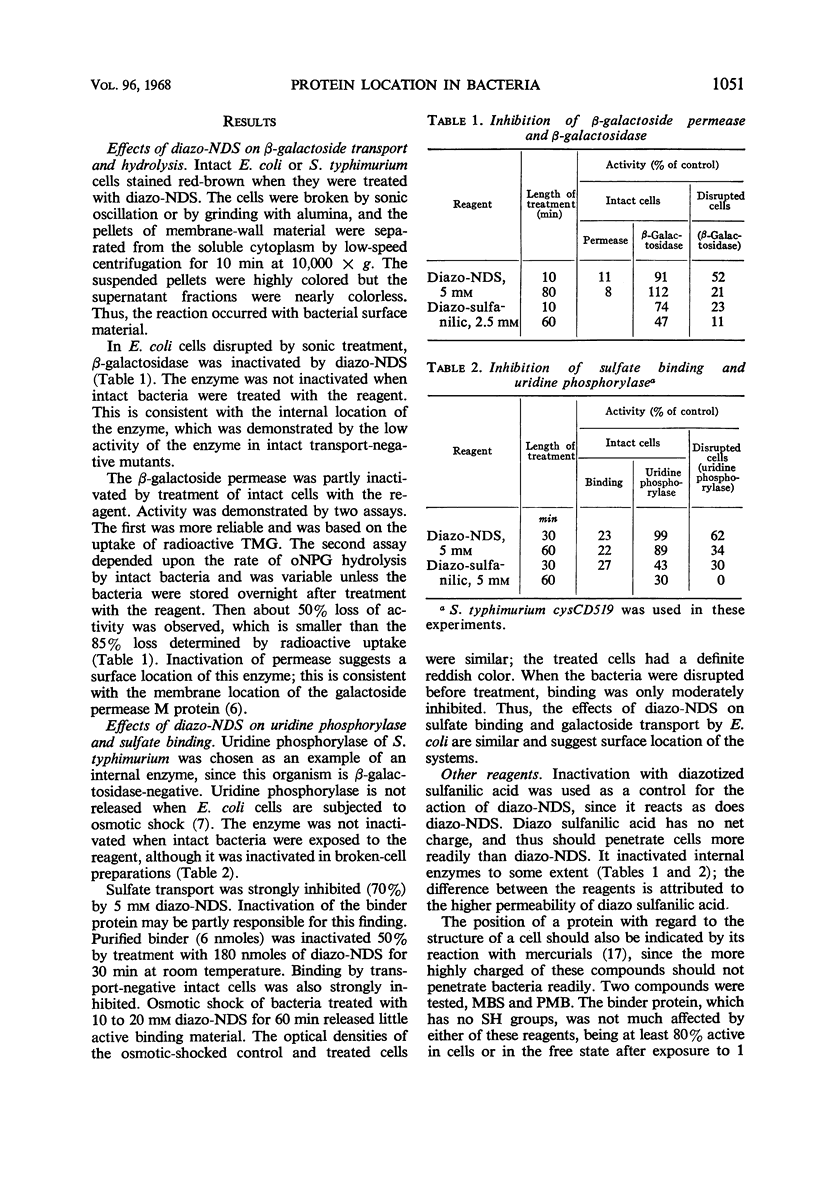
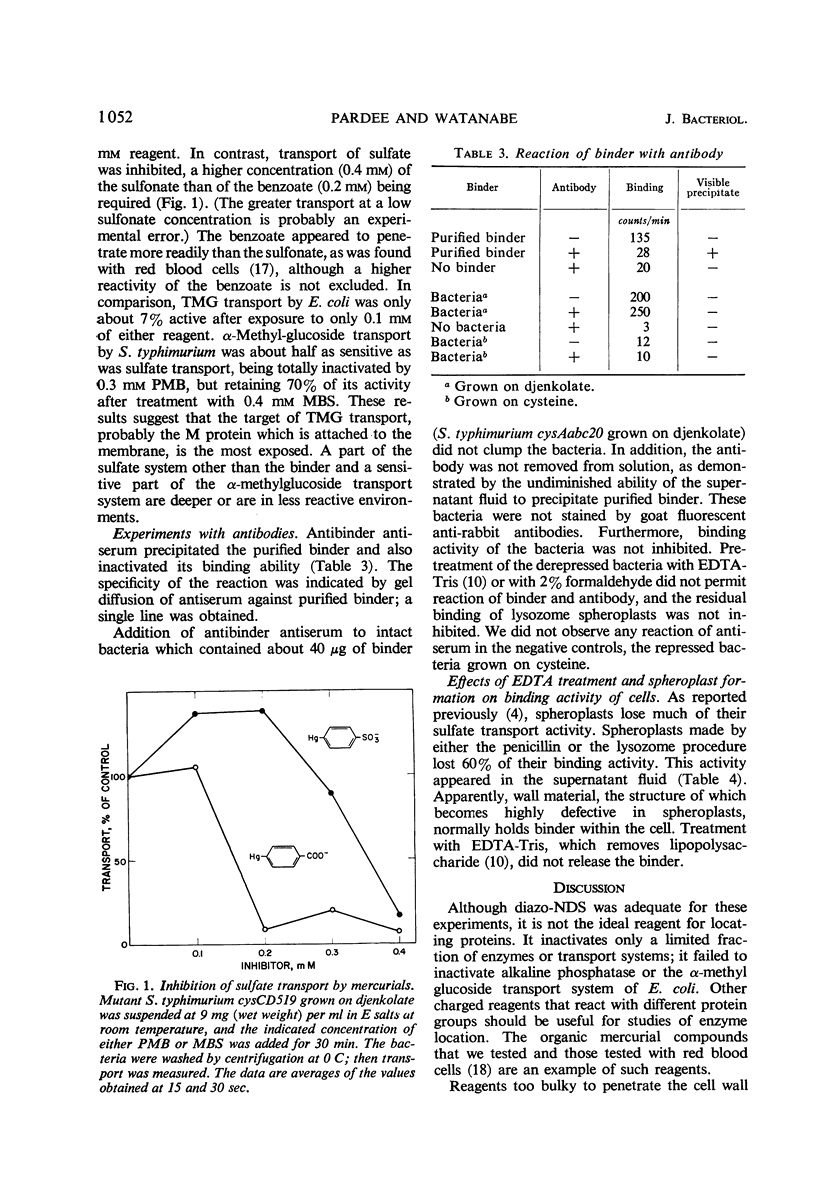
Abstract
A method is described for location of proteins in bacteria. It depends upon two techniques. One technique is the inactivation of the protein by a reagent which is incapable of penetrating the bacterial membrane (permeability barrier). Proteins inside this membrane cannot be inactivated unless the cells are disrupted; proteins on or outside the membrane can be inactivated. The second technique depends upon inactivation of the protein by specific antibody. Antibody should not penetrate the external bacterial wall, and therefore should only inactivate proteins that are on the wall surface. Thus, proteins can be localized inside the membrane, in the wall-membrane area, or outside the wall. One reagent developed for use with the first technique is diazo-7-amino-1,3-naphthalene-disulfonate. It inactivated β-galactoside transport, but not β-galactosidase of intact Escherichia coli. Similarly, it inactivated sulfate binding and transport but not uridine phosphorylase activity of Salmonella typhimurium. This indicates that the sulfate-binding protein is on or outside the cell membrane, and that uridine phosphorylase is inside the cell. The organic mercurial compounds used also showed that the sensitive parts of the sulfate and α-methylglucoside transport systems are less reactive than the sensitive part of the β-galactoside system. Antibody to the sulfate-binding protein inactivated the purified protein but did not inactivate this protein when intact bacteria were employed. Thus, it appears that the sulfate-binding protein does not protrude outside the cell wall. The conclusion that the binding protein is located in the wall-membrane region is supported by its release upon spheroplast formation or osmotic shock, and also by its ability to combine with sulfate in bacteria which cannot transport sulfate into the cell.