Abstract
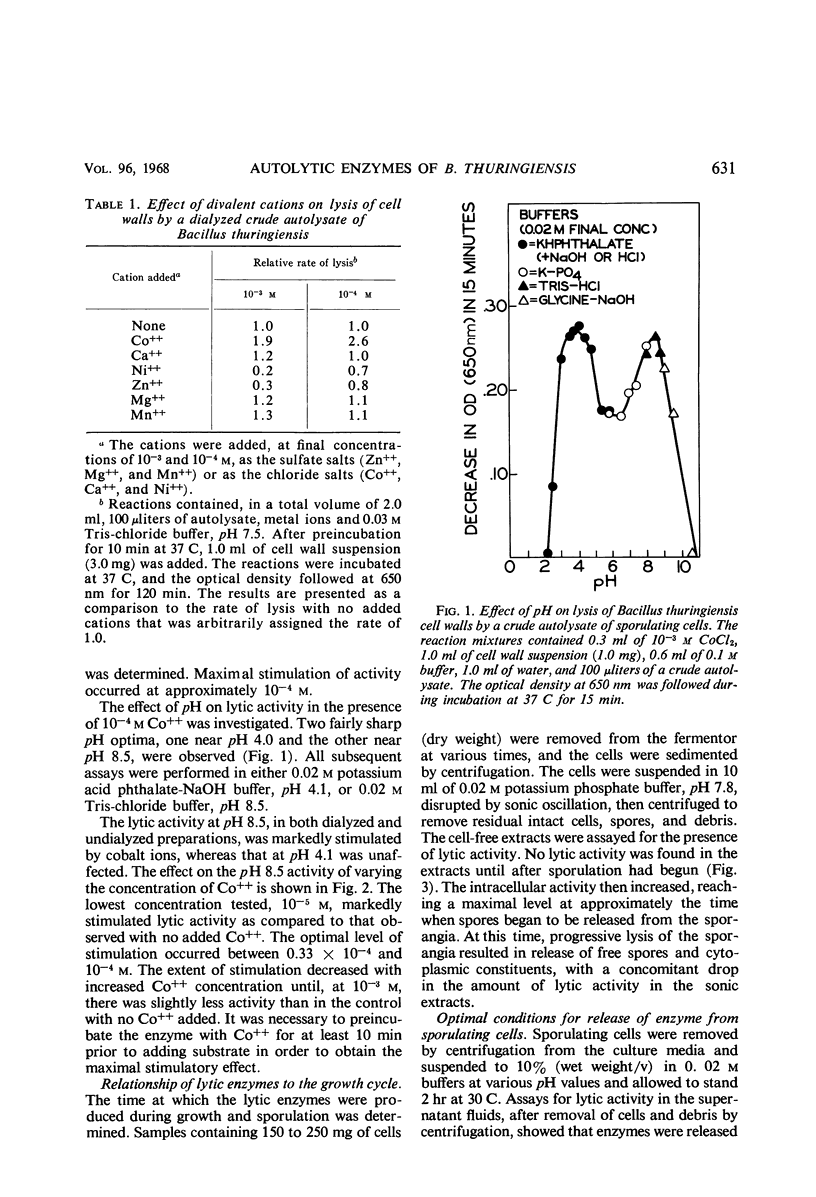
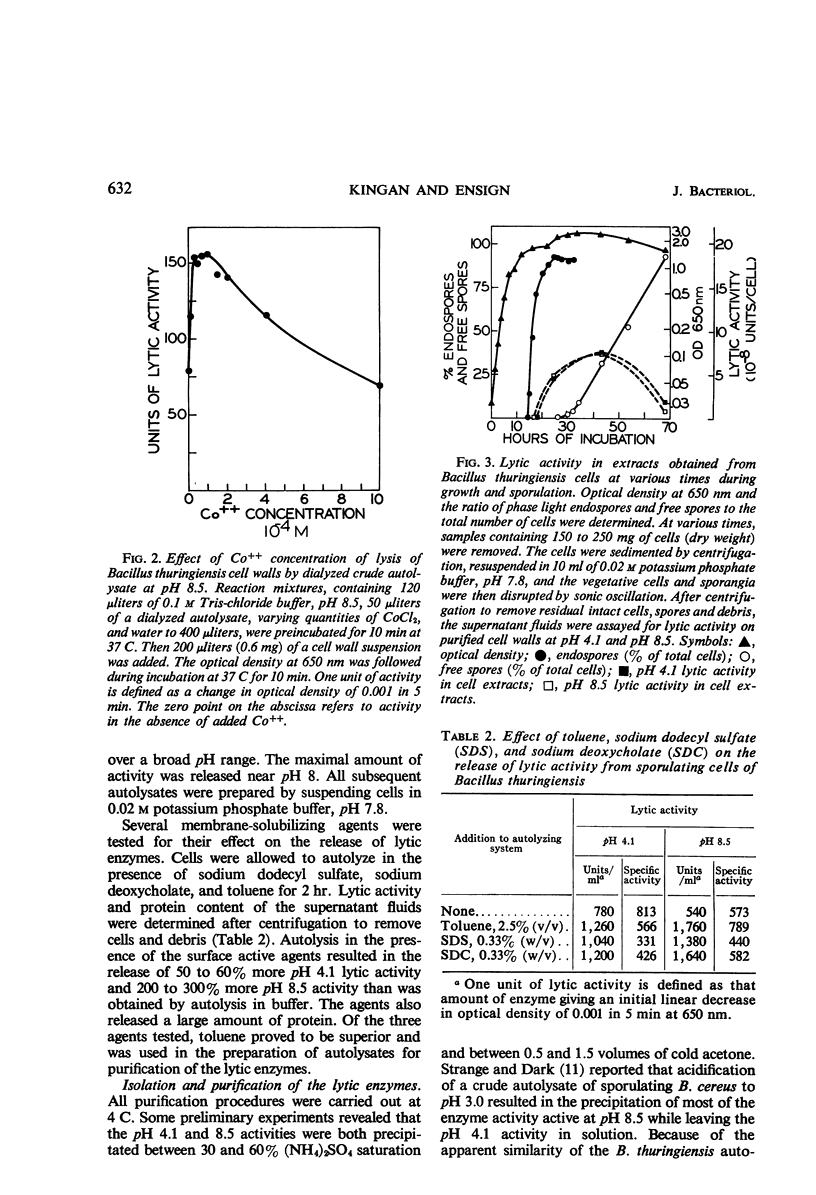
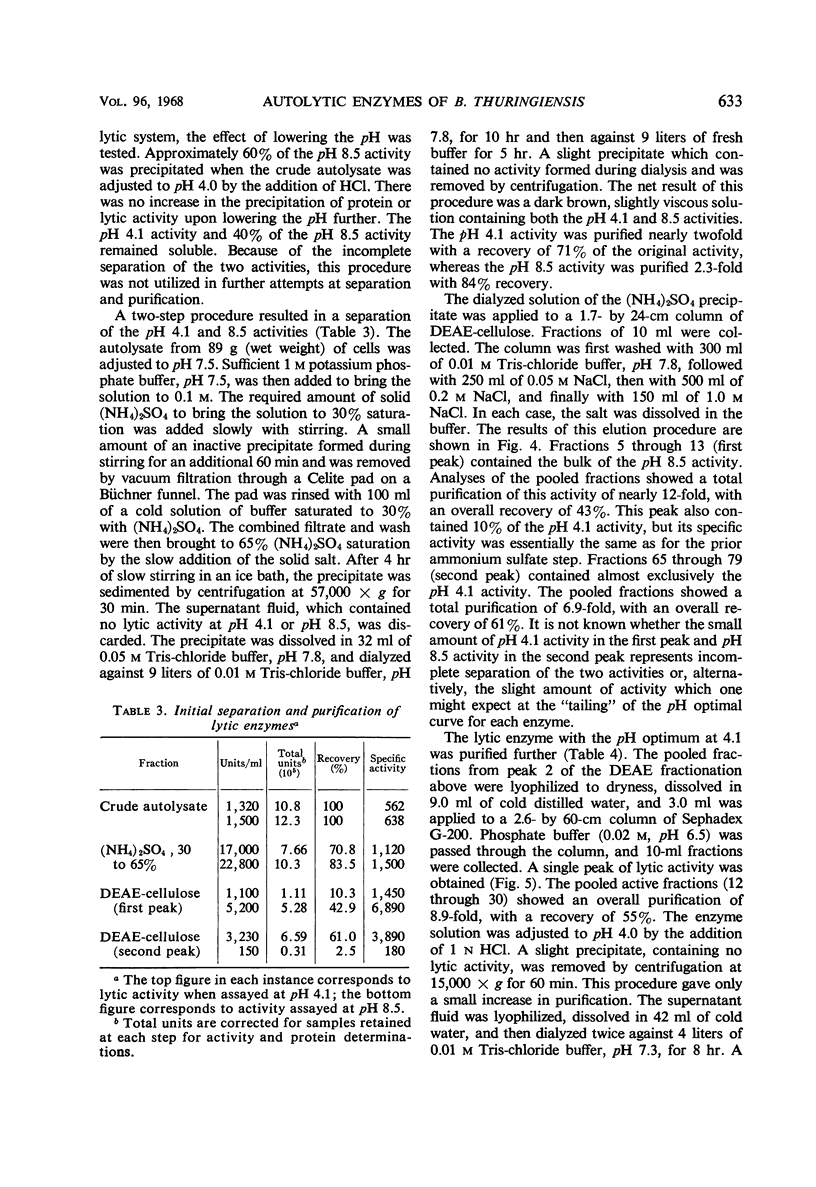
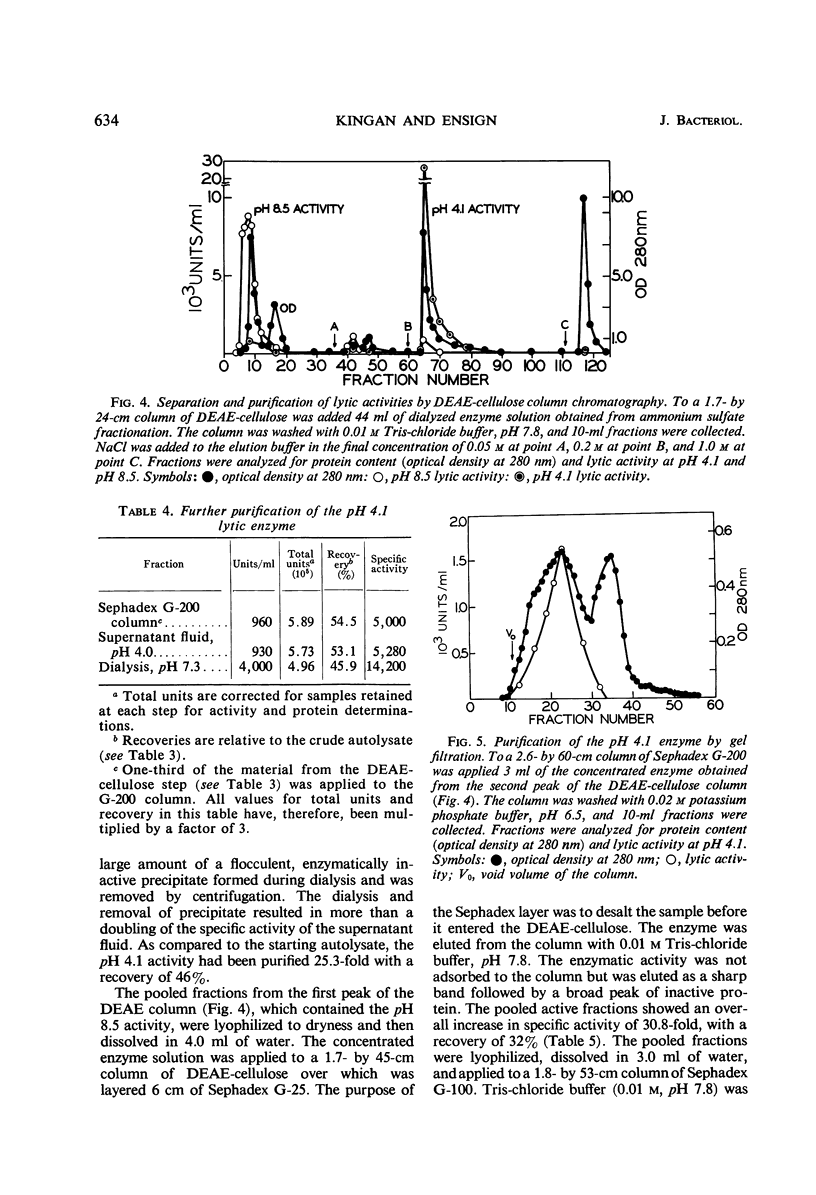
Cells of Bacillus thuringiensis containing refractile spores autolyzed readily when suspended in buffer. The autolysate contained enzymes which lysed vegetative cell walls of the organism. Three enzymes were isolated from the autolysate, and each was purified approximately 30-fold. One enzyme, most active near pH 4.0, was found to be an N-acetylmuramidase. The other two enzymes exhibited pH optima at 8.5. One was stimulated by cobalt ions and the other was not. The cobalt-stimulated enzyme was shown to be an N-acetylmuramyl-l-alanine amidase. The cobalt insensitive