Abstract
Anti–double-stranded DNA (dsDNA) antibodies are the serologic abnormality characteristically associated with systemic lupus erythematosus (SLE) and may play an important role in disease pathogenesis. Although the anti-dsDNA antibodies present in SLE are indicative of an antigen-driven response, the antigen has not been conclusively identified.
By screening a phage peptide display library, we demonstrated previously that the decapeptide DWEYSVWLSN is specifically bound by the pathogenic murine IgG2b anti-dsDNA antibody R4A. To investigate the possibility that a protein antigen might trigger lupus-like autoimmunity, we immunized BALB/c mice with DWEYSVWLSN in adjuvant. Mice developed significant titers of IgG anti-dsDNA antibodies 2–3 wk after the initial immunization. Immunized mice also developed antibodies against some other lupus autoantigens, and immunoglobulin deposition was present in renal glomeruli at 49 d. Although an immune response to peptide and dsDNA was evident in BALB/c mice, there was little response in other inbred strains.
This study demonstrates that lupus-like anti-dsDNA reactivity can be generated in nonautoimmune mice by immunization with a peptide antigen. Peptide-induced autoimmunity may prove useful in understanding the spreading of antigenic specificities targeted in SLE. However, most importantly, the demonstration that a peptide antigen can initiate a SLE-like immune response opens a new chapter on the potential antigenic stimuli that might trigger SLE.
Keywords: systemic lupus erythematosus, anti-DNA, peptide library, autoantibodies, inbred strains
Anti–double-stranded DNA (dsDNA)1 autoantibodies are a diagnostic feature of SLE. Many analyses of these antibodies have shown them to have the characteristics of antibodies arising in an antigen-driven response (1–3). Lupus-associated anti-dsDNA antibodies display high affinity for dsDNA, have undergone H chain class switching to IgG, and display somatic mutations in their variable region gene segments. Nevertheless, it remains uncertain what antigen triggers the production of these antibodies. Mammalian dsDNA is poorly immunogenic (4). Although some bacterial DNAs appear to be immunogenic in normal mouse strains, they do not elicit production of antibodies that cross-react with eukaryotic DNA (5, 6). Therefore, it remains unclear if DNA or nucleosomes induce anti-DNA antibodies in lupus-prone mice or if anti-dsDNA antibodies are made in response to some other antigen, either self or foreign. Specifically, anti-DNA antibodies cross-react with bacterial antigens (7, 8), and bacterial polysaccharide can trigger production of anti-DNA antibodies (9).
We have previously described R4A, a murine IgG2b anti-dsDNA mAb that deposits in glomeruli of nonautoimmune mouse strains (10). In an attempt to understand the fine specificity of the R4A antibody, we screened a phage decapeptide display library with R4A, and have reported that the peptide DWEYSVWLSN and the smaller peptide DWEYS that represents a consensus motif found in several peptides recognized by R4A, are bound by the antibody. DWEYS in either the L or the D form can inhibit the binding of R4A to dsDNA, and when administered in vivo to mice in the D-peptide configuration, can inhibit kidney deposition of R4A (11).
To test a possible role of protein antigens in the induction of anti-dsDNA antibodies, we immunized BALB/c mice and other nonautoimmune mouse strains with the R4A-specific peptide DWEYSVWLSN configured as a multimer on a multiple antigenic peptide (MAP) backbone (12, 13). Immunized BALB/c mice developed anti-dsDNA antibodies and some other autoantibodies, as well as glomerular immunoglobulin deposition suggestive of lupus nephritis.
Materials and Methods
Derivation of R4A and DWEYS.
The derivation of the anti-dsDNA antibody R4A (10) and the peptide DWEYSVWLSN have been described previously (11). In brief, R4A is a murine IgG2b antibody isolated from a nonautoimmune BALB/c mouse, encoded by an unmutated S107 V11 germline gene and an unmutated germline Vκ1 L chain gene. The R4A antibody binds dsDNA and deposits in glomeruli of SCID mice.
The DWEYSVWLSN peptide was identified by screening a phage decapeptide display library with R4A. After three rounds of selection, phage clones were amplified, and the oligonucleotide inserts were sequenced. The consensus peptide motif for the R4A-selected phage clones was D/E-W-D/E-Y-S/G. The soluble peptide DWEYS inhibited binding of R4A to dsDNA. The D configuration of the DWEYS peptide markedly inhibited R4A deposition in glomeruli of SCID mice in vivo (11).
Immunization.
