Abstract
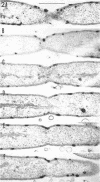
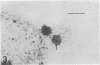
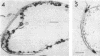
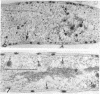
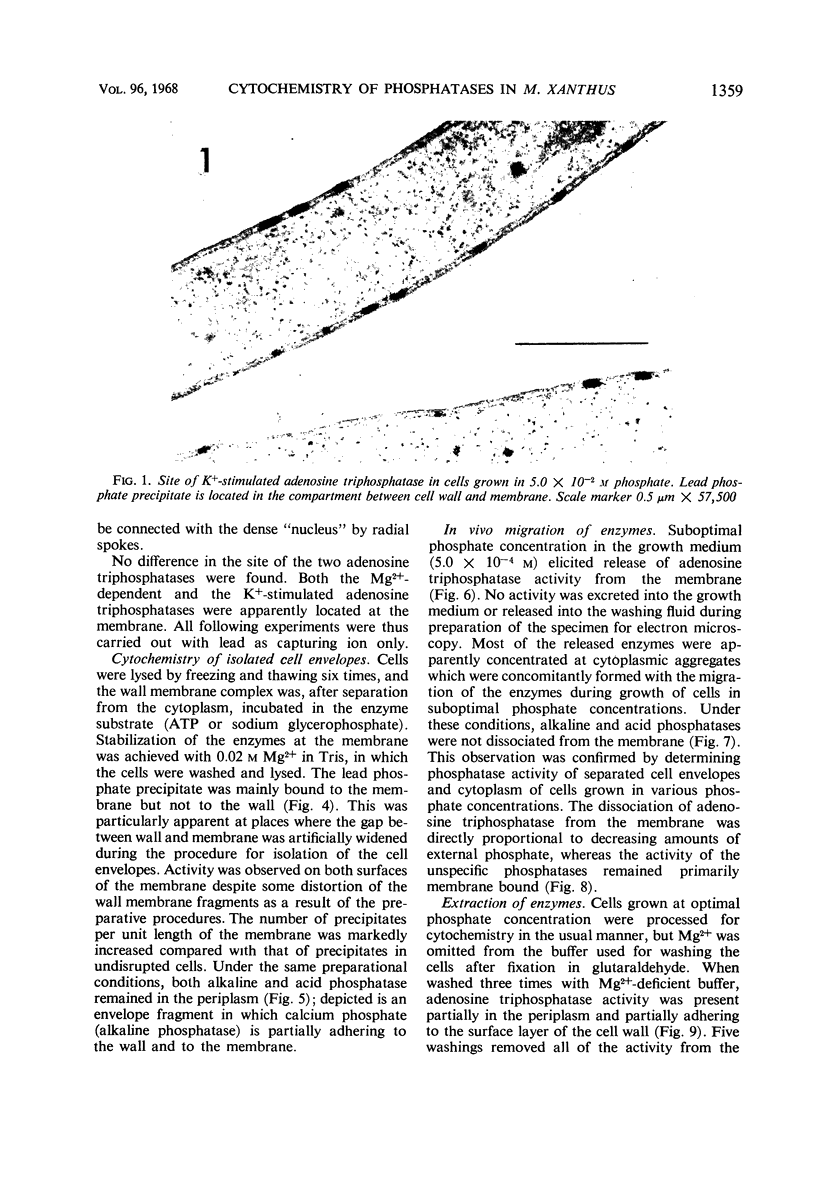
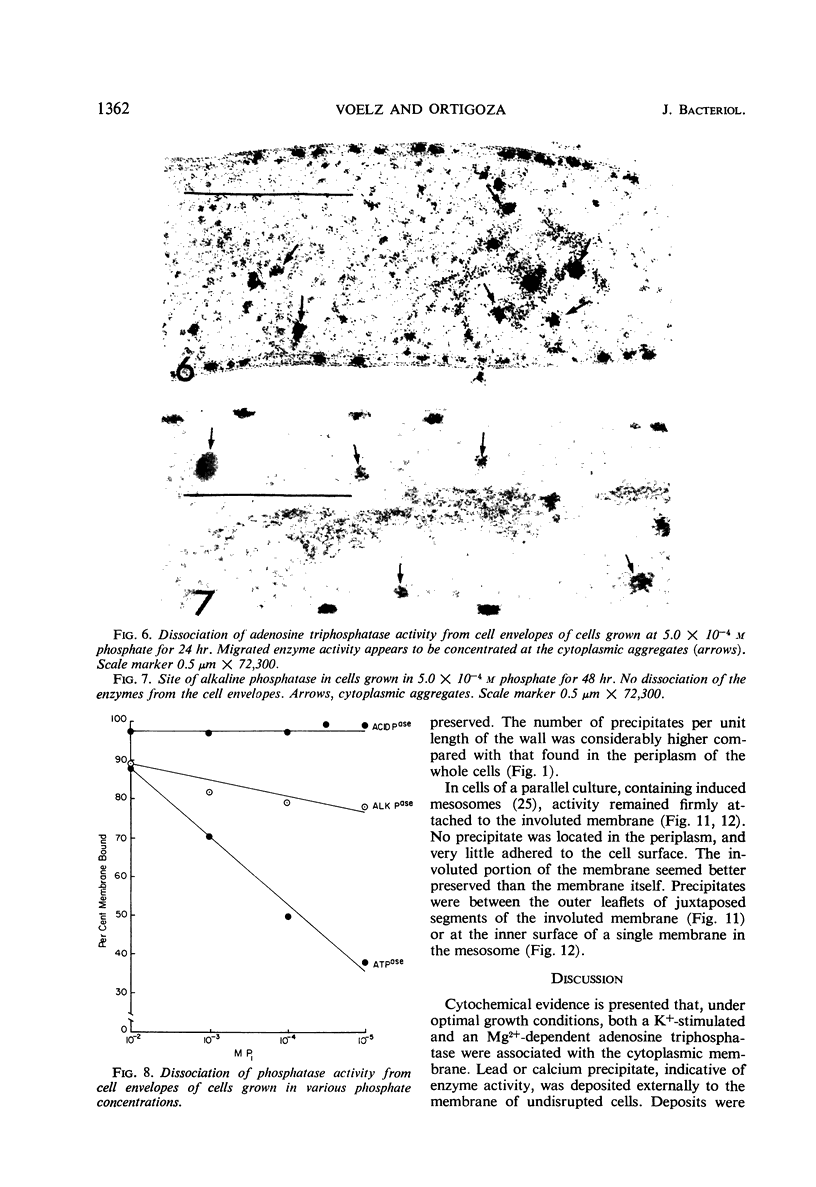
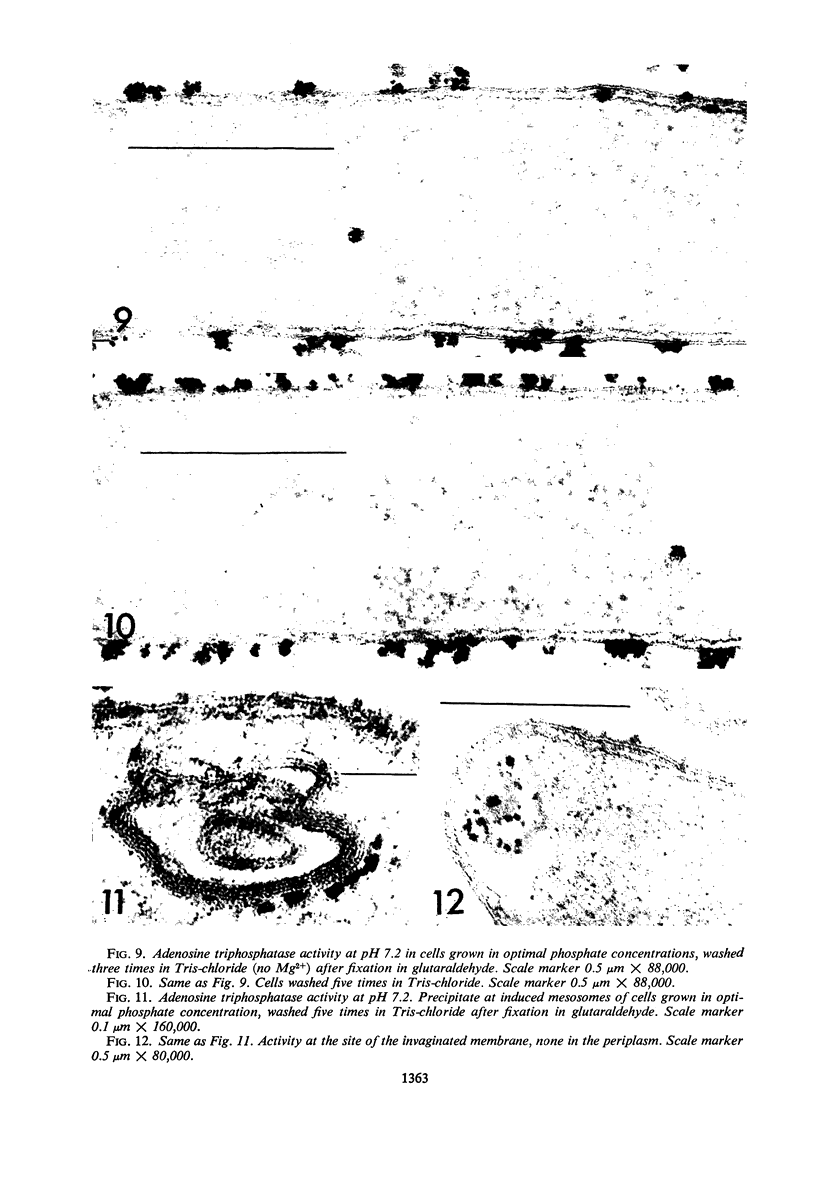
An Mg2+-dependent and a K+-stimulated adenosine triphosphatase were localized by cytochemistry at or near both surfaces of the cytoplasmic membrane of Myxococcus xanthus. An alkaline and an acid phosphatase resided at the external surface of the membrane or in the periplasm. All enzymes could be extracted from partially fixed cells with Mg2+-deficient buffers. Suboptimal external phosphate elicited dissociation of adenosine triphosphatase from the membrane but not that of the unspecific phosphatases. The dissociated enzymes migrated into the cytoplasm where they were associated mainly with cytoplasmic aggregates.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAMS A., McNAMARA P., JOHNSON F. B. Adenosine triphosphatase in isolated bacterial cell membranes. J Biol Chem. 1960 Dec;235:3649–3662. [PubMed] [Google Scholar]
- Abrams A., Baron C. Reversible attachment of adenosine triphosphatase to streptococcal membranes and the effect of magnesium ions. Biochemistry. 1968 Feb;7(2):501–507. doi: 10.1021/bi00842a003. [DOI] [PubMed] [Google Scholar]
- Abrams A. The release of bound adenosine triphosphatase from isolated bacterial membranes and the properties of the solubilized enzyme. J Biol Chem. 1965 Sep;240(9):3675–3681. [PubMed] [Google Scholar]
