Abstract
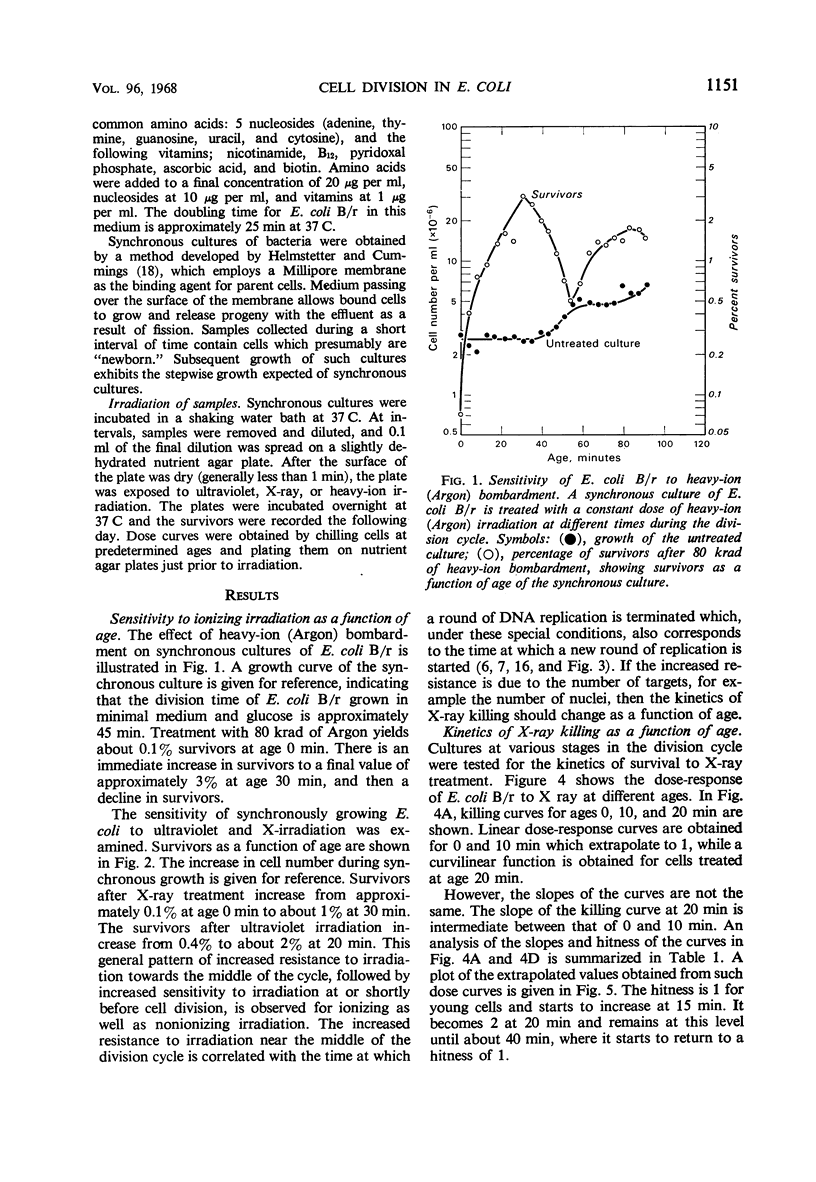
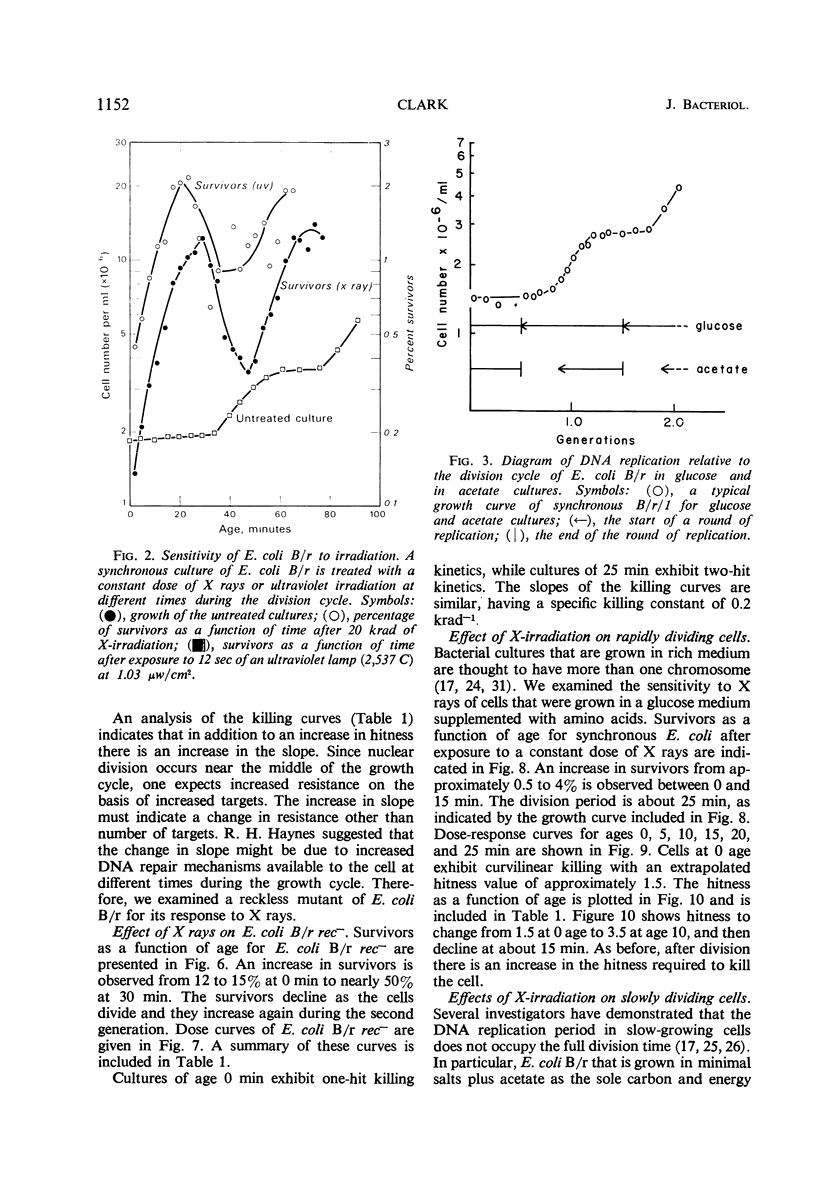
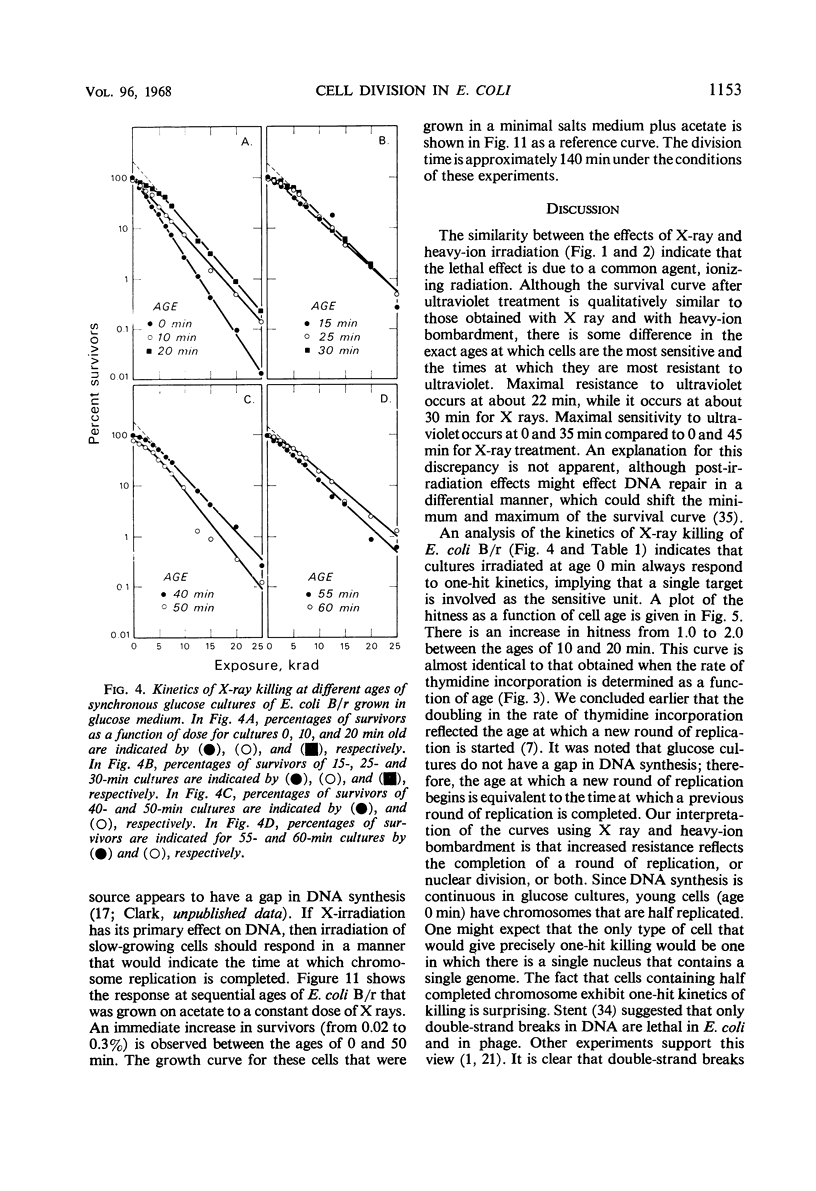
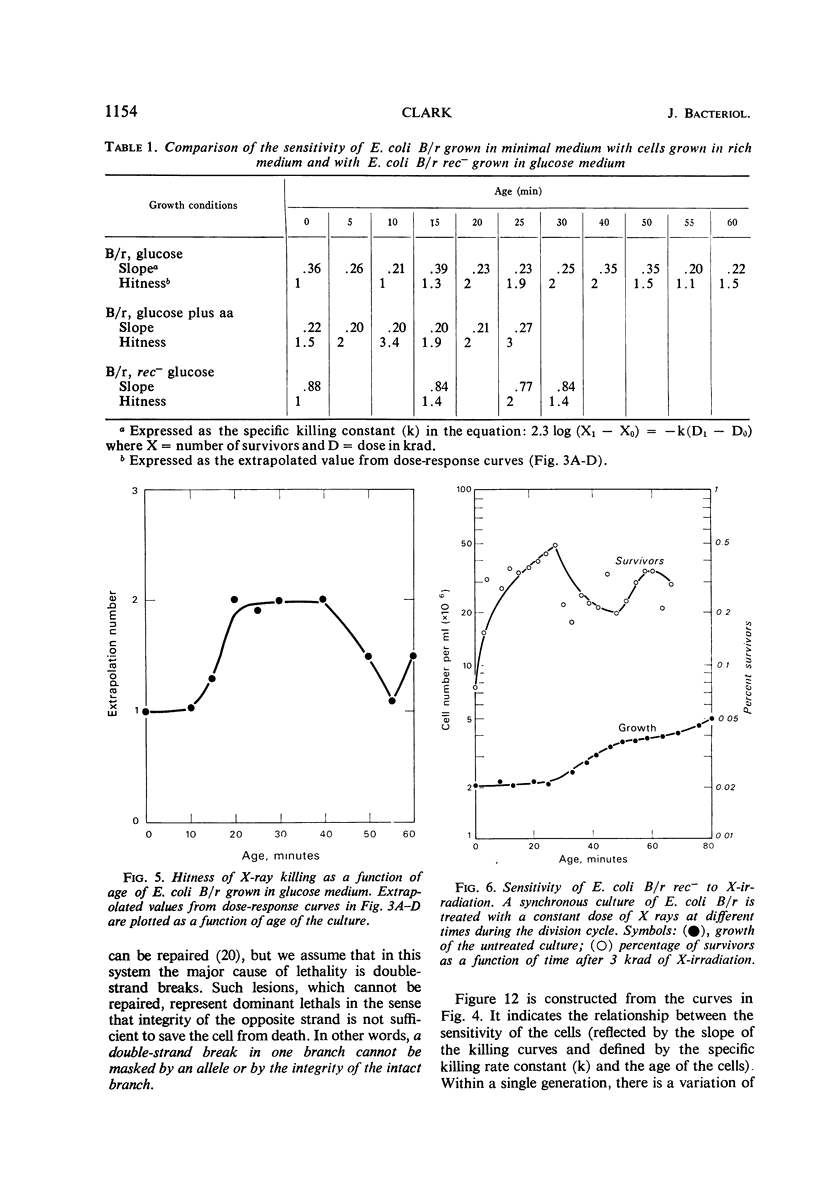
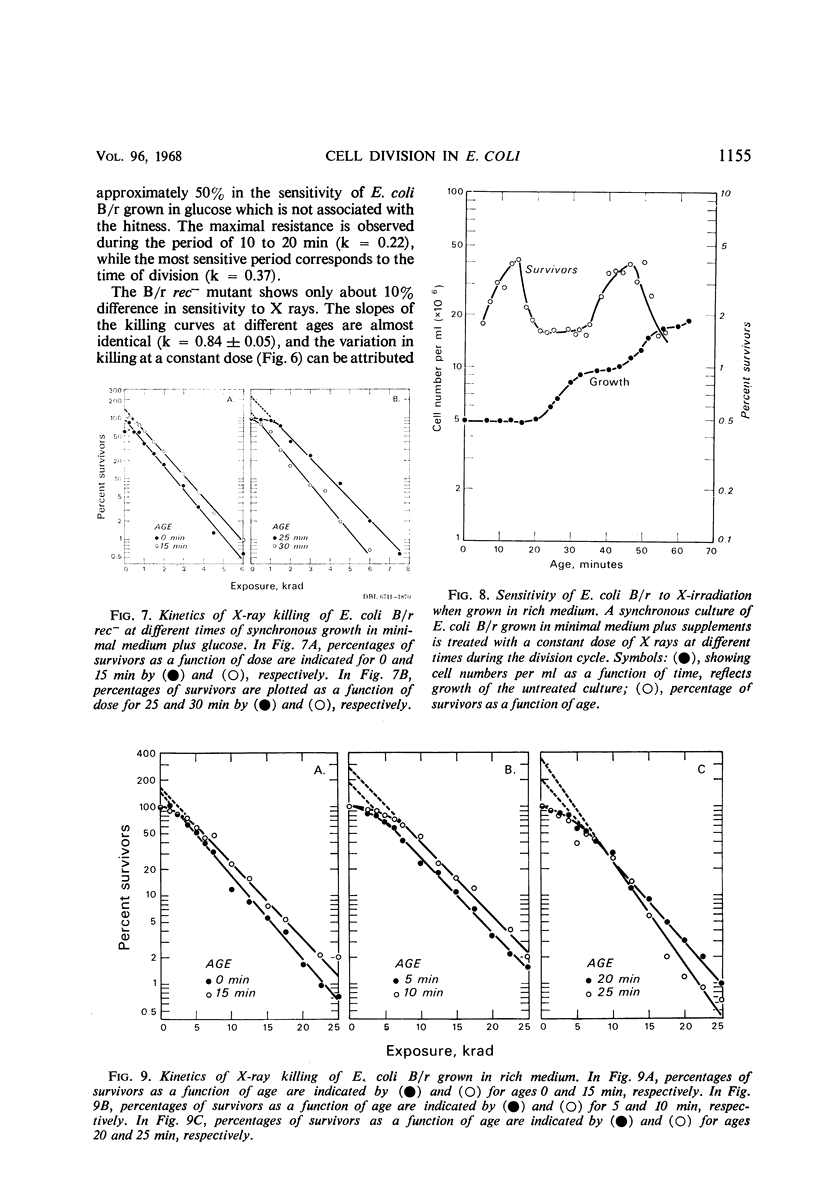
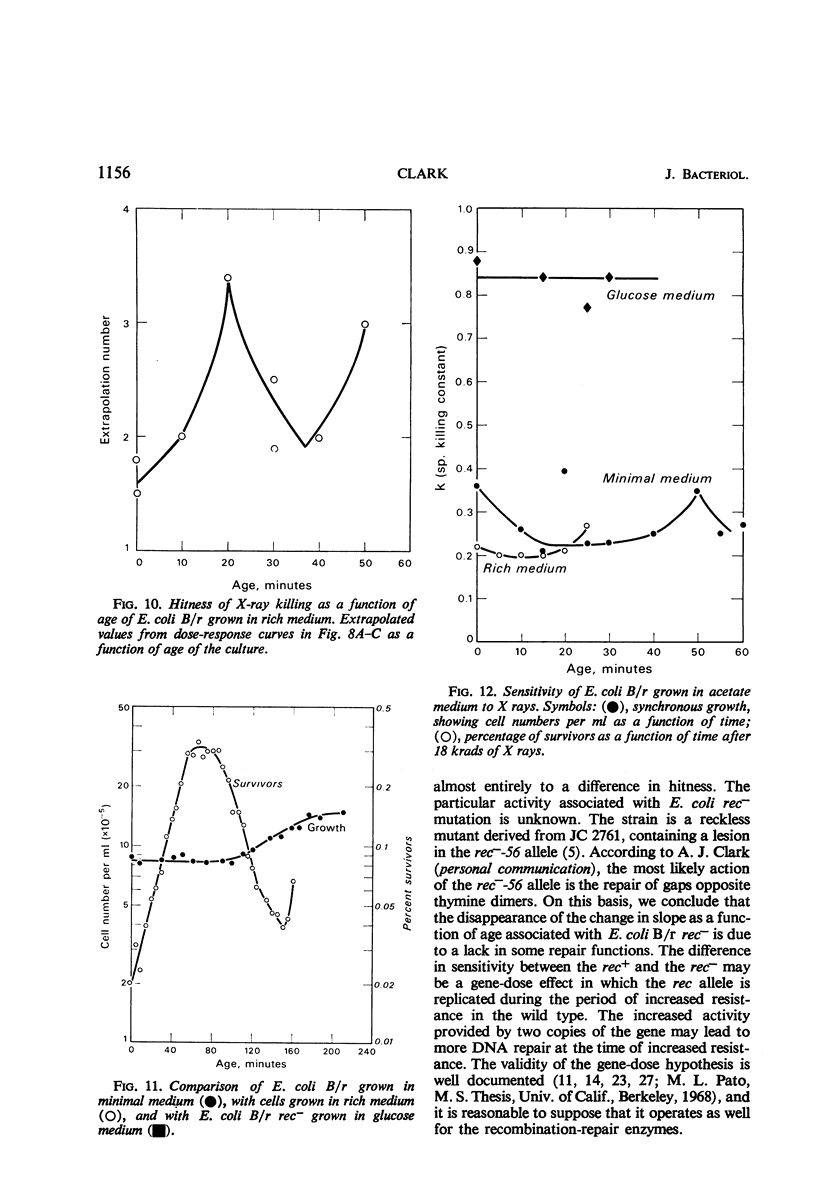
The sensitivity of Escherichia coli B/r to X-irradiation is correlated with the replication cycle of deoxyribonucleic acid (DNA). The sensitivity to X-irradiation in the wild type can be attributed to the presence of nuclear targets plus DNA repair mechanisms. The effects of nuclear targets are observed in the recombination-deficient (rec−) mutant B/r, but the sensitivity reflected by changes in the slope of killing curves is absent. A study of different growth conditions indicates that maximal resistance to X rays occurs toward the middle of the division cycle. Evidence is offered that branched chromosomes respond as one-hit targets to X-irradiation. The killing