4–6-wk-old female nonautoimmune BALB/cJ mice (The Jackson Laboratory, Bar Harbor, ME) were used for immunizations. DWEYSVWLSN was prepared for immunization on an eight-branched lysine backbone (MAP™; Applied Biosystems, Foster City, CA; peptide on MAP from Research Genetics, Huntsville, AL; references 12 and 13). Mice were immunized subcutaneously with 100 μg of MAP–peptide in CFA H37 Ra (Difco Laboratories, Detroit, MI) on day 0, followed by subcutaneous booster injections of 100 μg of MAP–peptide in IFA (Difco Laboratories) on days +7 and +14. Control mice matched for strain, sex, and age were immunized with the MAP backbone only (MAP core) in adjuvant using an identical schedule. Serum was obtained on days 0, +7, +14, +21, +35, and +49. Mice were housed in the Albert Einstein College of Medicine animal barrier facility.
Inbred Strains.
A/J, AKR/J, DBA/2, and C57BL/6 mice were obtained from The Jackson Laboratory. CBA/Ca mice were purchased from Harlan Sprague Dawley (Indianapolis, IN). 4–6-wk-old female mice were immunized in groups of five mice using the protocol detailed above for BALB/c mice.
Antipeptide Antibody ELISA.
Amidated, acetylated DWEYSVWLSN was synthesized at the peptide synthesis facility of the Albert Einstein College of Medicine. Peptide purity was ≥90% as assayed by HPLC. To assay for the presence of antipeptide antibodies, peptide at 20 μg/ml in PBS was adsorbed to Falcon Pro-Bind 96-well microtiter plates (Becton Dickinson, Lincoln Park, NJ) at 4°C overnight. Plates were blocked with 3% FCS (Hyclone Laboratories, Logan, UT) in PBS for 1 h at 37°C and then incubated with serum at a 1:500 dilution in PBS for 2 h at 37°C. The plates were then washed five times with PBS-Tween, and alkaline phosphatase–conjugated goat anti–mouse IgG or IgM (Southern Biotechnology Associates, Birmingham, AL) diluted 1:1,000 in 3% FCS/PBS was added for 1 h at 37°C, followed by p-nitrophenyl phosphate (alkaline phosphatase substrate) solution (Sigma Chemical Co., St. Louis, MO). OD was monitored at 405 nm.
Anti-dsDNA ELISA.
Salmon sperm dsDNA (Calbiochem Novabiochem, La Jolla, CA) was purified by filtration with a 0.45-μm filter (Millex HA; Millipore Corp., Bedford, MA). Salmon sperm dsDNA, 100 μl per well at 100 μg/ml was adsorbed to Immulon II 96-well microtiter plates (Dynatech Laboratories Inc., Chantilly, VA), dried overnight at 37°C, and blocked with 3% FCS in PBS for 1 h at 37°C. Serum at a 1:500 dilution in PBS was incubated for 2 h at 37°C, and the assay was performed as described above. Specificity for dsDNA was confirmed using murine anti–single-stranded DNA mAbs (gifts from Dr. G. Gilkeson, University of South Carolina, and Dr. J. Erikson, University of Pennsylvania) that showed no binding in this assay.
Isotype and IgG subclass analysis was performed as above, using the appropriate secondary alkaline phosphatase–linked antibodies obtained from Southern Biotechnology Associates. For analysis of S107 VH gene expression of the anti-dsDNA antibodies, serum at 1:100 dilution was incubated on dsDNA-coated plates, followed by supernatant from the TC54 cell line (rat anti– mouse S107 V1 and V11; reference 14) for 2 h at 37°C. Alkaline phosphatase–linked goat anti–rat IgG (H+L) antibody (Southern Biotechnology Associates) was added for 1 h at 37°C, and the assay was then developed with substrate and read as above.
Millipore Filter Assay for Anti-dsDNA Antibodies.
The Millipore filter assay for anti-dsDNA antibodies was performed as described (15). In brief, double-stranded Lambda DNA (Boehringer Mannheim Corp., Indianapolis, IN) was labeled with P32 using the Rediprime kit (Amersham Life Science, Little Chalfont, UK). Radioactive dsDNA was incubated with serum at a 1:5,000 dilution for 1 h at 37°C and filtered through a 0.45-μm filter (Millipore Corp.). The filters were washed with 1× SSC, and retained radioactivity was counted on a scintillation counter.
Isotype Quantitation.
Quantitation of IgG subclasses in the antipeptide and anti-dsDNA responses was performed using standard curves for subclass concentration. Antipeptide and anti-dsDNA ELISAs on serial dilutions of mouse serum starting from 1:100 were developed with alkaline phosphatase–linked antibodies to mouse IgG subclasses (IgG1, IgG2a, IgG2b, and IgG3) from Southern Biotechnology Associates. The Ig concentration was calculated directly from a standard curve generated concurrently using purified mouse myeloma proteins (MOPC 31C-IgG1,κ; UPC 10-IgG2a,κ; MOPC 141-IgG2b,κ; and FLOPC 21-IgG3,κ; Sigma Chemical Co.) and alkaline phosphatase–linked anti-IgG subclass antibodies as above.