- Burnham J. C., Hageage G. J., Jr Adenosine phosphate hydrolases in cell fractions of Vitreoscilla. J Bacteriol. 1967 Jan;93(1):191–198. doi: 10.1128/jb.93.1.191-198.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole H. A., Hughes D. E. The enzymic activity of the outer shell of Lactobacillus arabinosus. J Gen Microbiol. 1965 Jul;40(1):81–95. doi: 10.1099/00221287-40-1-81. [DOI] [PubMed] [Google Scholar]
- DRAPEAU G. R., MACLEOD R. A. NUTRITION AND METABOLISM OF MARINE BACTERIA. XII. ION ACTIVATION OF ADENOSINE TRIPHOSPHATASE IN MEMBRANES OF MARINE BACTERIAL CELLS. J Bacteriol. 1963 Jun;85:1413–1419. doi: 10.1128/jb.85.6.1413-1419.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DWORKIN M. NUTRITIONAL REGU.ATION OF MORPHOGENESIS IN MYXOCOCCUS XANTHUS. J Bacteriol. 1963 Jul;86:67–72. doi: 10.1128/jb.86.1.67-72.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDFISCHER S., ESSNER E., NOVIKOFF A. B. THE LOCALIZATION OF PHOSPHATASE ACTIVITIES AT THE LEVEL OF ULTRASTRUCTURE. J Histochem Cytochem. 1964 Feb;12:72–95. doi: 10.1177/12.2.72. [DOI] [PubMed] [Google Scholar]
- HUGHES D. E. The bacterial cytoplasmic membrane. J Gen Microbiol. 1962 Sep;29:39–46. doi: 10.1099/00221287-29-1-39. [DOI] [PubMed] [Google Scholar]
- Heppel L. A. Selective release of enzymes from bacteria. Science. 1967 Jun 16;156(3781):1451–1455. doi: 10.1126/science.156.3781.1451. [DOI] [PubMed] [Google Scholar]