effects of heavy-ion bombardment on E. coli are due primarily to ionizing radiation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALEXANDER P., STACEY K. A. Cross-linking of deoxyribonucleic acid in sperm heads by ionizing radiations. Nature. 1959 Sep 26;184:958–960. doi: 10.1038/184958a0. [DOI] [PubMed] [Google Scholar]
- BILLEN D. UNBALANCED DEOXYRIBONUCLEIC ACID SYNTHESIS: ITS ROLE IN X-RAY-INDUCED BACTERIAL DEATH. Biochim Biophys Acta. 1963 Aug 20;72:608–618. [PubMed] [Google Scholar]
- CAIRNS J. The bacterial chromosome and its manner of replication as seen by autoradiography. J Mol Biol. 1963 Mar;6:208–213. doi: 10.1016/s0022-2836(63)80070-4. [DOI] [PubMed] [Google Scholar]
- Clark A. J. The beginning of a genetic analysis of recombination proficiency. J Cell Physiol. 1967 Oct;70(2 Suppl):165–180. doi: 10.1002/jcp.1040700412. [DOI] [PubMed] [Google Scholar]
- DEERING R. A. Studies on division inhibition and filament formation of Escherichia coli by ultraviolet light. J Bacteriol. 1958 Aug;76(2):123–130. doi: 10.1128/jb.76.2.123-130.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEFILIPPES F. M., GUILD W. R. Irradiation of solutions of transforming DNA. Radiat Res. 1959 Jul;11(1):38–53. [PubMed] [Google Scholar]
- DREW R. M. Gamma-Irradiation of penumococcus deoxyribonucleic acid. Radiat Res. 1955 Oct;3(2):116–120. [PubMed] [Google Scholar]
- Donachie W. D. Control of enzyme steps during the bacterial cell cycle. Nature. 1965 Mar 13;205(976):1084–1086. doi: 10.1038/2051084a0. [DOI] [PubMed] [Google Scholar]
- Gorman J., Taruo P., LaBerge M., Halvorson H. Timing of enzyme synthesis during synchronous division in yeast. Biochem Biophys Res Commun. 1964 Feb 18;15(1):43–49. doi: 10.1016/0006-291x(64)90100-7. [DOI] [PubMed] [Google Scholar]
