Abstract
Posttranslationally modified forms of tubulin accumulate in the subset of stabilized microtubules (MTs) in cells but are not themselves involved in generating MT stability. We showed previously that stabilized, detyrosinated (Glu) MTs function to localize vimentin intermediate filaments (IFs) in fibroblasts. To determine whether tubulin detyrosination or MT stability is the critical element in the preferential association of IFs with Glu MTs, we microinjected nonpolymerizable Glu tubulin into cells. If detyrosination is critical, then soluble Glu tubulin should be a competitive inhibitor of the IF–MT interaction. Before microinjection, Glu tubulin was rendered nonpolymerizable and nontyrosinatable by treatment with iodoacetamide (IAA). Microinjected IAA-Glu tubulin disrupted the interaction of IFs with MTs, as assayed by the collapse of IFs to a perinuclear location, and had no detectable effect on the array of Glu or tyrosinated MTs in cells. Conversely, neither IAA-tyrosinated tubulin nor untreated Glu tubulin, which assembled into MTs, caused collapse of IFs when microinjected. The epitope on Glu tubulin responsible for interfering with the Glu MT–IF interaction was mapped by microinjecting tubulin fragments of α-tubulin. The 14-kDa C-terminal fragment of Glu tubulin (α-C Glu) induced IF collapse, whereas the 36-kDa N-terminal fragment of α-tubulin did not alter the IF array. The epitope required more than the detyrosination site at the C terminus, because a short peptide (a 7-mer) mimicking the C terminus of Glu tubulin did not disrupt the IF distribution. We previously showed that kinesin may mediate the interaction of Glu MTs and IFs. In this study we found that kinesin binding to MTs in vitro was inhibited by the same reagents (i.e., IAA-Glu tubulin and α-C Glu) that disrupted the IF–Glu MT interaction in vivo. These results demonstrate for the first time that tubulin detyrosination functions as a signal for the recruitment of IFs to MTs via a mechanism that is likely to involve kinesin.
INTRODUCTION
Tubulin is subjected to at least seven distinct posttranslational modifications, making it one of the most modified proteins known. Some of the modifications are unique to tubulin (detyrosination [Barra et al., 1973], glutamylation [Edde et al., 1990; Alexander et al., 1991], and polyglycylation [Redeker et al., 1994]), whereas others (acetylation [L’Hernault and Rosenbaum, 1985], phosphorylation of serine residues [Eipper, 1972; Alexander et al., 1991], and phosphorylation of tyrosine residues [Matten et al., 1990]) occur more widely. Although the individual modifications are chemically distinct, most of them share the property that they occur predominantly on polymeric tubulin, i.e., tubulin that has been incorporated into microtubules (MTs) (Kumar and Flavin, 1981; Gundersen et al., 1987; Matten et al., 1990). Modified forms of tubulin are known to accumulate in the subset of stabilized MTs in cells (Webster et al., 1987a; Khawaja et al., 1988; Bulinski and Gundersen, 1991), yet tubulin modifications do not seem to function directly in generating MT stability (Webster et al., 1987b, 1990; Khawaja et al., 1988; Cook et al., 1998).
The reversible detyrosination–retyrosination of the C terminus of the α-subunit of tubulin was first described in 1973 (Barra et al., 1973) and is the best characterized of the tubulin modifications. A tubulin carboxypeptidase catalyzes the removal of the C-terminal tyrosine residue from α-tubulin (Hallak et al., 1977), and a tubulin tyrosine ligase is responsible for readding the tyrosine residue (Raybin and Flavin, 1977). The ligase has been purified (Schroder et al., 1985) and cloned (Ersfeld et al., 1993). The two enzymes exhibit differential activities toward tubulin depending on its assembly status; the carboxypeptidase is preferentially active on polymeric tubulin (i.e., MTs) (Kumar and Flavin, 1981), whereas the ligase is only active on monomeric tubulin (Raybin and Flavin, 1977). In cultured cells, the ligase is able to retyrosinate efficiently all the monomeric tubulin, and as a result, at steady state, the monomer pool of tubulin is completely tyrosinated (Gundersen et al., 1987; Webster et al., 1987b).
The levels of tyrosinated (Tyr) and detyrosinated (Glu) tubulin in MTs in cells vary depending on the age of the MT (Gundersen et al., 1987; Schulze et al., 1987; Webster et al., 1987a). Because MTs polymerize from a pool of Tyr tubulin, they are initially composed completely of Tyr tubulin. In nonpolarized, proliferating cells, most MTs turnover so rapidly that the tubulin composing them is unavailable for detyrosination by the carboxypeptidase. However, a small number of MTs in proliferating cells and a larger number of MTs in differentiating cells become stabilized, and consequently the tubulin composing them is subjected to the carboxypeptidase for longer periods, resulting in MTs with high levels of Glu tubulin (Glu MTs) (Gundersen et al., 1984, 1987; Schulze et al., 1987; Webster et al., 1987a).
The increase in the number of stable, Glu MTs during differentiation suggests that these MTs may be important for the generation of cell polarity (Bulinski and Gundersen, 1991). This possibility is further supported by the observation that the generation of Glu MTs during cell polarization is not a global event; instead it is most pronounced along the axis defining cell polarity (Gundersen and Bulinski, 1988; Gundersen et al., 1989; Houliston and Maro, 1989; Baas and Black, 1990; Nagasaki et al., 1992; Cook, et al., 1998). Thus, understanding how stable MTs are generated and what functional significance is imparted by modifying the stable MTs will be important in defining the role of MTs in generating cell polarity.
One approach to understanding the function of tubulin detyrosination has been to look for MT-interacting proteins that exhibit differences in the binding to the two forms. Yet, in vitro studies examining the binding of classical structural MAPs, like MAP 2 and MAP 4, showed either no or only subtle differences in the binding to Glu and Tyr tubulin (Kumar and Flavin, 1982; Chapin and Bulinski, 1994). MAPs also do not appear to be preferentially localized on Glu or Tyr MTs in vivo (Chapin and Bulinski, 1994), so it seems likely that this class of MT-interacting proteins may not discriminate between Glu and Tyr tubulin. In a recent study, we found that the MT motor protein kinesin preferentially bound to Glu MTs compared with Tyr MTs (Liao and Gundersen, 1998). The differences in binding were significantly large (∼3-fold), raising the possibility that the preferential association of kinesin with Glu versus Tyr tubulin may have functional consequences in vivo.
Studies of the function of detyrosination or the other modifications in vivo have been even more difficult than studies in vitro. As noted above, virtually all of the modifications accumulate in stabilized MTs. This has limited the interpretation of many in vivo experiments that have attempted to attribute effects to modified MTs, because it is unclear whether the effect being measured is caused by MT stability or MT posttranslational modification. Mechanistically, this is an important distinction, because MT stability would be limited to regulating time-dependent interactions, whereas MT posttranslational modifications could potentially regulate interactions requiring molecular specificity. The specificity added by posttranslational modifications may be particularly important in the many cases in which cells have more than one posttranslationally modified form of tubulin (Schulze et al., 1987).
In this study, we report on an approach that allowed us to distinguish between MT posttranslational modification and MT stability. Our rationale was based on the observation that there is no soluble Glu tubulin in the subunit pool of tubulin in cells (Gundersen et al., 1987) and on the assumption that if we produce Glu tubulin in the subunit pool, it will act as a competitive inhibitor and interfere with Glu MT specific interactions. We describe here the preparation of nonpolymerizable Glu tubulin and show that, upon microinjection into cells, it remains in the subunit pool and disrupts the preferential localization of IFs on Glu MTs, which we had characterized in a previous study (Gurland and Gundersen, 1995). Importantly, the nonpolymerizable Glu tubulin did not disrupt the endogenous stabilized, Glu MTs. Nonpolymerizable Tyr tubulin or polymerizable Glu tubulin did not disrupt the IF–MT association. Using tubulin peptides and fragments, we map the epitope on Glu tubulin responsible for interfering with the IF–MT interaction and show that it encompasses the C terminus of α-tubulin. We show that the same reagents that interfere with the IF–MT interaction in vivo are also capable of inhibiting the binding of kinesin to MTs in vitro. These results demonstrate, for the first time, that tubulin detyrosination functions as a signal for the recruitment of IFs to MTs and strongly suggest that kinesin may mediate the interaction in vivo.
MATERIALS AND METHODS
Cell Culture and Treatments
NIH3T3 fibroblasts were cultured in DMEM (Life Technologies, Gaithersburg, MD) supplemented with 10% calf serum as described previously (Gundersen and Bulinski, 1988; Nagasaki et al., 1992). Cells were seeded onto acid-washed sterile glass coverslips for immunofluorescence and grown until confluent (2 d). Monolayers were wounded as described previously (Gundersen and Bulinski, 1988; Gurland and Gundersen, 1993) to generate a homogeneous population of cells at the wound edge containing arrays of Glu MTs oriented toward the wound-edge and were then treated as described in the RESULTS. Cells were fixed in −20°C methanol for immunofluorescence as described previously (Gundersen et al., 1984).