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anraku Y. The reduction and restoration of galactose transport in osmotically shocked cells of Escherichia coli. J Biol Chem. 1967 Mar 10;242(5):793–800. [PubMed] [Google Scholar]
- DREYFUSS J. CHARACTERIZATION OF A SULFATE- AND THIOSULFATE-TRANSPORTING SYSTEM IN SALMONELLA TYPHIMURIUM. J Biol Chem. 1964 Jul;239:2292–2297. [PubMed] [Google Scholar]
- Dreyfuss J., Pardee A. B. Evidence for a sulfate-binding site external to the cell membrane of Salmonella typhimurium. Biochim Biophys Acta. 1965 Jun 15;104(1):308–310. doi: 10.1016/0304-4165(65)90256-4. [DOI] [PubMed] [Google Scholar]
- ECHOLS H., GAREN A., GAREN S., TORRIANI A. Genetic control of repression of alkaline phosphatase in E. coli. J Mol Biol. 1961 Aug;3:425–438. doi: 10.1016/s0022-2836(61)80055-7. [DOI] [PubMed] [Google Scholar]
- Fox C. F., Carter J. R., Kennedy E. P. GENETIC CONTROL OF THE MEMBRANE PROTEIN COMPONENT OF THE LACTOSE TRANSPORT SYSTEM OF Escherichia coli. Proc Natl Acad Sci U S A. 1967 Mar;57(3):698–705. doi: 10.1073/pnas.57.3.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppel L. A. Selective release of enzymes from bacteria. Science. 1967 Jun 16;156(3781):1451–1455. doi: 10.1126/science.156.3781.1451. [DOI] [PubMed] [Google Scholar]
- Kaback H. R., Stadtman E. R. Proline uptake by an isolated cytoplasmic membrane preparation of Escherichia coli. Proc Natl Acad Sci U S A. 1966 Apr;55(4):920–927. doi: 10.1073/pnas.55.4.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leive L., Kollin V. Controlling EDTA treatment to produce permeable Escherichia coli with normal metabolic processes. Biochem Biophys Res Commun. 1967 Jul 21;28(2):229–236. doi: 10.1016/0006-291x(67)90434-2. [DOI] [PubMed] [Google Scholar]
