phase of Paracoccidioides brasiliensis and Blastomyces dermatitidis reveals that both fungi are very much alike, containing multiple nuclei and nuclear pores, mitochondria, ribosomes, scarce endoplasmic reticulum, intracytoplasmic membrane systems, glycogen, and vacuoles. Shadowed cell walls show fine fibrillar surfaces that contrast with those in the yeast phase. The intracytoplasmic membrane system is continuous with the plasma membrane and is similar to bacterial mesosomes. Granules with light cores and dark rims are observed in the plasma membrane. Live hyphae growing inside a dead hypha are found much more frequently in immersed cultures than in solid-medium cultures, suggesting that breakage of the hypha could elicit this phenomenon.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLONDEL B., TURIAN G. Relation between basophilia and fine structure of cytoplasm in the fungus Allomyces macrogynus Em. J Biophys Biochem Cytol. 1960 Feb;7:127–134. doi: 10.1083/jcb.7.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasie J. K., Dewey M. M., Blaurock A. E., Worthington C. R. Electron microscope and low-angle x-ray diffraction studies on outer segment membranes from the retina of the frog. J Mol Biol. 1965 Nov;14(1):143–152. doi: 10.1016/s0022-2836(65)80236-4. [DOI] [PubMed] [Google Scholar]
- CARBONELL L. M., POLLAK L. "Myelin figures" in yeast cultures of Paracoccidioides brasiliensis. J Bacteriol. 1962 Jun;83:1356–1357. doi: 10.1128/jb.83.6.1356-1357.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARBONELL L. M., POLLAK L. ULTRAESTRUCTURA DEL PARACOCCIDIOIDES BRASILIENSIS EN CULTIVOS DE LA FASE LEVADURIFORME. Mycopathol Mycol Appl. 1963 Jun 15;19:184–204. doi: 10.1007/BF02051247. [DOI] [PubMed] [Google Scholar]
- CARBONELL L. M., RODRIGUEZ J. TRANSFORMATION OF MYCELIAL AND YEAST FORMS OF PARACOCCIDIOIDES BRASILIENSIS IN CULTURES AND IN EXPERIMENTAL INOCULATIONS. J Bacteriol. 1965 Aug;90:504–510. doi: 10.1128/jb.90.2.504-510.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonell L. M. Cell wall changes during the budding process of Paracoccidioides brasiliensis and Blastomyces dermatitidis. J Bacteriol. 1967 Jul;94(1):213–223. doi: 10.1128/jb.94.1.213-223.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellar D. J., Lundgren D. G., Slepecky R. A. Fine structure of Bacillus megaterium during synchronous growth. J Bacteriol. 1967 Oct;94(4):1189–1205. doi: 10.1128/jb.94.4.1189-1205.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREEMAN J. A., SPURLOCK B. O. A new epoxy embedment for electron microscopy. J Cell Biol. 1962 Jun;13:437–443. doi: 10.1083/jcb.13.3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROCOTT R. G. A stain for fungi in tissue sections and smears using Gomori's methenamine-silver nitrate technic. Am J Clin Pathol. 1955 Aug;25(8):975–979. doi: 10.1093/ajcp/25.8_ts.0975. [DOI] [PubMed] [Google Scholar]
- HAWKER L. E. FINE STRUCTURE OF FUNGI AS REVEALED BY ELECTRON MICROSCOPY. Biol Rev Camb Philos Soc. 1965 Feb;40:52–92. doi: 10.1111/j.1469-185x.1965.tb00795.x. [DOI] [PubMed] [Google Scholar]
- IMAEDA T., OGURA M. Formation of intracytoplasmic membrane system of mycobacteria related to cell division. J Bacteriol. 1963 Jan;85:150–163. doi: 10.1128/jb.85.1.150-163.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUFT J. H. Permanganate; a new fixative for electron microscopy. J Biophys Biochem Cytol. 1956 Nov 25;2(6):799–802. doi: 10.1083/jcb.2.6.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry R. J., Sussman A. S. Intra-hyphal hyphae in "clock" mutants of Neurospora. Mycologia. 1966 Jul-Aug;58(4):541–548. [PubMed] [Google Scholar]
- McALEAR J. H., EDWARDS G. A. [Continuity of plasma membrane and nuclear membrane]. Exp Cell Res. 1959 Mar;16(3):689–692. doi: 10.1016/0014-4827(59)90139-9. [DOI] [PubMed] [Google Scholar]
- McDonough E. S., Lewis A. L. Blastomyces dermatitidis: production of the sexual stage. Science. 1967 Apr 28;156(3774):528–529. doi: 10.1126/science.156.3774.528. [DOI] [PubMed] [Google Scholar]
- SHATKIN A. J., TATUM E. L. Electron microscopy of Neurospora crassa mycelia. J Biophys Biochem Cytol. 1959 Dec;6:423–426. doi: 10.1083/jcb.6.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman A. S., Durkee T. L., Lowry R. J. A model for rhythmic and temperature-independent growth in 'clock' mutants of Neurospora. Mycopathol Mycol Appl. 1965 Apr 14;25(3):381–396. doi: 10.1007/BF02049924. [DOI] [PubMed] [Google Scholar]
- TSUDA S. Electron microscopical studies of ultrathin sections in Penicillium chrysogenum. J Bacteriol. 1956 Apr;71(4):450–453. doi: 10.1128/jb.71.4.450-453.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weier T. E., Bisalputra T., Harrison A. Subunits in chloroplast membranes of Scenedesmus quadricauda. J Ultrastruct Res. 1966 Apr;15(1):38–56. doi: 10.1016/s0022-5320(66)80092-8. [DOI] [PubMed] [Google Scholar]
- Wilsenach R., Kessel M. The role of lomasomes in wall formation in Penicillium vermiculatum. J Gen Microbiol. 1965 Sep;40(3):401–404. doi: 10.1099/00221287-40-3-401. [DOI] [PubMed] [Google Scholar]