Preparation of Tubulin for Microinjection
Tubulin from calf brain and HeLa cell extracts was purified by two cycles of assembly–disassembly and DEAE-Sephadex A-50 chromatography as described (Gundersen et al. [1987] and Chapin and Bulinski [1991], respectively) and then was concentrated by vacuum dialysis. DEAE-purified HeLa tubulin was comprised of 90–95% tyrosinated tubulin as determined by quantitative Western blot analysis. Pure Glu tubulin was prepared by incubating either DEAE-purified brain or HeLa tubulin with PMSF-treated pancreatic carboxypeptidase A (CPA; 10 μg/ml; Sigma, St. Louis, MO) for 20 min at 37°C. The reaction was stopped by the addition of 20 mM DTT for 10 min at 37°C (Kumar and Flavin, 1981). Because CPA does not remove C-terminal amino acid residues, CPA treatment of tubulin removes only the C-terminal Tyr from α-tubulin (Kumar and Flavin, 1981). Nonpolymerizable Glu (from brain or from HeLa cell extracts) and Tyr (from HeLa cell extracts) tubulin was prepared by treating tubulin with 2 mM iodoacetamide (IAA) for 30 min at 37°C as described previously (Luduena and Roach, 1981) except that the IAA and tubulin mixture was incubated on ice for 20 min before the 37°C incubation. The reaction was stopped by adding β-mercaptoethanol to 2 mM, incubating for 5 min at room temperature, and then dialyzing against 0.1 M piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES), pH 6.9, 1 mM EGTA, 1 mM MgCl2, and 0.1 mM GTP. Identical results were obtained with tubulin preparations that were detyrosinated after the IAA treatment. IAA-treated tubulin was dialyzed exhaustively against 10 mM HEPES, pH 7.4, and 140 mM KCl before microinjection.
Assembly competence of the IAA-tubulin was determined by in vitro sedimentation assay. Briefly, IAA-tubulin (40 μM in 0.1 M PIPES, pH 6.9, 1 mM EGTA, 1 mM MgCl2, and 1 mM GTP) was polymerized for 30 min at 37°C followed by sedimentation (100,000 × g for 7 min) at 37°C. The concentration of tubulin in the pellet (MTs) and supernatant (monomeric tubulin) was determined by the bicinchoninic acid protein assay (Sigma). Pellets obtained in this assay were examined for MTs by negative stain electron microscopy as described (Bulinski and Bossler, 1994).
Preparation of α-Tubulin Fragments for Microinjection
DEAE-purified tubulin from calf brain extract was digested with 0.5% trypsin (wt/wt) (DPCC-treated type XI from bovine pancreas; Sigma) for 30 min at 30°C. The reaction was stopped by the addition of PMSF to 2 mM. Digested tubulin was diluted in SDS sample buffer and immediately separated on a preparative 12% polyacrylamide gel. The C-terminal fragment (α-C, ∼14 kDa) and N-terminal fragment (α-N, ∼36 kDa) generated by trypsin digestion were identified by their position in the gel in reference to known standards, cut out from the gel, and eluted with an electroelution apparatus (Schleicher & Schuell, Keene, NH). Eluted tubulin fragments were concentrated by vacuum dialysis against 10 mM HEPES and 140 mM KCl, pH 7.4, before microinjection. α-C Glu tubulin (α-C Glu) was prepared by treating brain tubulin with 15 μg/ml pancreatic CPA for 20 min at 37°C. Immediately after the CPA treatment, trypsin was added to the sample to a final concentration of 0.5% (wt/wt), and the tubulin was incubated for 30 min at 30°C. CPA and trypsin were inactivated by addition of DTT to 20 mM and PMSF to 2 mM, respectively. The α-C Glu fragment was then gel purified as described above.
Western Blotting
Brain and HeLa tubulin samples were subjected to SDS-PAGE on 7.5% polyacrylamide gels, transferred to nitrocellulose sheets, and blocked as described (Gundersen et al., 1994). Blots were then reacted with either SG or W2 rabbit polyclonal antisera against Glu and Tyr tubulin (Gundersen et al., 1984), respectively, at a dilution of 1:60,000. Alkaline phosphatase-conjugated secondary antibody against rabbit IgG (1:12,000) (Promega, Madison, WI) was used to detect SG and W2 antibody reactivity. The blots were developed with nitro blue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate.
Microinjection
3T3 cells at the edge of a wound were pressure microinjected with the tubulin preparations at the concentrations indicated in the RESULTS. Human IgG (HuIgG, 2 mg/ml) was coinjected to provide a marker for the injected cells. Microinjection was performed as described previously (Mikhailov and Gundersen, 1995). Before injection, tubulin or tubulin fragments were centrifuged (100,000 × g for 7 min) at 4°C to remove aggregates. We routinely performed protein assays after centrifugation and normalized the amount of tubulin or tubulin fragments injected. We estimate that ∼5–10% of the cell volume was routinely introduced into injected cells. After injection, cells were maintained at 37°C in a humidified CO2 environment for 2 h after which time they were fixed in −20°C methanol and immunofluorescently stained.
Immunofluorescence
Cells were quadruple stained for Glu MTs, Tyr MTs, vimentin IFs, and the injected HuIgG marker by incubating fixed cells with primary antibodies diluted in Tris-buffered saline, pH 7.4, containing 10% normal goat serum. The HuIgG was visualized by direct immunofluorescence staining with an appropriate fluorescently conjugated secondary antibody. Glu MTs, Tyr MTs, and vimentin IFs were visualized by indirect immunofluorescence with antibodies to Glu tubulin, Tyr tubulin, and IFs. A rabbit polyclonal antibody specific for Glu tubulin (SG) (Gundersen et al., 1984) was used at a dilution of 1:400 (of serum); a rat monoclonal antibody specific for Tyr tubulin (YL1/2) (Kilmartin et al., 1982) was used at a dilution of 1:10 of culture supernatant (YL1/2 hybridoma cells were purchased from the European Collection of Animal Cell Cultures, Salisbury, UK); a mouse monoclonal IgM (56B5) specific for IF rod domains (Kaplan et al., 1991) was used at a dilution of 1:4 (of culture supernatant) and was generously provided by Dr. R. Liem (Columbia University, New York, NY). Secondary antibodies were fluorescein-conjugated goat anti-rabbit IgG (Cappel, Durham, NC), aminomethylcoumarin-conjugated donkey anti-human IgG, indodicarbocyanin (Cy5)-conjugated donkey anti-rat IgG (minimum cross-reaction with mouse and rabbit IgGs), and tetramethyl rhodamine-conjugated goat anti-mouse IgM (Jackson ImmunoResearch, West Grove, PA). The secondary antibodies exhibited no detectable cross-reaction with the inappropriate primary antibodies as judged by fluorescence microscopy.
Fluorescence microscopy was performed on a Nikon Optiphot microscope using a narrow excitation band fluorescein cube (DM510, B1E; Nikon, Garden City, NY), a rhodamine cube (DM590, 565DR-P; Omega Optical, Battleboro, VT), a coumarin cube (DM400, UV2A; Nikon) with a 420–490 nm bandpass barrier filter to avoid overlapping fluorescein fluorescence, and a Cy5 cube (DM508, Cy5; Nikon). No channel cross-over was observed between rhodamine and Cy5. Images were recorded with a Micromax cooled charge-coupled device camera (Princeton Instruments, Trenton, NJ) with a Kodak KAF 1400 chip (1317 × 1035 pixels; Rochester, NY) and were processed with MetaMorph imaging software (Universal Imaging, West Chester, PA).
Binding of K394 to MTs
Purified recombinant squid kinesin head, K394 (Song and Mandelkow, 1993), was generously provided by Dr. E. Mandelkow (Max Plank, Hamburg, Germany). K394 in 25P150 buffer (25 mM PIPES, pH 6.9, 1 mM MgCl2, 1 mM EGTA, 150 mM KCl) and various tubulin preparations, fragments, or peptides were added to MTs assembled from DEAE-purified bovine brain tubulin in the presence of 20 μM taxol. The final concentrations of K394 and MTs in the incubation mixtures were 1.4 and 2 μM, respectively. When present, IAA-HeLa Glu or Tyr tubulin and α-tubulin fragments were used at 7–14 μM, and the C-terminal peptides of Glu and Tyr tubulin were used at 150 μM. After incubation for 15 min at room temperature, MTs and MT-bound K394 were separated from soluble proteins by sedimentation (100,000 × g for 7 min at 32°C). The levels of K394 in the supernatants and pellets were analyzed by SDS-PAGE and Coomassie staining. After digitization using DeskScan II (Hewlett Packard, Palo Alto, CA), the density of the bands was quantified using the MetaMorph “show region statistics” function.
RESULTS
Preparation of Nonpolymerizable, Glu Tubulin
Previously, we showed that vimentin IFs were colocalized with Glu MTs, rather than Tyr MTs, and that microinjection of affinity-purified antibodies to Glu tubulin, but not to Tyr tubulin, caused the redistribution of the IFs to a perinuclear location (collapse) (Gurland and Gundersen, 1995). A previous study showed that glial filaments in astrocytes were frequently colocalized with stable, posttranslationally modified MTs (Cambray-Deakin et al., 1988). However, in neither study was it established whether the IFs were on the stabilized, modified MTs because of their stability or because of their content of modified tubulin. If Glu tubulin is responsible for the preferential interaction of IFs with stabilized, Glu MTs in fibroblasts, then soluble monomeric Glu tubulin should act as a competitive inhibitor of the interaction and cause IFs to collapse.
