Abstract
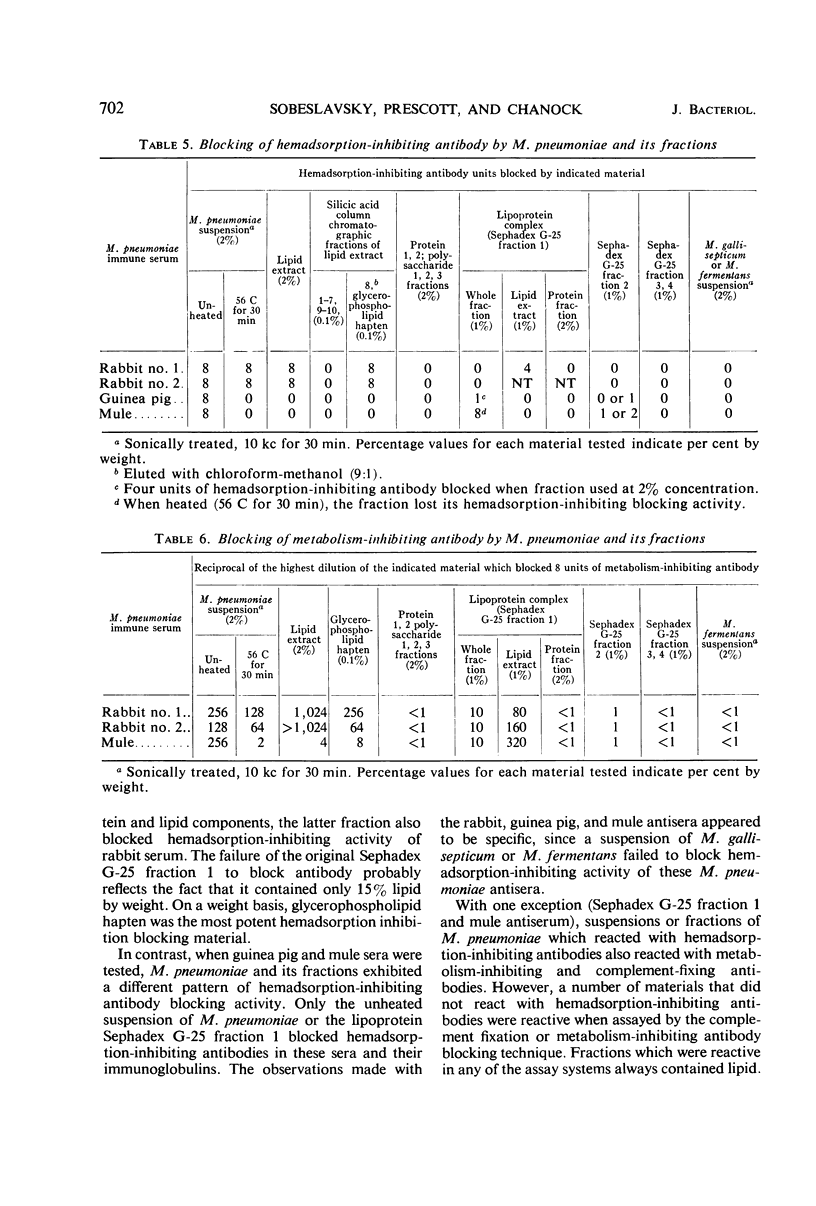
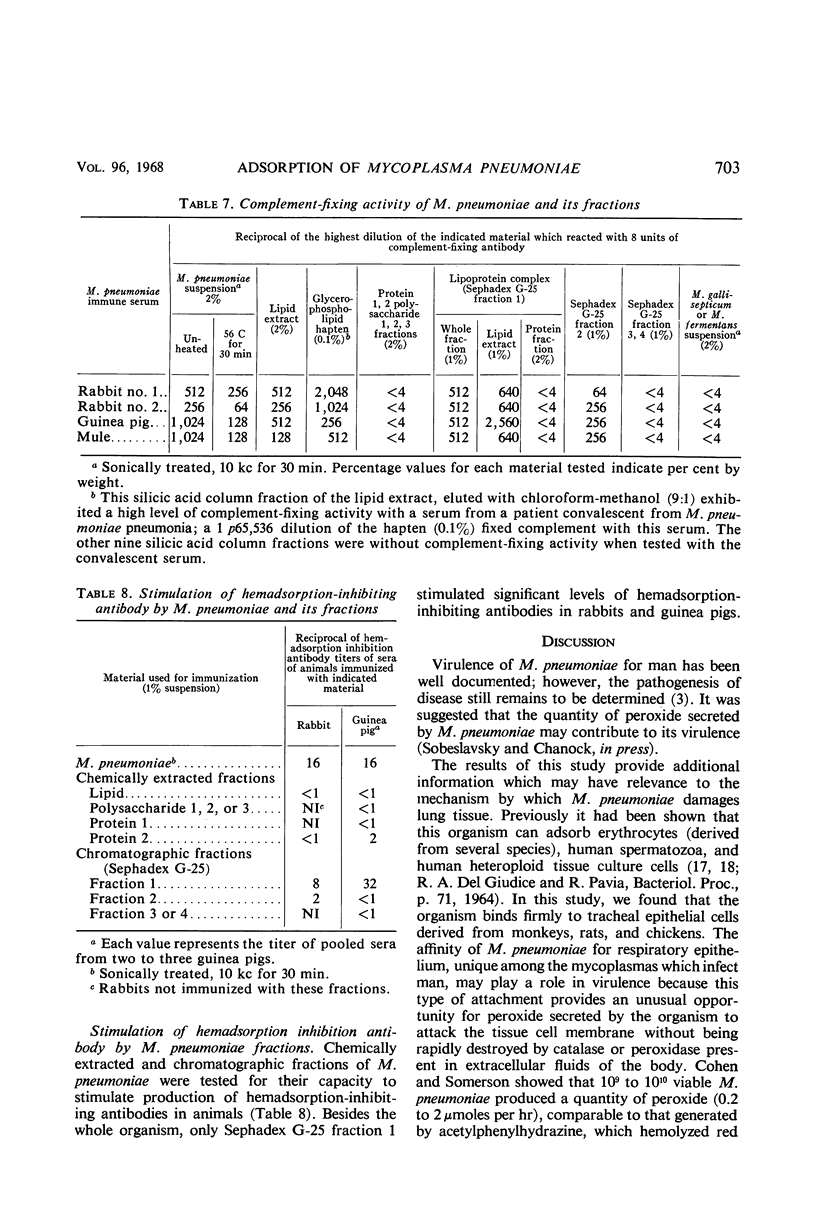
Monkey, rat, and chicken tracheal epithelial cells, as well as monkey, rat, guinea pig, and chicken erythrocytes, adsorbed firmly to colonies of Mycoplasma pneumoniae and M. gallisepticum. Colonies of M. pulmonis also adsorbed erythrocytes but with less avidity than M. pneumoniae or M. gallisepticum; unlike the latter organisms, M. pulmonis did not adsorb tracheal epithelial cells. Colonies of M. orale type 1 and M. orale type 3 adsorbed only chicken red cells. Other mycoplasma species tested, including four of human origin and one of animal origin, did not adsorb red cells or epithelial cells. M. pneumoniae and M. gallisepticum appeared to attach to erythrocytes or tracheal epithelial cells by neuraminic acid receptors on these cells, whereas M. orale types 1 and 3 and M. pulmonis seemed to utilize another type or other types of receptors. Pretreatment of red cells or tracheal epithelial cells with receptor-destroying enzyme, neuraminidase, or influenza B virus removed the adsorption receptors for M. pneumoniae. Similarly, pretreatment of M. pneumoniae colonies with neuraminic acid-containing materials prevented adsorption of erythrocytes or respiratory tract cells. The adsorption sites on M. pneumoniae were specifically blocked by homologous but not heterologous antisera. This property made it possible to study the nature of the mycoplasma adsorption sites by testing the capacity of different fractions of the organism to block the action of adsorption-inhibiting antibodies. Such studies suggested that the mycoplasma binding sites were probably lipid or lipoprotein in nature. The glycerophospholipid hapten was implicated as one such site, since this serologically active hapten blocked the action of hemadsorption-inhibiting antibodies in M. pneumoniae rabbit antiserum. The affinity of M. pneumoniae for