- HELMSTETTER C. E., CUMMINGS D. J. AN IMPROVED METHOD FOR THE SELECTION OF BACTERIAL CELLS AT DIVISION. Biochim Biophys Acta. 1964 Mar 16;82:608–610. doi: 10.1016/0304-4165(64)90453-2. [DOI] [PubMed] [Google Scholar]
- HELMSTETTER C. E., URETZ R. B. X-ray and ultraviolet sensitivity of synchronously dividing Escherichia coli. Biophys J. 1963 Jan;3:35–47. doi: 10.1016/s0006-3495(63)86802-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAPLAN H. S., MOSES L. E. BIOLOGICAL COMPLEXITY AND RADIOSENSITIVITY. RADIATION LETHALITY IN CELLS AND VIRUSES IS CORRELATED WITH NUCLEIC ACID CONTENT, STRUCTURE, AND PLOIDY. Science. 1964 Jul 3;145(3627):21–25. doi: 10.1126/science.145.3627.21. [DOI] [PubMed] [Google Scholar]
- KAPLAN H. S., SMITH K. C., TOMLIN P. Radiosensitization of E. coli by purine and pyrimidine analogues incorporated in deoxyribonucleic acid. Nature. 1961 May 27;190:794–796. doi: 10.1038/190794a0. [DOI] [PubMed] [Google Scholar]