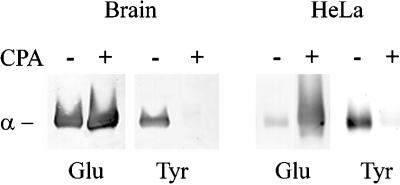
To test this idea, we have used the wounded monolayer model in which the fibroblasts at the edge of the wound generate highly polarized arrays of Glu MTs oriented toward the wound edge (Gundersen and Bulinski, 1988; Nagasaki et al., 1992). To produce monomeric Glu tubulin in the wound-edge cells, we microinjected Glu tubulin that had been chemically modified so that it could not assemble or copolymerize with existing MTs. Isolated tubulin from brain is an approximately equal mixture of Glu and Tyr tubulin (Gundersen et al., 1987), so we first prepared pure Glu tubulin by CPA treatment (Figure 1) (Webster et al., 1987b). This treatment resulted in complete detyrosination of tubulin as determined by Western blot analysis using Glu and Tyr tubulin-specific antibodies (Figure 1). The increase in reactivity of the CPA-treated tubulin with the Glu tubulin antibody demonstrates that CPA does not remove additional amino acids from the C terminus, because the Glu tubulin antibody does not recognize tubulin lacking the penultimate Glu residue (Paturle et al., 1989). To render the Glu tubulin nonpolymerizable, we treated Glu tubulin with IAA, which has been shown to modify sulfhydryls on tubulin and prevent tubulin polymerization (Luduena and Roach, 1981). We confirmed by in vitro sedimentation analysis and by negative-staining electron microscopy that the IAA-treated Glu tubulin (IAA-Glu tubulin) was unable to polymerize (our unpublished results).
Figure 1.
Preparation of pure Glu and Tyr tubulin. Tubulin, purified from brain tissue (left) and from HeLa cells (right), was treated with CPA to remove the C-terminal tyrosine residues. Samples of the CPA-treated (+) or untreated (−) preparations were analyzed by Western blot to determine the levels of Glu and Tyr tubulin. Blots were loaded with 0.5 μg of tubulin per lane and were reacted with antibodies specific to Glu tubulin (Glu) and to Tyr tubulin (Tyr). Only the region corresponding to α-tubulin is shown. Note that brain tubulin is a mixture of Glu and Tyr tubulin before CPA treatment (∼50:50) whereas HeLa tubulin is predominantly Tyr tubulin (92% Tyr vs. 8% Glu). CPA treatment of brain or HeLa tubulin almost completely eliminated reactivity with the Tyr tubulin antibody and increased reactivity with the Glu tubulin antibody.
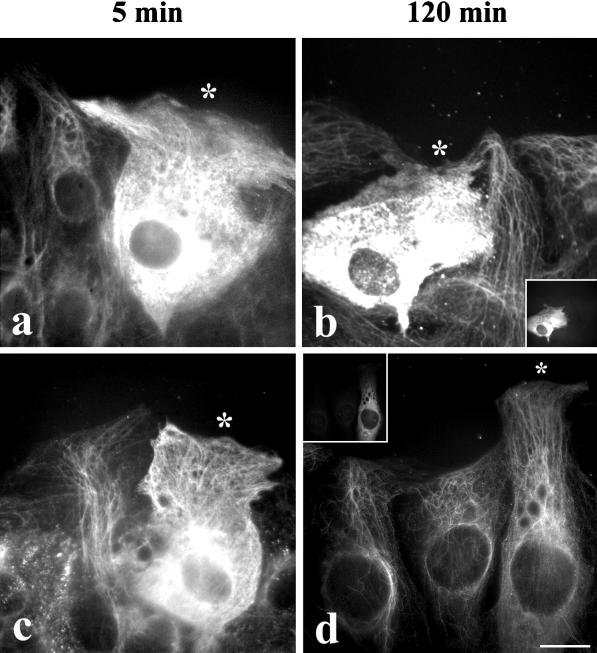
In addition to preventing the reassembly of the injected Glu tubulin, it was important to prevent the retyrosination of the soluble Glu tubulin after it was injected into cells. When Glu tubulin and IAA-Glu tubulin were microinjected into cells and the cells were fixed 5 min after the microinjection, we detected both types of tubulin in the soluble pool by indirect immunofluorescence using antibodies to Glu tubulin (Figure 2, a and c). Some of the preexisting endogenous Glu MTs are also visible through the diffuse Glu tubulin staining. When cells were fixed 2 h after microinjection, IAA-Glu tubulin was still detected in the soluble pool, with antibodies to Glu tubulin indicating that most of it did not assemble into MTs and that most of the introduced Glu tubulin was not retyrosinated (Figure 2b). The inability of IAA-Glu tubulin to be retyrosinated after injection was confirmed by the fact that we did not detect any soluble Tyr tubulin by immunofluorescence during the course of these experiments (our unpublished results).
Figure 2.
Microinjected IAA-Glu tubulin does not polymerize and is not retyrosinated. Wound-edge 3T3 cells were injected with 140 μM IAA-treated Glu tubulin (a and b) or 100 μM untreated Glu tubulin (c and d) and incubated for 5 or 120 min at 37°C before fixation. Human IgG (2 mg/ml) was coinjected as a marker. Cells were immunostained for Glu tubulin (a–d), human IgG (insets in b and d), and Tyr tubulin. Injected cells are marked with asterisks at the cell periphery. Note that after 5 min both IAA-Glu tubulin and untreated Glu tubulin in injected cells are detected as diffuse immunofluorescence in the cytoplasm with anti-Glu tubulin antibodies. The filamentous Glu MT staining observed is attributable to the staining of endogenous Glu MTs. After 120 min only those cells injected with IAA-Glu tubulin are identifiable by a diffuse, anti-Glu tubulin immunofluorescent staining pattern (b), indicative of the presence of nonpolymerizable Glu tubulin that is not retyrosinated after microinjection into cells. Conversely, cells injected with untreated Glu tubulin must be identified by human IgG immunoreactivity (d) because most of the injected Glu tubulin has been retyrosinated (our unpublished results) and incorporated into the MT network. Bar, 10 μm.
In contrast, microinjected Glu tubulin (not IAA treated) did not remain in the soluble pool of tubulin (Figure 2d). Indeed, after 2 h, cells injected with Glu tubulin did not contain elevated Glu tubulin either in the soluble pool or in MTs and were only detectable by staining for the coinjected human IgG marker (Figure 2d, inset). We did not follow the fate of the injected Glu tubulin in detail because this had been done in a previous study (Webster et al., 1987b). However, as in the previous study, we did observe elevated Glu tubulin in MTs at intermediate times (≥10 min), consistent with the ability of Glu tubulin to polymerize, and a diminution of Glu tubulin levels at later times (>60 min), consistent with its rapid retyrosination. This confirms that the chemical modification of Glu tubulin by IAA prevented both the assembly and the retyrosination of injected tubulin and provided us with the appropriate tool for testing the hypothesis that soluble Glu tubulin may be a competitive inhibitor of the IF–MT interaction in vivo.