- Kushnarev V. M., Smirnova T. A. Electron microscopy of alkaline phosphatase of Escherichia coli. Can J Microbiol. 1966 Aug;12(4):605–607. doi: 10.1139/m66-086. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marchesi V. T., Palade G. E. The localization of Mg-Na-K-activated adenosine triphosphatase on red cell ghost membranes. J Cell Biol. 1967 Nov;35(2):385–404. doi: 10.1083/jcb.35.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima S., Ishida M., Miura T. Subfractionation of protoplast membrane and enzyme localization in Bacillus megaterium. J Biochem. 1966 Sep;60(3):256–261. doi: 10.1093/oxfordjournals.jbchem.a128431. [DOI] [PubMed] [Google Scholar]
- Munkres M., Wachtel A. Histochemical localization of phosphatases in Mycoplasma gallisepticum. J Bacteriol. 1967 Mar;93(3):1096–1103. doi: 10.1128/jb.93.3.1096-1103.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. On the surface localization of enzymes in E. coli. Biochem Biophys Res Commun. 1964 Oct 14;17(3):215–219. doi: 10.1016/0006-291x(64)90386-9. [DOI] [PubMed] [Google Scholar]
- Neujahr H. Y. Transport of B-vitamins in microorganisms. V. Comparative studies on the ATP-hydrolyzing activities of cell fractions obtained from thiamine sufficient and thiamine deficient cells of L. fermenti. Acta Chem Scand. 1966;20(6):1518–1528. doi: 10.3891/acta.chem.scand.20-1518. [DOI] [PubMed] [Google Scholar]
- Nossal N. G., Heppel L. A. The release of enzymes by osmotic shock from Escherichia coli in exponential phase. J Biol Chem. 1966 Jul 10;241(13):3055–3062. [PubMed] [Google Scholar]
- PADYKULA H. A., HERMAN E. The specificity of the histochemical method for adenosine triphosphatase. J Histochem Cytochem. 1955 May;3(3):170–195. doi: 10.1177/3.3.170. [DOI] [PubMed] [Google Scholar]