- KUEMPEL P. L., PARDEE A. B. THE CYCLE OF BACTERIAL DUPLICATION. J Cell Physiol. 1963 Oct;62:SUPPL1–SUPPL1:30. doi: 10.1002/jcp.1030620404. [DOI] [PubMed] [Google Scholar]
- Kaplan H. S. DNA-strand scission and loss of viability after x irradiation of normal and sensitized bacterial cells. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1442–1446. doi: 10.1073/pnas.55.6.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lark C. Regulation of deoxyribonucleic acid synthesis in Escherichia coli: dependence on growth rates. Biochim Biophys Acta. 1966 Jun 22;119(3):517–525. doi: 10.1016/0005-2787(66)90128-6. [DOI] [PubMed] [Google Scholar]
- Lark K. G. Regulation of chromosome replication and segregation in bacteria. Bacteriol Rev. 1966 Mar;30(1):3–32. doi: 10.1128/br.30.1.3-32.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAALOE O., HANAWALT P. C. Thymine deficiency and the normal DNA replication cycle. I. J Mol Biol. 1961 Apr;3:144–155. doi: 10.1016/s0022-2836(61)80041-7. [DOI] [PubMed] [Google Scholar]
- MUNSON R. J., MACLEAN F. I. The nature and radiation sensitivity of the long forms of Escherichia coli strain B/r. J Gen Microbiol. 1961 May;25:29–39. doi: 10.1099/00221287-25-1-29. [DOI] [PubMed] [Google Scholar]
- Masters M., Kuempel P. L., Pardee A. B. Enzyme synthesis in synchronous cultures of bacteria. Biochem Biophys Res Commun. 1964 Feb 18;15(1):38–42. doi: 10.1016/0006-291x(64)90099-3. [DOI] [PubMed] [Google Scholar]
- OWEN M. E., MORTIMER R. K. Dominant lethality induced by x-rays in haploid and diploid Saccharomyces cerevisiae. Nature. 1956 Mar 31;177(4509):625–626. doi: 10.1038/177625b0. [DOI] [PubMed] [Google Scholar]
- Person S. Comparative Killing Efficiencies for Decays of Tritiated Compounds Incorporated into E. coli. Biophys J. 1963 May;3(3):183–187. doi: 10.1016/s0006-3495(63)86814-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHAECHTER M., WILLIAMSON J. P., HOOD J. R., Jr, KOCH A. L. Growth, cell and nuclear divisions in some bacteria. J Gen Microbiol. 1962 Nov;29:421–434. doi: 10.1099/00221287-29-3-421. [DOI] [PubMed] [Google Scholar]
- STAPLETON G. E. Variations in the sensitivity of escherichia coli to ionizing radiations during the growth cycle. J Bacteriol. 1955 Oct;70(4):357–362. doi: 10.1128/jb.70.4.357-362.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WITKIN E. M. THE EFFECT OF ACRIFLAVINE ON PHOTOREVERSAL OF LETHAL AND MUTAGENIC DAMAGE PRODUCED IN BACTERIA BY ULTRAVIOLET LIGHT. Proc Natl Acad Sci U S A. 1963 Sep;50:425–430. doi: 10.1073/pnas.50.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANAGITA T., MARUYAMA Y., TAKEBE I. Cellular response to deleterious agents during the course of synchronous growth of Escherichia coli. J Bacteriol. 1958 May;75(5):523–529. doi: 10.1128/jb.75.5.523-529.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]