Microinjection of Assembly-incompetent, but not Assembly-competent, Glu Tubulin Causes Collapse of the IF Network
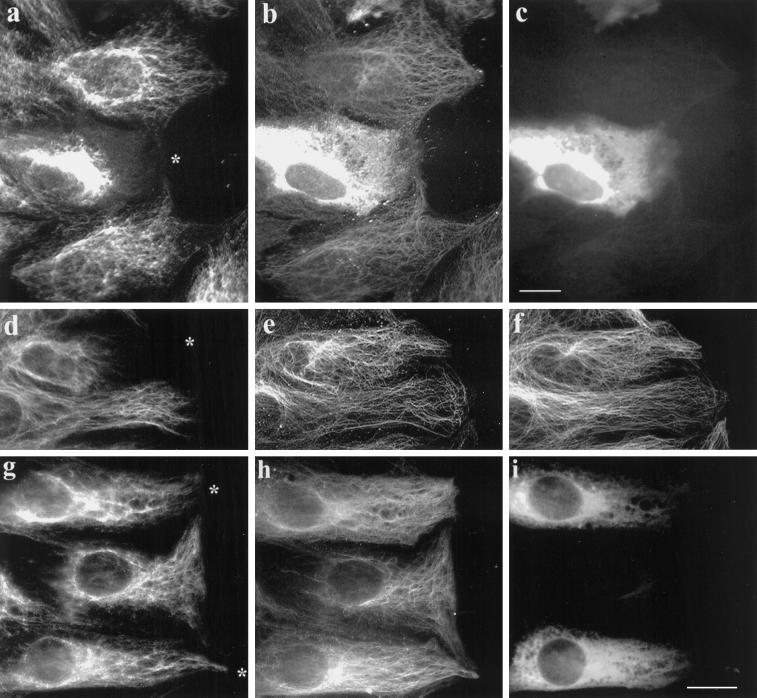
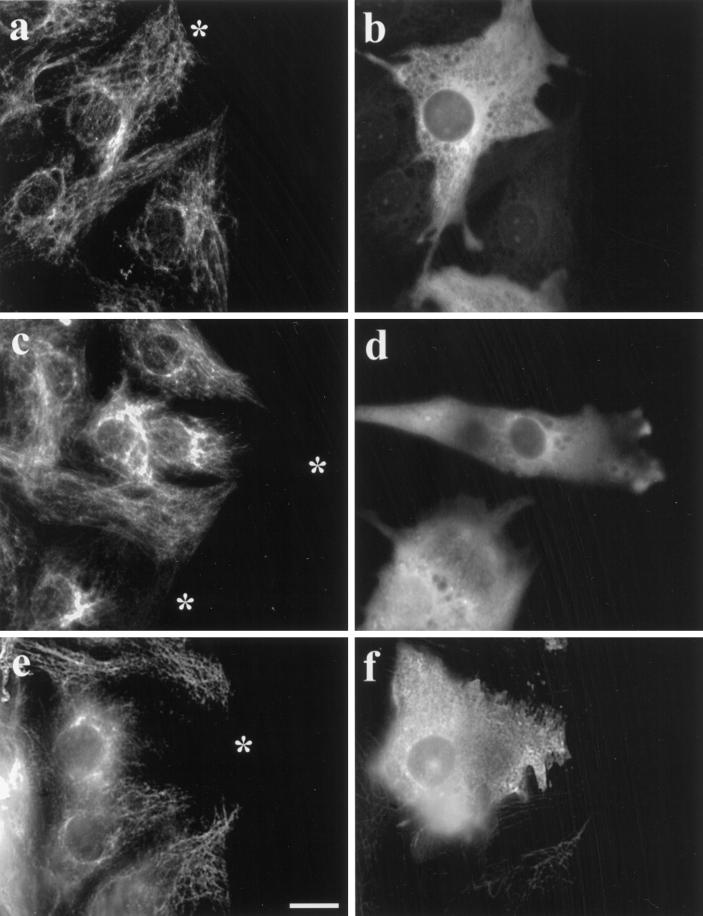
When 3T3 cells at the edge of a wound were microinjected with the IAA-Glu tubulin at concentrations between 100 and 150 μM, the IFs collapsed to a perinuclear region in 74% (n = 410) of the cells (Figure 3a–c). In cells microinjected with IAA-Glu tubulin at ≥100 μM, Glu tubulin was detected with antibody as diffuse fluorescence in the cytoplasm for several hours, confirming that the IAA-Glu tubulin remained assembly incompetent and resistant to retyrosination in vivo (Figure 3b). However, this diffuse cytoplasmic staining made it difficult to detect the endogenous Glu MTs and to determine whether the IAA-Glu tubulin interfered with the endogenous Glu MTs. Accordingly, we injected cells with lower concentrations of IAA-Glu tubulin (50–100 μM) and found that the IFs were still collapsed in a majority of the cells (Figure 3d). The amount of soluble IAA-Glu tubulin was low enough in some of these cells that it was now possible to detect the endogenous Glu MTs. As shown for a typical example in Figure 3d–f, the injected IAA-Glu tubulin did not significantly alter the array of Glu MTs (Figure 3e) or Tyr MTs (Figure 3f), even though the IFs were collapsed in the injected cell (Figure 3d). This shows that IAA-Glu tubulin acts by competitively inhibiting the IF–Glu MT interaction rather than by altering the Glu MTs.
Figure 3.
Microinjection of nonpolymerizable, IAA-Glu tubulin causes collapse of the IF network. 3T3 cells at the edge of an in vitro wound were injected with IAA-treated Glu tubulin (from calf brain) at 140 μM (a–c) or at 70 μM (d–f) or with untreated Glu tubulin at 100 μM (g–i). A marker protein, human IgG (2 mg/ml), was coinjected in all cases. Cells were fixed 2 h after injection and immunofluorescently stained to reveal vimentin (a, d, and g), Glu tubulin (b, e, and h), human IgG (c and i), and Tyr tubulin (f). The asterisks in a, d, and g indicate the cell periphery of the injected cell (the human IgG marker is not shown for d–f). Note that in the cells injected with IAA-Glu the IFs are collapsed around the nucleus and do not extend to the cell edge, whereas in cells injected with untreated, assembly-competent Glu tubulin the IFs remain extended in the cytoplasm. Microinjection of human IgG alone had no apparent effect on the distributions of either the MTs or the IFs (our unpublished results). Bar, 10 μm.
As reported previously (Webster et al., 1987b) and shown in Figure 2d, we found that microinjected brain Glu tubulin (not IAA treated) was rapidly incorporated into MTs. To determine whether the IF-collapsing activity of Glu tubulin was dependent on maintaining Glu tubulin in the monomer pool, we examined the distribution of IFs in cells microinjected with untreated brain Glu tubulin, which was assembly competent. Although IFs collapsed to a perinuclear region in 62% of the cells injected with 50–150 μM brain IAA-Glu tubulin (see Figures 3, a and d, and 5), microinjection of an equivalent amount of polymerization-competent brain Glu tubulin did not induce IF collapse (see Figures 3, g–i, and 5). Thus, the inhibitory effect of Glu tubulin on the maintenance of an extended IF array was dependent on maintaining Glu tubulin in its soluble form. Indeed, we would expect that increasing Glu tubulin in MTs would lead to an increase in IF–MT interaction. Although we have not directly examined this here, we showed previously that increasing Glu tubulin in MTs by taxol treatment increased IF–MT interaction (Gurland and Gundersen, 1995).
Nonpolymerizable HeLa Glu Tubulin, but not HeLa Tyr Tubulin, Disrupts the IF Network
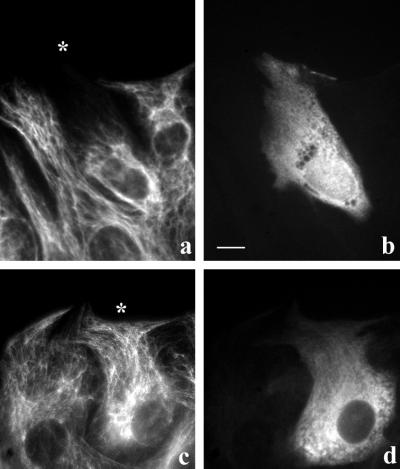
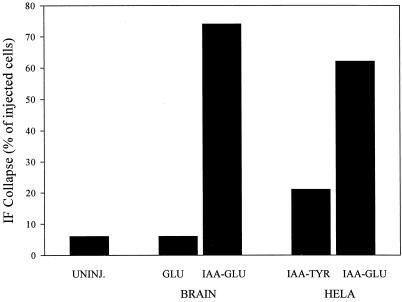
To test whether the coalignment of IFs with MTs was specifically inhibited by the detyrosinated form of tubulin, we prepared IAA-tubulin from HeLa cells for microinjection. Whereas brain tubulin is approximately a 50:50 mixture of Glu and Tyr tubulin (Gundersen et al., 1987), tubulin purified from HeLa cells is reported to be predominantly Tyr tubulin (Chapin and Bulinski, 1991). In our preparations of HeLa cell tubulin, we found that Tyr tubulin was the predominant species (Figure 1); quantitative Western blot analysis showed that our HeLa tubulin was ≥92% Tyr tubulin. HeLa Tyr tubulin treated with IAA to inhibit its ability to polymerize did not induce IF collapse in a significant number of cells after microinjection (Figure 4, c and d). In contrast, when we converted IAA-HeLa Tyr tubulin to the Glu form by CPA treatment (which resulted in a conversion to 95% Glu tubulin, see Figure 1), microinjected IAA-HeLa Glu tubulin did cause collapse of the IF network (Figure 4, a and b). At identical concentrations in the microinjection needle (70 μM), IAA-HeLa Glu tubulin induced IF collapse in 62% (n = 265) of the injected cells, whereas IAA-HeLa Tyr tubulin only induced IF collapse in 21% (n = 121) of the injected cells (Figure 5). This shows directly that Glu tubulin is more effective than is Tyr tubulin in disrupting the coalignment of IFs and MTs. Also, because HeLa tubulin does not contain a number of the other posttranslational modifications found in brain tubulin (e.g., phosphorylation, glutamylation, etc.) (Chapin and Bulinski, 1991), the collapsing activity of IAA-Glu tubulin does not depend on these modifications. At this point, we do not know whether the small degree of IF collapse observed with the IAA-HeLa Tyr tubulin (Figure 5, 21 vs. 6% in uninjected controls) is caused by a lower intrinsic activity of the Tyr tubulin or by the low level of Glu tubulin present in the preparation (see Figure 1).
Figure 4.
Microinjected IAA-HeLa Glu tubulin, but not IAA-HeLa Tyr tubulin, induces collapse of the IF network. 3T3 cells at the edge of a wound were injected with 100 μM IAA-HeLa Glu tubulin (a and b) or 140 μM IAA-HeLa Tyr tubulin (c and d), fixed 2 h later, and immunofluorescently stained as described above. Human IgG (2 mg/ml) was coinjected as a marker to identify the injected cells. Shown are the vimentin IFs (a and c) and the injected human IgG marker (b and d). The asterisks indicate the peripheral edge of injected cells in a and c. Bar, 10 μm.
Figure 5.