enzyme exhibited both N-acetylmuramyl-l-alanine amidase and endopeptidase activity. The amidase activity may reflect incomplete separation of the cobalt-stimulated enzyme. The endopeptidase cleaved the peptide bond between l-alanine d-glutamic acid. A cell wall lytic endopeptidase with this specificity has not been previously reported. All three enzymes were extremely limited in the range of bacterial cell walls which they attacked. Except for cell walls of Micrococcus lysodeikticus, which were lysed by the muramidase, only cell walls of members of the genus Bacillus were attacked.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- EGGSTEIN M., KREUTZ F. H. Vergleichende Untersuchungen zur quantitativen Eiweissbestimmung im Liquor und eiweissarmen Lösungen. Klin Wochenschr. 1955 Oct 1;33(37-38):879–884. doi: 10.1007/BF01473099. [DOI] [PubMed] [Google Scholar]
- GREENBERG R. A., HALVORSON H. O. Studies on an autolytic substance produced by an aerobic sporeforming bacterium. J Bacteriol. 1955 Jan;69(1):45–50. doi: 10.1128/jb.69.1.45-50.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEIMPEL A. M., ANGUS T. A. The taxonomy of insect pathogens related to Bacillus cereus Frankland and Frankland. Can J Microbiol. 1958 Oct;4(5):531–541. doi: 10.1139/m58-058. [DOI] [PubMed] [Google Scholar]
- Kingan S. L., Ensign J. C. Chemical composition of the cell wall of Bacillus thuringiensis var. thuringiensis. J Bacteriol. 1968 Feb;95(2):724–726. doi: 10.1128/jb.95.2.724-726.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAMANNA C., JONES L. Antigenic relationship of the endospores of Bacillus cereus-like insect pathogens to Bacillus cereus and Bacillus anthracis. J Bacteriol. 1961 Apr;81:622–625. doi: 10.1128/jb.81.4.622-625.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- STRANGE R. E., DARK F. A. A cell-wall lytic enzyme associated with spores of Bacillus species. J Gen Microbiol. 1957 Feb;16(1):236–249. doi: 10.1099/00221287-16-1-236. [DOI] [PubMed] [Google Scholar]
- STRANGE R. E., DARK F. A. Cell-wall lytic enzymes at sporulation and spore germination in Bacillus species. J Gen Microbiol. 1957 Oct;17(2):525–537. doi: 10.1099/00221287-17-2-525. [DOI] [PubMed] [Google Scholar]


