Abstract
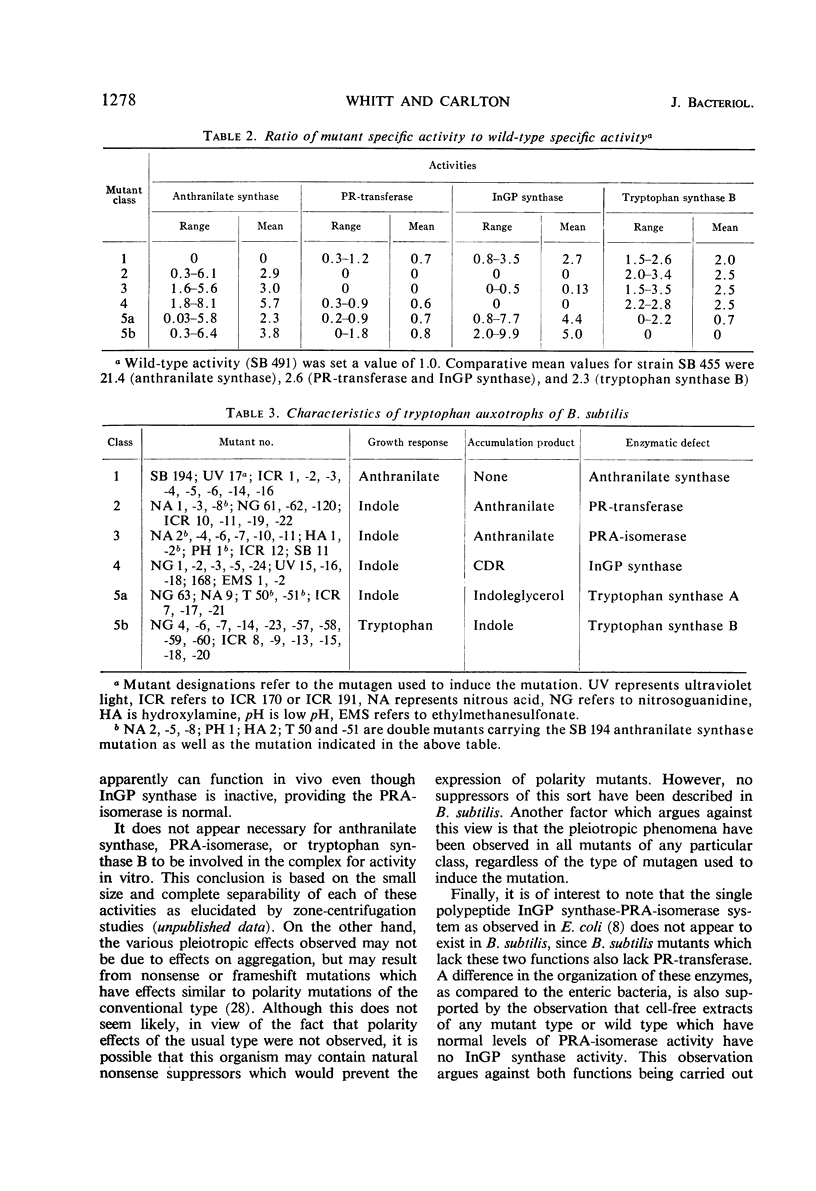
Sixty-five tryptophan auxotrophs which map in a cluster on the genome of Bacillus subtilis were characterized on the basis of (i) growth response, (ii) accumulation of intermediate compounds, and (iii) determination of enzymatic defects. They could be placed into six phenotypic classes. Certain of the mutants exhibited pleiotropic effects on more than one enzymatic activity in a manner different from those effects reported for the tryptophan pathway in other organisms. Invariably, mutations in the second gene, that coding for phosphoribosyl transferase activity, were found to lack the indoleglycerol phosphate synthase activity specified by the third gene in the cluster; however, this polarity did not extend to genes more distal in the cluster. Furthermore, mutations in the gene which codes for phosphoribosyl-anthranilate isomerase not only led to a loss of this enzyme but also to a loss of phosphoribosyl transferase and indoleglycerol phosphate synthase. In contrast, mutations in either of the loci coding for these latter functions had no apparent effect on isomerase activity. No polarity of the conventional type was found, e.g., none of the mutations in any gene led to polarized effects on the levels of the enzymes specified by the other genes of the cluster. These observations indicated a possible in vivo aggregation involving the transferase, isomerase, and synthase enzymes, with the isomerase acting as the “key” enzyme in the aggregate.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANAGNOSTOPOULOS C., CRAWFORD I. P. Transformation studies on the linkage of markers in the tryptophan pathway in Bacillus subtilis. Proc Natl Acad Sci U S A. 1961 Mar 15;47:378–390. doi: 10.1073/pnas.47.3.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostopoulos C., Crawford I. P. Le groupe des gènes régissant la biosynthèse du tryptophane chez Bacillus subtilis. C R Acad Sci Hebd Seances Acad Sci D. 1967 Jul 3;265(1):93–96. [PubMed] [Google Scholar]
- Bauerle R. H., Margolin P. A multifunctional enzyme complex in the tryptophan pathway of Salmonella typhimurium: comparison of polarity and pseudopolarity mutations. Cold Spring Harb Symp Quant Biol. 1966;31:203–214. doi: 10.1101/sqb.1966.031.01.028. [DOI] [PubMed] [Google Scholar]
- Bauerle R. H., Margolin P. The functional organization of the tryptophan gene cluster in Salmonella typhimurium. Proc Natl Acad Sci U S A. 1966 Jul;56(1):111–118. doi: 10.1073/pnas.56.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume A. J., Balbinder E. The tryptophan operon of Salmonella typhimurium. Fine structure analysis by deletion mapping and abortive transduction. Genetics. 1966 Mar;53(3):577–592. doi: 10.1093/genetics/53.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton B. C. Fine-structure mapping by transformation in the tryptophan region of Bacillus subtilis. J Bacteriol. 1966 May;91(5):1795–1803. doi: 10.1128/jb.91.5.1795-1803.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton B. C. Transformation mapping of the genes controlling tryptophan biosynthesis in Bacillus subtilis. J Bacteriol. 1967 Sep;94(3):660–665. doi: 10.1128/jb.94.3.660-665.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creighton T. E., Yanofsky C. Indole-3-glycerol phosphate synthetase of Escherichia coli, an enzyme of the tryptophan operon. J Biol Chem. 1966 Oct 25;241(20):4616–4624. [PubMed] [Google Scholar]