Effect of microinjected monomeric tubulins on IF distribution. Wound-edge 3T3 cells were microinjected with polymerization-competent brain Glu tubulin, IAA-treated brain Glu tubulin, IAA-treated HeLa Tyr tubulin, or IAA-treated HeLa Glu tubulin. The IF collapse in each injected cell was determined visually by fluorescence microscopy. IFs were considered to be collapsed when the entire IF network extended <30% of the distance from the nucleus to the leading edge of the cell. Brain Glu and IAA-Glu tubulins were microinjected at 100 μM; HeLa IAA-Glu and IAA-Tyr tubulins were microinjected at 70 μM. Data are pooled from two or more experiments that gave similar results. For uninjected cells (UNINJ.), n = 200; for brain Glu tubulin, n = 51; for brain IAA-Glu tubulin, n = 410; for HeLa IAA-Tyr tubulin, n = 121; and for HeLa IAA-Glu tubulin, n = 265.
C-Terminal Fragments of α-Tubulin Induce Collapse of IFs
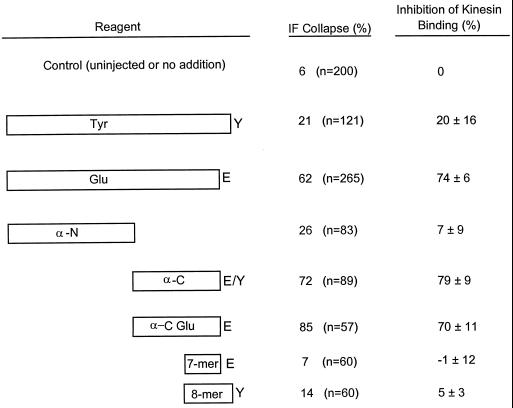
We showed above that monomeric, nonpolymerizable Glu tubulin was sufficient to induce collapse of IFs in vivo. Because the Glu tubulin antibodies, which induced collapse of the IF network in our previous study (Gurland and Gundersen, 1995), were raised against synthetic peptides corresponding to the last seven amino acid residues of Glu tubulin (Gundersen et al., 1984), we hypothesized that a linear sequence in Glu tubulin may contain the epitope involved in the MT–IF interaction. To determine whether the extreme C terminus of detyrosinated α-tubulin alone is sufficient to mediate the MT–IF interaction in vivo, we microinjected wound-edge cells with an excess of the C-terminal 7-aa Glu peptide and, as a control, the C-terminal 8-aa Tyr peptide. The final concentration of both peptides after injection was estimated to be 2 mM. Two hours after injection, the peptides were still present in cells, as determined by a diffuse immunofluorescent staining pattern; however neither the Glu nor Tyr peptide induced collapse of the IFs (our unpublished results).
The above results showed that the interaction of IFs with Glu MTs may require additional determinants other than the seven amino acids at the C terminus of α-tubulin. To test this, we prepared larger C-terminal α-tubulin fragments by digesting tubulin with trypsin. Trypsin preferentially proteolyzes the α-tubulin subunit and generates α-tubulin fragments of 14 kDa from the C terminus (α-C) and 36 kDa from the N terminus (α-N) (Serrano et al., 1984). From a limited trypsin digestion of purified brain tubulin, we isolated and purified 14-kDa α-C and 36-kDa α-N fragments by SDS-PAGE and electroelution as described in MATERIALS AND METHODS. We confirmed that the α-C fragments were reactive with the α-tubulin-specific antibody DM1A as reported previously (Breitling and Little, 1986) as well as with anti-Glu and anti-Tyr tubulin antibodies by Western blot analysis (our unpublished results). The α-N fragment was reactive with an antibody to acetylated α-tubulin, which occurs on Lys 40 within the α-N fragment (our unpublished results).
In cells injected with the α-N fragment (at concentrations as high as 170 μM), there was little observable change in the distribution of IFs (Figure 6, a and b). Conversely, in cells injected with the α-C fragment (at 145 μM), the IFs collapsed to a perinuclear area in 72% (n = 89) of the cells (Figure 6, c and d). As described previously, α-C from brain tubulin contains a mixture of Glu and Tyr tubulin fragments. To test whether the Glu tubulin fragment was sufficient to inhibit the MT–IF interaction, we prepared a detyrosinated α-C tubulin (α-C Glu) fragment by predigesting brain tubulin with CPA before trypsin proteolysis. We confirmed that this α-C Glu fragment reacted with Glu but not Tyr antibodies (our unpublished results). In cells microinjected with the α-C Glu (at 235 μM), the IFs collapsed in 85% (n = 57) of the cells (Figures 6, e and f, and 8). Combined with our previous results showing that IAA-Glu tubulin was more effective in collapsing IFs than was IAA-Tyr tubulin, these results suggest that the C terminus of detyrosinated α-tubulin provides a preferential site for the interaction of IFs with MTs.
Figure 6.
C-terminal fragments of α-tubulin induce collapse of IFs. Wound-edge cells were microinjected with N-terminal (α-N) or C-terminal (α-C and α-C Glu) α-tubulin trypsin fragments, incubated for 2 h at 37°C, fixed, and immunofluorescently stained for vimentin IFs (a, c, and e), human IgG marker (b and d), Glu tubulin (f), and Tyr tubulin. IFs are unaltered in cells injected with 70 μM α-N (a and b) but are collapsed to a perinuclear region in cells injected with 145 μM α-C (c and d) or 235 μM α-C Glu (e and f). Asterisks denote the peripheral edge of injected cells. Bar, 10 μm.
Nonpolymerizable HeLa Glu Tubulin and C-Terminal Fragments of α-Tubulin Inhibit the Binding of Kinesin to MTs
The mechanism by which IFs preferentially associate with Glu MTs in vivo is unknown. However, several lines of evidence point to the possibility that kinesin may be involved. Gyoeva and Gelfand (1991) found that microinjection of anti-kinesin antibodies into fibroblasts results in the collapse of IFs, and more recently, we found that conventional kinesin binds to Glu MTs with an affinity approximately threefold higher than that with which it binds to Tyr MTs and it interacts specifically with vimentin IFs in vitro (Liao and Gundersen, 1998). On the basis of these findings, we hypothesized that the microinjected IAA-Glu tubulin and the α-C and α-C Glu fragments collapsed IFs in vivo by blocking the interaction of kinesin with MTs.
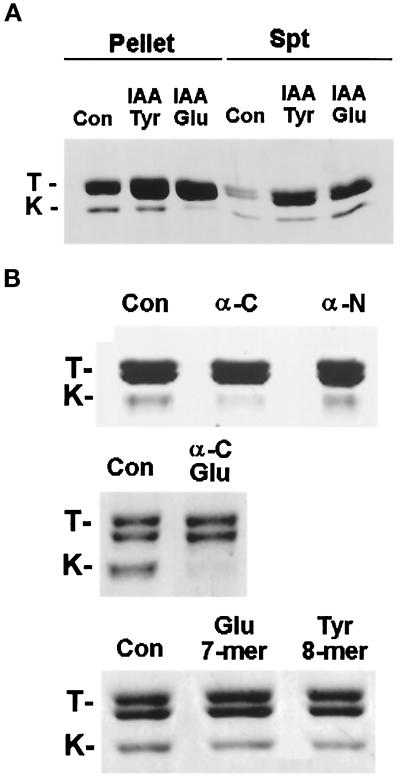
To test this hypothesis directly, we incubated taxol-stabilized brain MTs with the recombinant conventional kinesin head K394 in the presence of IAA-HeLa Glu or Tyr tubulins or the tubulin peptides or fragments that were used in the microinjection studies described above. The binding incubations were done such that the IAA-tubulins and tubulin fragments or peptides were at sevenfold molar excess over the MT concentration and at a final concentration similar to that used in vivo (estimated as one-tenth of the injected concentration). As shown in Figures 7 and 8, we observed significant inhibition of kinesin binding to MTs only in the presence of IAA-Glu tubulin (Figure 7A), the α-C fragment, or the α-C Glu fragment (Figure 7B). IAA-Tyr tubulin, the α-N fragment, the 7-aa Glu peptide, and the 8-aa Tyr peptide had no significant effect on the binding of kinesin to MTs (Figures 7 and 8). These results are in close agreement with our in vivo observations; i.e., IAA-HeLa Glu tubulin and α-C and α-C Glu fragments disrupted the distribution of IFs, whereas IAA-HeLa Tyr tubulin, the α-N fragment, the Glu peptide, and the Tyr peptide failed to do so (Figure 8). Taken together, these results suggest that the inhibition of the IF–MT interaction by IAA-Glu tubulin and the α-C and α-C Glu fragments is attributable to the competitive inhibition of kinesin binding to MTs.
Figure 7.
Effect of nonpolymerizable IAA-tubulins and α-tubulin fragments and peptides on binding of kinesin to MTs. The kinesin head (K394) was incubated with MTs in the absence or presence of IAA-HeLa Glu or IAA-HeLa Tyr tubulin (A), α-tubulin fragments (B; α-C, α-N, and α-C Glu), or C-terminal peptides of α-tubulin (B), was pelleted by centrifugation, and was analyzed by SDS-PAGE and Coomassie staining. Pellet and supernatant (Spt) fractions are shown in A, whereas only pellet fractions are shown in B. Bands marked T and K are tubulin and the kinesin head K394, respectively. Note, in A, that little kinesin head is detected in the MT pellet in the sample incubated with IAA-HeLa Glu tubulin (kinesin head is instead recovered in the supernatant), but significant kinesin head is recovered in MT pellets in the sample incubated with IAA-HeLa Tyr tubulin. The additional tubulin recovered in the supernatants in the IAA-HeLa Glu and Tyr tubulin samples reflects the added nonpolymerizable tubulin. Con, control.