respiratory tract epithelium, unique among the mycoplasmas that infect man, may play a role in virulence, since this type of attachment provides an unusual opportunity for peroxide, secreted by the organism, to attack the tissue cell membrane without being rapidly destroyed by catalase or peroxidase present in extracellular body fluids.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADA G. L., STONE J. D. Electrophoretic studies of virus-red cell interaction: mobility gradient of cells treated with viruses of the influenza group and the receptor-destroying enzyme of V. cholerae. Br J Exp Pathol. 1950 Jun;31(3):263–274. [PMC free article] [PubMed] [Google Scholar]
- BELLANTI J. A., RUSS S. B., HOLMES G. E., BUESCHER E. L. THE NATURE OF ANTIBODIES FOLLOWING EXPERIMENTAL ARBOVIRUS INFECTION IN GUINEA PIGS. J Immunol. 1965 Jan;94:1–11. [PubMed] [Google Scholar]
- CHANOCK R. M., HAYFLICK L., BARILE M. F. Growth on artificial medium of an agent associated with atypical pneumonia and its identification as a PPLO. Proc Natl Acad Sci U S A. 1962 Jan 15;48:41–49. doi: 10.1073/pnas.48.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN G., HOCHSTEIN P. GLUTATHIONE PEROXIDASE: THE PRIMARY AGENT FOR THE ELIMINATION OF HYDROGEN PEROXIDE IN ERYTHROCYTES. Biochemistry. 1963 Nov-Dec;2:1420–1428. doi: 10.1021/bi00906a038. [DOI] [PubMed] [Google Scholar]
- Chanock R. M. Mycoplasma infections of man. N Engl J Med. 1965 Nov 25;273(22):1199–contd. doi: 10.1056/NEJM196511252732206. [DOI] [PubMed] [Google Scholar]
- Cohen G., Somerson N. L. Mycoplasma pneumoniae: hydrogen peroxide secretion and its possible role in virulence. Ann N Y Acad Sci. 1967 Jul 28;143(1):85–87. doi: 10.1111/j.1749-6632.1967.tb27648.x. [DOI] [PubMed] [Google Scholar]