- DOY C. H., GIBSON F. 1-(o-Carboxyphenylamino)-1-deoxyribulose. A compound formed by mutant strains of Aerobacter aerogenes and Escherichia coli blocked in the biosynthesis of tryptophan. Biochem J. 1959 Aug;72:586–597. doi: 10.1042/bj0720586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EATON N. R. New press for disruption of microorganisms. J Bacteriol. 1962 Jun;83:1359–1360. doi: 10.1128/jb.83.6.1359-1360.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EISENSTEIN R. B., YANOFSKY C. Tryptophan synthetase levels in Escherichia coli, Shigella dysenteriae, and transduction hybrids. J Bacteriol. 1962 Jan;83:193–204. doi: 10.1128/jb.83.1.193-204.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson F. Chorismic acid: purification and some chemical and physical studies. Biochem J. 1964 Feb;90(2):256–261. doi: 10.1042/bj0900256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hütter R., DeMoss J. A. Organization of the tryptophan pathway: a phylogenetic study of the fungi. J Bacteriol. 1967 Dec;94(6):1896–1907. doi: 10.1128/jb.94.6.1896-1907.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito J., Crawford I. P. Regulation of the enzymes of the tryptophan pathway in Escherichia coli. Genetics. 1965 Dec;52(6):1303–1316. doi: 10.1093/genetics/52.6.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito J., Yanofsky C. The nature of the anthranilic acid synthetase complex of Escherichia coli. J Biol Chem. 1966 Sep 10;241(17):4112–4114. [PubMed] [Google Scholar]
- JACOB F., MONOD J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 1961 Jun;3:318–356. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MATSUSHIRO A., SATO K., ITO J., KIDA S., IMAMOTO F. ON THE TRANSCRIPTION OF THE TRYTOPHAN OPERON IN ESCHERICHIA COLI. I. THE TRYPTOPHAN OPERATOR. J Mol Biol. 1965 Jan;11:54–63. doi: 10.1016/s0022-2836(65)80170-x. [DOI] [PubMed] [Google Scholar]
- SCHWARTZ A. K., BONNER D. M. TRYPTOPHAN SYNTHETASE IN BACILLUS SUBTILIS: EFFECTS OF HIGH POTASSIUM ION CONCENTRATION ON A TWO COMPONENT ENZYME. Biochim Biophys Acta. 1964 Aug 26;89:337–347. doi: 10.1016/0926-6569(64)90223-8. [DOI] [PubMed] [Google Scholar]
- SMITH O. H., YANOFSKY C. 1-(o-Carboxyphenylamino)-1-deoxyribulose 5-phosphate, a new intermediate in the biosynthesis of tryptophan. J Biol Chem. 1960 Jul;235:2051–2057. [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANOFSKY C., LENNOX E. S. Transduction and recombination study of linkage relationships among the genes controlling tryptophan synthesis in Escherichia coli. Virology. 1959 Aug;8:425–447. doi: 10.1016/0042-6822(59)90046-7. [DOI] [PubMed] [Google Scholar]
- YANOFSKY C. The enzymatic conversion of anthranilic acid to indole. J Biol Chem. 1956 Nov;223(1):171–184. [PubMed] [Google Scholar]
- YANOFSKY C. The tryptophan synthetase system. Bacteriol Rev. 1960 Jun;24(2):221–245. doi: 10.1128/br.24.2.221-245.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]