Figure 8.
Effectiveness of nonpolymerizable IAA-tubulin and tubulin fragments and peptides in collapsing IFs in vivo and in inhibiting kinesin binding to MTs in vitro. For determination of the effect on IF distribution in vivo, wound-edge 3T3 cells were microinjected with IAA-HeLa Glu tubulin (Glu), IAA-HeLa Tyr tubulin (Tyr), α-tubulin fragments (α-C, α-N, and α-C Glu), the C-terminal 7-mer peptide of Glu tubulin (7-mer), and the C-terminal 8-mer peptide of Tyr tubulin (8-mer). Injected cells were assayed for IF collapse as described in Figure 5. IAA-HeLa Glu tubulin and IAA-HeLa Tyr tubulin were microinjected at 70 μM; α-N, α-C, and α-C Glu were microinjected at 170, 145, and 235 μM, respectively; and C-terminal Glu and Tyr tubulin peptides (7-mer and 8-mer) were microinjected at 20 mM. All concentrations refer to concentrations in the microinjection needle; the final concentration in the cells would be approximately one-tenth of that microinjected (see text). For determination of the effect on kinesin binding to MTs, the conventional kinesin head (K394) (1.4 μM) was incubated with taxol-stabilized brain MTs (2 μM) in the presence of the same tubulin and tubulin fragments described above, and samples were incubated as described in MATERIALS AND METHODS. IAA-HeLa Glu or IAA-HeLa Tyr tubulin and α-tubulin fragments were used at 14 μM, and the C-terminal peptides of Glu and Tyr tubulin were used at 150 μM. The amount of K394 that cosedimented with MTs and the level of tubulin in the MT pellets were quantified by SDS-PAGE and densitometry analysis. The amount of K394 cosedimenting with MTs was normalized to the level of tubulin in the MT pellet and compared with that of the control sample to obtain the inhibition of kinesin binding (%).
DISCUSSION
Our results demonstrate, for the first time, a specific function for one of the tubulin posttranslational modifications and point more generally to a fundamental role for tubulin posttranslational modifications in the segregation of monomeric from polymeric functions of tubulin. That IAA-Glu tubulin disrupted the coalignment of IFs and MTs but equivalent concentrations of IAA-Tyr tubulin were ineffective shows directly that the posttranslational detyrosination of tubulin itself is critical for maintaining an extended distribution of IFs on MTs. The alternative explanation that IFs accumulate on the most stable MTs in the cell, which are the Glu MTs, cannot be correct because the injected IAA-Glu tubulin collapsed the IFs without significantly affecting the distribution of the stable, Glu MTs (Figure 3d–f). Our current results are supported by a previous study in which antibodies to Glu tubulin, but not to Tyr tubulin, were able to induce the collapse of IFs (Gurland and Gundersen, 1995). Without the association with Glu tubulin in MTs, the IFs are not capable of withstanding the forces that collapse the IFs toward the cell center. This collapsing activity is thought to involve centripetal actin flow (Hollenbeck et al., 1989). The wound-edge cells we have used in this study may have a particularly active centripetal actin flow because the cells are highly polarized and locomoting (Mikhailov and Gundersen, 1995).
What does the cell gain by using posttranslational detyrosination to regulate the interaction of IFs with MTs? Indeed, polarization of the IFs could be accomplished by the time-dependent association of IFs with the most stable MTs in the cell; for the 3T3 cells used in this study, this would result in the codistribution of IFs and Glu MTs we observed previously (Gurland and Gundersen, 1995). We think our current results with the nonpolymerizable tubulins point to a more fundamental role for detyrosination. We have shown that only one form, Glu tubulin, acts as an effective inhibitor of the IF–MT interaction when introduced into the soluble pool in vivo. Combining our current results with previous work demonstrating that Tyr tubulin is normally the only form found at steady state in the monomer pool (Gundersen et al., 1987), we hypothesize that one form of tubulin, Tyr tubulin, is used for polymerization and MT dynamics, whereas the other form, Glu tubulin, is used to maintain IF–MT interactions. Thus, the fundamental role for tubulin posttranslational modification may be to segregate the two functional activities of tubulin: MT–tubulin interactions (i.e., polymerization) and MT–organelle interactions.
There may be important advantages in segregating the two activities of tubulin to different biochemical forms of tubulin. By the use of distinct forms for polymerization and stable organelle interactions, potential conflicts between the two activities may be minimized. Theoretically, it might be possible to reduce interference between the two activities of tubulin by maintaining the soluble pool of tubulin at a very low level in the cell. However, restricting monomeric tubulin to such low levels would have the deleterious consequences of limiting the rates of MT polymerization and lowering the level of MTs that could be maintained at steady state. As a direct consequence of lowered MT polymerization and decreased MT number, the cell might not be able to support the rapid dynamics necessary for proper spindle or morphogenetic functions of MTs. Thus, we suggest that the evolution of tubulin posttranslational modification permits both rapid changes in MT polymer and the efficient use of MTs for maintaining organelle interactions. The separation of tubulin activities by the use of biochemically distinct forms of tubulin is already a well known feature of tubulin; the hydrolysis of GTP by tubulin after polymerization allows for GTP–tubulin to polymerize and GDP–tubulin to depolymerize, a critical aspect of dynamic instability (Mitchison and Kirschner, 1984).
In our study, we found that nonpolymerizable IAA-Glu tubulin blocked the interaction of kinesin heads with MTs, suggesting that kinesin can bind to monomeric tubulin. Kinesin binding to monomeric tubulin has not been reported previously, which may be attributable to a lack of experiments designed to detect such an interaction or to difficulties in measuring the interaction. It is also clear from our study and a previous study that used a peptide corresponding to the C terminus of β-tubulin (Tucker and Goldstein, 1997) that portions of the tubulin molecule are capable of interacting with kinesin.
Kinesin has been implicated in maintaining the extended distribution of IFs. In the initial study, Gyoeva and Gelfand (1991) found that microinjection of antibodies to kinesin heavy chain collapsed IFs to a perinuclear location. This suggested that kinesin was necessary for maintaining the extended distribution of IFs in cells. Recently, we showed that conventional kinesin binds to Glu MTs with a higher affinity than to Tyr MTs and also binds specifically to vimentin IFs in vitro (Liao and Gundersen, 1998), raising the possibility that kinesin directly mediates the preferential association of IFs with Glu MTs that is observed in vivo (Gurland and Gundersen, 1995). Our current results demonstrating that the same set of reagents that induces IF collapse in vivo also acts as inhibitors of conventional kinesin head binding to MTs strongly support the hypothesis that kinesin is critical in maintaining the distribution of IFs on Glu MTs.
A key question that remains is how kinesin establishes the preferential association of IFs on Glu MTs. The agreement between the results of our in vivo and in vitro experiments with the tubulin fragments suggests that kinesin may directly link IFs and Glu MTs in vivo. Yet, to date we have found no evidence that kinesin is localized between Glu MTs and IFs in vivo (our unpublished results). However, recent work by Prahlad et al. (1998) has shown that kinesin localizes with small IF fragments in spreading cells and can also be detected on IFs when cells are pretreated at 4°C before fixation. Using the evidence to date, we can conclude that kinesin is important for establishing the preferential distribution of IFs on Glu MTs in vivo, but we cannot be certain that it directly links the two filaments together.
An alternate possibility is that kinesin does not act as a stable cross-bridging molecule between IFs and Glu MTs but instead is only involved with extending IFs on (Glu) MTs. After IFs have been extended, another molecule responsible for maintaining the stable IF–MT interaction may be engaged. This two-component model would not require that kinesin perform a novel activity (i.e., acting as a stable cross-bridging molecule) in addition to its well-characterized motor activity. One possible candidate for the stable cross-bridging molecule is the protein plectin. Plectin binds to both MTs and IFs (Foisner and Wiche, 1991) and has been localized in cross-bridging structures between MTs and IFs in vivo (Svitkina et al., 1996). Plectin is not preferentially localized on Glu MTs (Svitkina et al., 1996), suggesting that it is not involved in the mechanism that results in the preferential localization of IFs with Glu MTs. Nonetheless, if kinesin were responsible for the selective association of IFs with Glu MTs, plectin need not preferentially interact with one type of MT compared with the other.
Critical for either the kinesin-transport and -anchoring model or the kinesin-transport and plectin-anchoring model is the idea that IFs are actively translocated along MTs by kinesin. To date, kinesin has not been shown to move IFs on MTs; however, there is indirect evidence that IF may be a cargo of kinesin. As noted above, we demonstrated that conventional kinesin interacts specifically with vimentin IFs and this association appears to be mediated by the tail of kinesin where cargo is thought to interact (Liao and Gundersen, 1998). Additionally, in a study using GFP–vimentin, we found that IFs actively extended into the periphery of cells by a process that was dependent on MTs (Ho et al., 1998). In the study by Prahlad et al. (1998), the small vimentin filaments, which contained kinesin immunoreactivity, were observed to move in a MT-dependent manner, and we have found similar results (Martys et al., 1999). All of these studies point to the possibility that IFs are a cargo of kinesin, and it will be interesting to see whether there is a specific kinesin involved in this activity as has been suggested by in vitro binding studies (Liao and Gundersen, 1998).