- RYTER A., KELLENBERGER E., BIRCHANDERSEN A., MAALOE O. Etude au microscope électronique de plasmas contenant de l'acide désoxyribonucliéique. I. Les nucléoides des bactéries en croissance active. Z Naturforsch B. 1958 Sep;13B(9):597–605. [PubMed] [Google Scholar]
- TORRIANI A. Influence of inorganic phosphate in the formation of phosphatases by Escherichia coli. Biochim Biophys Acta. 1960 Mar 11;38:460–469. doi: 10.1016/0006-3002(60)91281-6. [DOI] [PubMed] [Google Scholar]
- VOELZ H. FORMATION AND STRUCTURE OF MESOSOMES IN MYXOCOCCUS XANTHUS. Arch Mikrobiol. 1965 May 28;51:60–70. doi: 10.1007/BF00406850. [DOI] [PubMed] [Google Scholar]
- VOELZ H. SITES OF ADENOSINE TRIPHOSPHATASE ACTIVITY IN BACTERIA. J Bacteriol. 1964 Oct;88:1196–1198. doi: 10.1128/jb.88.4.1196-1198.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelz H., Voelz U., Ortigoza R. O. The "polyphosphate overplus" phenomenon in Myxococcus xanthus and its influence on the architecture of the cell. Arch Mikrobiol. 1966 May 9;53(4):371–388. doi: 10.1007/BF00409874. [DOI] [PubMed] [Google Scholar]
- WEIBULL C., GREENWALT J. W., LOW H. The hydrolysis of adenosine triphosphate by cell fractions of Bacillus megaterium. I. Localization and general characteristics of the enzymic activities. J Biol Chem. 1962 Mar;237:847–852. [PubMed] [Google Scholar]
- Yayashi M., Uchida R. A cation activated adenosinetriphosphatase in cell membranes of halophilic Vibrio parahaemolyticus. Biochim Biophys Acta. 1965 Oct 25;110(1):207–209. doi: 10.1016/s0926-6593(65)80113-8. [DOI] [PubMed] [Google Scholar]