- MADDY A. H. A FLUORESCENT LABEL FOR THE OUTER COMPONENTS OF THE PLASMA MEMBRANE. Biochim Biophys Acta. 1964 Sep 25;88:390–399. doi: 10.1016/0926-6577(64)90194-9. [DOI] [PubMed] [Google Scholar]
- PRESTIDGE L. S., PARDEE A. B. A SECOND PERMEASE FOR METHYL-THIO-BETA-D-GALACTOSIDE IN ESCHERICHIA COLI. Biochim Biophys Acta. 1965 May 4;100:591–593. doi: 10.1016/0304-4165(65)90029-2. [DOI] [PubMed] [Google Scholar]
- Pardee A. B., Prestidge L. S., Whipple M. B., Dreyfuss J. A binding site for sulfate and its relation to sulfate transport into Salmonella typhimurium. J Biol Chem. 1966 Sep 10;241(17):3962–3969. [PubMed] [Google Scholar]
- Piperno J. R., Oxender D. L. Amino-acid-binding protein released from Escherichia coli by osmotic shock. J Biol Chem. 1966 Dec 10;241(23):5732–5734. [PubMed] [Google Scholar]
- STEIN W. D. N-terminal histidine at the active centre of a permeability mechanism. Nature. 1958 Jun 14;181(4624):1662–1663. doi: 10.1038/1811662b0. [DOI] [PubMed] [Google Scholar]
- TONOMURA K., TANABE O. LOCALIZATION OF CELL-BOUND ALPHA-AMYLASE IN ASPERGILLUS ORYZAE DEMONSTRATED BY FLUORESCENT-ANTIBODY TECHNIQUE. J Bacteriol. 1964 Jan;87:226–227. doi: 10.1128/jb.87.1.226-227.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VANSTEVENINCK J., WEED R. I., ROTHSTEIN A. LOCALIZATION OF ERYTHROCYTE MEMBRANE SULFHYDRYL GROUPS ESSENTIAL FOR GLUCOSE TRANSPORT. J Gen Physiol. 1965 Mar;48:617–632. doi: 10.1085/jgp.48.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]