Some human fibroblasts do not have MTs with elevated levels of Glu tubulin (i.e., Glu MTs) even though they have detectable levels of Glu tubulin by Western blotting (Webster et al., 1987b; our unpublished results). These cells have extended IFs, and this raises the question of whether Glu MTs are necessary for maintaining an extended IF array in all cells. We have done a limited number of experiments in cells that do not have Glu MTs (serum-starved 3T3 cells [Gundersen et al., 1994] and human A549 cells) and found that microinjected affinity-purified antibodies to Glu tubulin are still capable of collapsing IFs in these cells (Kreitzer and Gundersen, unpublished observations). This suggests that the maintenance of extended IFs is still dependent on Glu tubulin in cells without detectable Glu MTs. We have not yet determined whether the nonpolymerizable Glu tubulin is capable of collapsing IFs in these cells.
In addition to that of IFs, the distribution of other peripherally localized organelles such as the ER (Terasaki et al., 1986) and mitochondria (Ball and Singer, 1982) is also dependent on MTs. As MT-based, plus-end–directed motors, kinesins have been implicated in the transport and maintenance of the organization of the ER (Dabora and Sheetz, 1988; Vale and Hotani, 1988), mitochondria (Nangaku et al., 1994), and membrane-bound vesicles (Yamazaki et al., 1995; Hirokawa, 1996). Our results demonstrating the preferential interaction of conventional kinesin with Glu MTs (Liao and Gundersen, 1998) and the competitive inhibition of kinesin binding by IAA-Glu tubulin but not by IAA-Tyr tubulin (this study) raise the possibility that Glu MTs may also be the preferred MT substrates in other MT–organelle interactions. It will be important to determine whether other members of the kinesin superfamily are also regulated by posttranslational detyrosination.
There are a number of other forms of posttranslationally modified tubulin (Eipper, 1972; Barra et al., 1973; L’Hernault and Rosenbaum, 1985; Edde et al., 1990; Matten et al., 1990; Alexander et al., 1991; Redeker et al., 1994), and little is known about their function. It is possible that they are also regulating the interaction of MTs with organelles. Perhaps the diversity of modifications allows for specific regulation of different organelles with MTs: a one-modification, one-organelle model. Alternatively, separate modifications may specify regulation of different cellular pathways or localizations. In any case, we think that because most of these modifications are restricted to stable MTs, their primary function will be to separate monomer (polymerization) and polymer (organelle interactions) functions of MTs.
ACKNOWLEDGMENTS
We thank Yarong Li for preparing some of the tubulin used in this study. We thank James Goldman for use of his electroelutor. G.K. was supported in part by a predoctoral training grant from the National Institute on Aging. This work was supported by a grant from the National Institutes of Health (GM-42026) to G.G.G.
REFERENCES
- Alexander JE, Hunt DF, Lee MK, Shabanowitz J, Michel H, Berlin SC, MacDonald TL, Sundberg RJ, Rebhun LI, Frankfurter A. Characterization of posttranslational modifications in neuron-specific class III β-tubulin by mass spectrometry. Proc Natl Acad Sci USA. 1991;88:4685–4689. doi: 10.1073/pnas.88.11.4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas PW, Black MM. Individual microtubules in the axon consist of domains that differ in both composition and stability. J Cell Biol. 1990;111:495–509. doi: 10.1083/jcb.111.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball EH, Singer SJ. Mitochondria are associated with microtubules and not with intermediate filaments in cultured fibroblasts. Proc Natl Acad Sci USA. 1982;79:123–126. doi: 10.1073/pnas.79.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barra HS, Rodriguez JA, Arce CA, Caputto R. A soluble preparation from rat brain that incorporates into its own proteins [14C]-arginine by a ribonuclease-sensitive system and [14C]-tyrosine by a ribonuclease-insensitive system. J Neurochem. 1973;20:97–108. doi: 10.1111/j.1471-4159.1973.tb12108.x. [DOI] [PubMed] [Google Scholar]
- Breitling F, Little M. Carboxy-terminal regions on the surface of tubulin and microtubules. Epitope locations of YOL1/34, DM1A and DM1B. J Mol Biol. 1986;189:367–370. doi: 10.1016/0022-2836(86)90517-6. [DOI] [PubMed] [Google Scholar]
- Bulinski JC, Bossler A. Purification and characterization of ensconsin, a novel microtubule stabilizing protein. J Cell Sci. 1994;107:2389–2349. doi: 10.1242/jcs.107.10.2839. [DOI] [PubMed] [Google Scholar]
- Bulinski JC, Gundersen GG. Stabilization and posttranslational modification of microtubules during cellular morphogenesis. Bioessays. 1991;13:285–293. doi: 10.1002/bies.950130605. [DOI] [PubMed] [Google Scholar]
- Cambray-Deakin MA, Robson SJ, Burgoyne RD. Colocalisation of acetylated microtubules, glial filaments, and mitochondria in astrocytes in vitro. Cell Motil Cytoskeleton. 1988;10:438–449. doi: 10.1002/cm.970100311. [DOI] [PubMed] [Google Scholar]
- Chapin S, Bulinski JC. Preparation and functional assay of pure populations of tyrosinated and detyrosinated tubulin. Methods Enzymol. 1991;196:254–264. doi: 10.1016/0076-6879(91)96024-l. [DOI] [PubMed] [Google Scholar]
- Chapin SJ, Bulinski JC. Cellular microtubules heterogenous in their content of microtubule-associated protein 4 (MAP4) Cell Motil Cytoskeleton. 1994;27:133–149. doi: 10.1002/cm.970270205. [DOI] [PubMed] [Google Scholar]
- Cook TA, Nagasaki T, Gundersen GG. Rho guanosine triphosphatase mediates the selective stabilization of microtubules induced by lysophosphatidic acid. J Cell Biol. 1998;141:175–185. doi: 10.1083/jcb.141.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabora SL, Sheetz MP. The microtubule-dependent formation of a tubulovesicular network with characteristics of the ER from cultured cell extracts. Cell. 1988;54:27–35. doi: 10.1016/0092-8674(88)90176-6. [DOI] [PubMed] [Google Scholar]
- Edde B, Rossier J, LeCaer J-P, Desbruyeres E, Gros F, Denoulet P. Posttranslational glutamylation of α-tubulin. Science. 1990;247:83–85. doi: 10.1126/science.1967194. [DOI] [PubMed] [Google Scholar]
- Eipper BA. Rat brain microtubule protein: purification and determination of covalently bound phosphate and carbohydrate. Proc Natl Acad Sci USA. 1972;69:2283–2287. doi: 10.1073/pnas.69.8.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersfeld K, Wehland J, Plessman U, Dodemont H, Gerke V, Weber K. Characterization of the tubulin-tyrosine ligase. J Cell Biol . 1993;120:725–732. doi: 10.1083/jcb.120.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foisner R, Wiche G. Intermediate filament-associated proteins. Curr Opin Cell Biol. 1991;3:75–81. doi: 10.1016/0955-0674(91)90168-x. [DOI] [PubMed] [Google Scholar]
- Gundersen GG, Bulinski JC. Selective stabilization of microtubules oriented toward the direction of cell migration. Proc Natl Acad Sci USA. 1988;85:5946–5950. doi: 10.1073/pnas.85.16.5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen GG, Kalnoski MH, Bulinski JC. Distinct populations of microtubules: tyrosinated and nontyrosinated α-tubulin are distributed differently in cells. Cell. 1984;38:779–789. doi: 10.1016/0092-8674(84)90273-3. [DOI] [PubMed] [Google Scholar]
- Gundersen GG, Khawaja S, Bulinski JC. Postpolymerization detyrosination of α-tubulin: a mechanism for subcellular differentiation of microtubules. J Cell Biol. 1987;105:251–264. doi: 10.1083/jcb.105.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen GG, Khawaja S, Bulinski JC. Generation of a stable, posttranslationally modified microtubule array is an early event in myogenic differentiation. J Cell Biol. 1989;109:2275–2288. doi: 10.1083/jcb.109.5.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen GG, Kim I, Chapin CJ. Regulation of microtubule stability in fibroblasts by serum and TGF-β. J Cell Sci. 1994;107:645–659. doi: 10.1242/jcs.107.3.645. [DOI] [PubMed] [Google Scholar]
- Gurland G, Gundersen GG. Protein phosphatase inhibitors induce the selective breakdown of stable microtubules in fibroblasts and epithelial cells. Proc Natl Acad Sci USA. 1993;90:8827–8831. doi: 10.1073/pnas.90.19.8827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurland G, Gundersen GG. Stable, detyrosinated microtubules function to localize vimentin intermediate filaments. J Cell Biol. 1995;131:1275–1290. doi: 10.1083/jcb.131.5.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyoeva FK, Gelfand VI. Coalignment of vimentin intermediate filaments with microtubules depends on kinesin. Nature. 1991;353:445–448. doi: 10.1038/353445a0. [DOI] [PubMed] [Google Scholar]