- Gesner B., Thomas L. Sialic acid binding sites: role in hemagglutination by Mycoplasma gallisepticum. Science. 1966 Feb 4;151(3710):590–591. doi: 10.1126/science.151.3710.590. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Prescott B., Sobeslavsky O., Caldes G., Chanock R. M. Isolation and characterization of fractions of Mycoplasma pneumoniae. I. Chemical and chromatographic separation. J Bacteriol. 1966 Jun;91(6):2117–2125. doi: 10.1128/jb.91.6.2117-2125.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH P. F., HENDRIKSON C. V. GLUCOSE-CONTAINING PHOSPHOLIPIDS IN MYCOPLASMA LAIDLAWII, STRAIN B. J Lipid Res. 1965 Jan;6:106–111. [PubMed] [Google Scholar]
- Sobeslavsky O., Prescott B., James W. D., Chanock R. M. Isolation and characterization of fractions of Mycoplasma pneumoniae. II. Antigenicity and immunogenicity. J Bacteriol. 1966 Jun;91(6):2126–2138. doi: 10.1128/jb.91.6.2126-2138.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerson N. L., James W. D., Walls B. E., Chanock R. M. Growth of Mycoplasma pneumoniae on a glass surface. Ann N Y Acad Sci. 1967 Jul 28;143(1):384–389. doi: 10.1111/j.1749-6632.1967.tb27680.x. [DOI] [PubMed] [Google Scholar]
- Somerson N. L., Walls B. E., Chanock R. M. Hemolysin of Mycoplasma pneumoniae: tentative identification as a peroxide. Science. 1965 Oct 8;150(3693):226–228. doi: 10.1126/science.150.3693.226. [DOI] [PubMed] [Google Scholar]
- TAYLOR-ROBINSON D., SOMERSON N. L., TURNER H. C., CHANOCK R. M. SEROLOGICAL RELATIONSHIPS AMONG HUMAN MYCOPLASMAS AS SHOWN BY COMPLEMENT-FIXATION AND GEL DIFFUSION. J Bacteriol. 1963 Jun;85:1261–1273. doi: 10.1128/jb.85.6.1261-1273.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Robinson D., Manchee R. J. Novel approach to studying relationships between mycoplasmas and tissue culture cells. Nature. 1967 Dec 30;216(5122):1306–1307. doi: 10.1038/2161306a0. [DOI] [PubMed] [Google Scholar]
- Taylor-Robinson D., Manchee R. J. Spermadsorption and spermagglutination by mycoplasmas. Nature. 1967 Jul 29;215(5100):484–487. doi: 10.1038/215484a0. [DOI] [PubMed] [Google Scholar]