- Hallak ME, Rodriguez JA, Barra HS, Caputto R. Release of tyrosine from tubulin. Some common factors that affect this process and the assembly of tubulin. FEBS Lett. 1977;73:147–150. doi: 10.1016/0014-5793(77)80968-x. [DOI] [PubMed] [Google Scholar]
- Hirokawa N. Organelle transport along microtubules—the role of KIFs. Trends Cell Biol. 1996;6:135–141. doi: 10.1016/0962-8924(96)10003-9. [DOI] [PubMed] [Google Scholar]
- Ho C-L, Martys J, Mikhailov A, Gundersen GG, Liem RKH. Novel features of intermediate filament dynamics revealed by green fluorescent protein fusion proteins. J Cell Sci. 1998;111:1767–1778. doi: 10.1242/jcs.111.13.1767. [DOI] [PubMed] [Google Scholar]
- Hollenbeck PJ, Bershadsky AD, Pletjushkina OY, Tint IS, Vasiliev JM. Intermediate filament collapse is an ATP dependent and actin-dependent process. J Cell Sci. 1989;92:621–631. doi: 10.1242/jcs.92.4.621. [DOI] [PubMed] [Google Scholar]
- Houliston E, Maro B. Posttranslational modification of distinct microtubule subpopulations during cell polarization and differentiation in the mouse preimplantation embryo. J Cell Biol. 1989;108:543–551. doi: 10.1083/jcb.108.2.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan M, Chin SSM, Maciose P, Srinawasan J, Hashim G, Liem RKH. Characterization of a panel of neurofilament antibodies recognizing N-terminal epitopes. J Neurosci Res. 1991;30:545–554. doi: 10.1002/jnr.490300312. [DOI] [PubMed] [Google Scholar]
- Khawaja S, Gundersen GG, Bulinski JC. Enhanced stability of microtubules enriched in detyrosinated tubulin is not a direct function of detyrosination level. J Cell Biol. 1988;106:141–149. doi: 10.1083/jcb.106.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilmartin JV, Wright B, Milstein C. Rat monoclonal anti-tubulin antibodies derived by using a new nonsecreting rat cell line. J Cell Biol. 1982;93:576–582. doi: 10.1083/jcb.93.3.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N, Flavin M. Preferential action of a brain detyrosinolating carboxypeptidase on polymerized tubulin. J Biol Chem. 1981;256:7678–7686. [PubMed] [Google Scholar]
- Kumar N, Flavin M. Modulation of some parameters of assembly of microtubules in vitro by tyrosination of tubulin. Eur J Biochem. 1982;128:215–222. doi: 10.1111/j.1432-1033.1982.tb06954.x. [DOI] [PubMed] [Google Scholar]
- L’Hernault S, Rosenbaum J. Chlamydomonas α-tubulin is posttranslationally modified by acetylation on the ε-amino group of a lysine. Biochemistry. 1985;24:473–478. doi: 10.1021/bi00323a034. [DOI] [PubMed] [Google Scholar]
- Liao G, Gundersen GG. Kinesin is a candidate for cross-bridging microtubules and intermediate filaments: selective binding of kinesin to detyrosinated tubulin and vimentin. J Biol Chem. 1998;273:9797–9803. doi: 10.1074/jbc.273.16.9797. [DOI] [PubMed] [Google Scholar]
- Luduena RF, Roach MC. Interaction of tubulin with drugs and alkylating agents. 2. Effects of colchicine, podophyllotoxin and vinblastine on the alkylation of tubulin. Biochemistry. 1981;20:4437–4444. doi: 10.1021/bi00518a032. [DOI] [PubMed] [Google Scholar]
- Martys, J.L., Ho, C.L., Liem, R.K.H., and Gundersen, G.G. (1999). IFs in motion: observations of intermediate filaments in cells using GFP-vimentin. Mol. Biol. Cell (in press). [DOI] [PMC free article] [PubMed]
- Matten WT, Aubrey M, West J, Maness PF. Tubulin is phosphorylated at tyrosine by pp60c-src in nerve growth cone membranes. J Cell Biol. 1990;111:1959–1969. doi: 10.1083/jcb.111.5.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikhailov A, Gundersen GG. The centripetal transport of microtubules in motile cells. Cell Motil Cytoskeleton. 1995;32:173–186. doi: 10.1002/cm.970320303. [DOI] [PubMed] [Google Scholar]
- Mitchison TJ, Kirschner MW. Dynamic instability of microtubule growth. Nature. 1984;312:237–242. doi: 10.1038/312237a0. [DOI] [PubMed] [Google Scholar]
- Nagasaki T, Chapin CJ, Gundersen GG. Distribution of detyrosinated microtubules in motile NRK fibroblasts is rapidly altered upon cell-cell contact: implications for contact inhibition of locomotion. Cell Motil Cytoskeleton. 1992;23:45–60. doi: 10.1002/cm.970230106. [DOI] [PubMed] [Google Scholar]
- Nangaku M, Sato-Yoshitake R, Okada Y, Noda Y, Takemura R, Yamazaki H, Hirokawa N. KIF1B, a novel microtubule plus end-directed monomeric motor protein for transport of mitochondria. Cell. 1994;79:1209–1220. doi: 10.1016/0092-8674(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Paturle L, Wehland J, Margolis RL, Job D. Complete separation of tyrosinated, detyrosinated, and nontyrosinated brain tubulin subpopulations using affinity chromatography. Biochemistry. 1989;21:2698–2704. doi: 10.1021/bi00432a050. [DOI] [PubMed] [Google Scholar]
- Prahlad V, Uyoon N, Moir RD, Vale RD, Goldman RD. Rapid movement of vimentin on microtubule tracks: kinesin-dependent assembly of intermediate filament networks. J Cell Biol. 1998;143:159–170. doi: 10.1083/jcb.143.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybin D, Flavin M. Enzyme which adds tyrosine to the α-chain of tubulin. Biochemistry. 1977;16:2189–2194. doi: 10.1021/bi00629a023. [DOI] [PubMed] [Google Scholar]
- Redeker V, Levilliers N, Schmitter JM, LeCaer JP, Rossier J, Adoutte A, Bre MH. Polyglycylation of tubulin: a posttranslational modification in axonemal microtubules. Science. 1994;266:1688–1691. doi: 10.1126/science.7992051. [DOI] [PubMed] [Google Scholar]
- Schroder HC, Wehland J, Weber K. Purification of brain tubulin-tyrosine ligase by biochemical and immunological methods. J Cell Biol. 1985;106:276–281. doi: 10.1083/jcb.100.1.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze E, Asai DJ, Bulinski JC, Kirschner M. Posttranslational modifications and microtubule stability. J Cell Biol. 1987;105:2167–2177. doi: 10.1083/jcb.105.5.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano L, Avila J, Maccioni RB. Limited proteolysis of tubulin and the localization of the binding site for colchicine. J Biol Chem. 1984;259:6607–6611. [PubMed] [Google Scholar]
- Song YH, Mandelkow E. Recombinant kinesin motor domain binds to β-tubulin and decorates microtubules with a B surface lattice. Proc Natl Acad Sci USA. 1993;90:1671–1675. doi: 10.1073/pnas.90.5.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svitkina TM, Verkovsky AB, Borisy GG. Plectin sidearms mediate the interaction of intermediate filaments with microtubules and other components of the cytoskeleton. J Cell Biol. 1996;135:991–1007. doi: 10.1083/jcb.135.4.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasaki M, Chen LB, Fujiwara K. Microtubules and the endoplasmic reticulum are highly interdependent structures. J Cell Biol. 1986;103:1557–1568. doi: 10.1083/jcb.103.4.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker C, Goldstein LSB. Probing the kinesin-microtubule interaction. J Biol Chem. 1997;272:9481–9488. doi: 10.1074/jbc.272.14.9481. [DOI] [PubMed] [Google Scholar]
- Vale RD, Hotani H. Formation of membrane networks in vitro by kinesin-driven microtubule movement. J Cell Biol. 1988;107:2233–2241. doi: 10.1083/jcb.107.6.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster DR, Gundersen GG, Bulinski JC, Borisy GG. Differential turnover of tyrosinated and detyrosinated microtubules. Proc Natl Acad Sci USA. 1987a;84:9040–9044. doi: 10.1073/pnas.84.24.9040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster DR, Gundersen GG, Bulinski JC, Borisy GG. Assembly and turnover of detyrosinated tubulin in vivo. J Cell Biol. 1987b;105:265–276. doi: 10.1083/jcb.105.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster DR, Wheland J, Weber K, Borisy GG. Detyrosination of α-tubulin does not stabilize MTs in vivo. J Cell Biol. 1990;111:113–122. doi: 10.1083/jcb.111.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki H, Nakata T, Okada Y, Hirokawa N. KIF3A/B: a heterodimeric kinesin superfamily protein that works as a microtubule plus end-directed motor for membrane organelle transport. J Cell Biol. 1995;130:1387–1399. doi: 10.1083/jcb.130.6.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]