Nuclear Antigen ELISA.
Purified histone and cardiolipin were obtained commercially. Histone (Boehringer Mannheim Corp.) was adsorbed to Immulon II (Dynatech Laboratories, Inc.) 96-well microtiter plates overnight at 4°C at a concentration of 10 μg/ml (in PBS). Cardiolipin (Fluka Chemical Corp., Ronkonkoma, NY) was adsorbed to Immulon II 96-well microtiter plates (Dynatech Laboratories Inc.) at room temperature overnight at a concentration of 75 μg/ml in ethanol. The assays were then performed as described above for measuring antipeptide antibodies.
Inhibition ELISAs.
Plates were coated with dsDNA or histone as described above. l-DWEYS at an initial concentration of 6.4 mM (5 mg/ml), was assessed for its ability to inhibit binding of sera from peptide-immunized BALB/c mice to nuclear antigens. Plates were blocked with 3% FCS in PBS for 1 h at 37°C, and serial 1:2 dilutions of inhibitor (50 μl) were added to the antigen-coated wells. Sera from day +49 at 1:250 (50 μl; final concentration 1:500) was added to the inhibitor solution and then incubated for 2 h at 37°C. The assay was then performed as described above.
Antinuclear Antibodies by Immunofluorescence.
Antinuclear antibodies (ANA) were assayed on Hep-2 cells according to the protocol supplied by the manufacturer (Immunoconcepts, Sacramento, CA). Serial dilutions of serum beginning at 1:40 were incubated on the slides for 30 min followed by a fluoresceinated goat anti–mouse IgG antibody (Southern Biotechnology Associates) for 30 min at room temperature. Slides were rinsed, washed, mounted with coverslips, and viewed with a fluorescence microscope (Carl Zeiss, Inc., Thornwood, NY). Results were reported as the last dilution at which fluorescence was clearly detectable.
Renal Deposition of Ig.
The technique for the assessment of kidney Ig deposition has been reported previously (16). In brief, one kidney from each animal was fixed in 10% formalin and embedded in paraffin. 4-μm-thick sections were cut, deparaffinized, rehydrated, blocked with 2% filtered horse serum in PBS in moist chambers, and stained for 1 h with biotinylated goat anti–mouse IgG (Vector Laboratories, Inc., Burlingame, CA) or each biotinylated goat anti–mouse IgG subclass (IgG1, IgG2a, IgG2b, and IgG3; Southern Biotechnology Associates) at a 1:800 dilution at room temperature. Sections were washed, incubated for 45 min with streptavidin-alkaline phosphatase, and developed with BCIP and NBT (GIBCO BRL, Gaithersburg, MD). Color development was stopped after 30 min by washing in distilled water. Coverslips were mounted on the stained sections with cytosol mounting solution (Aqua Polymount; Polysciences Inc., Warrington, PA). The sections were then sealed and viewed with a Zeiss microscope.
Results
DWEYSVWLSN Induces ANA.
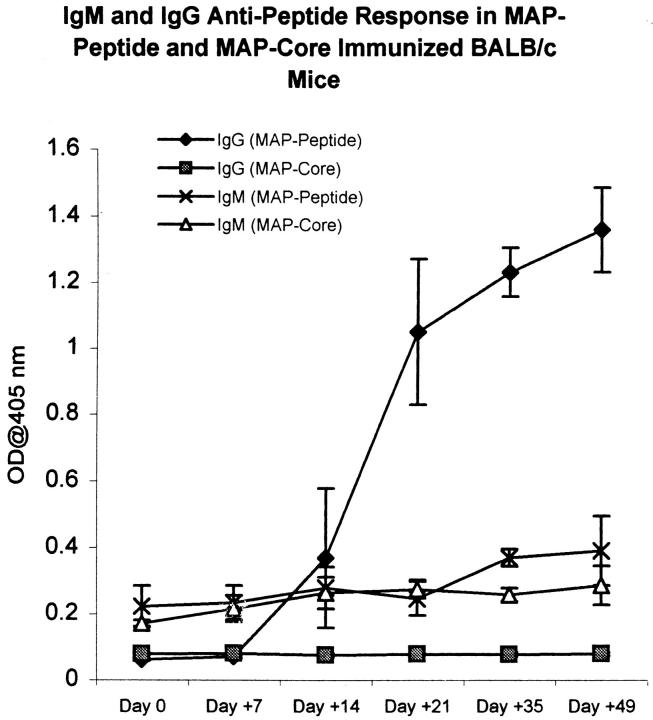
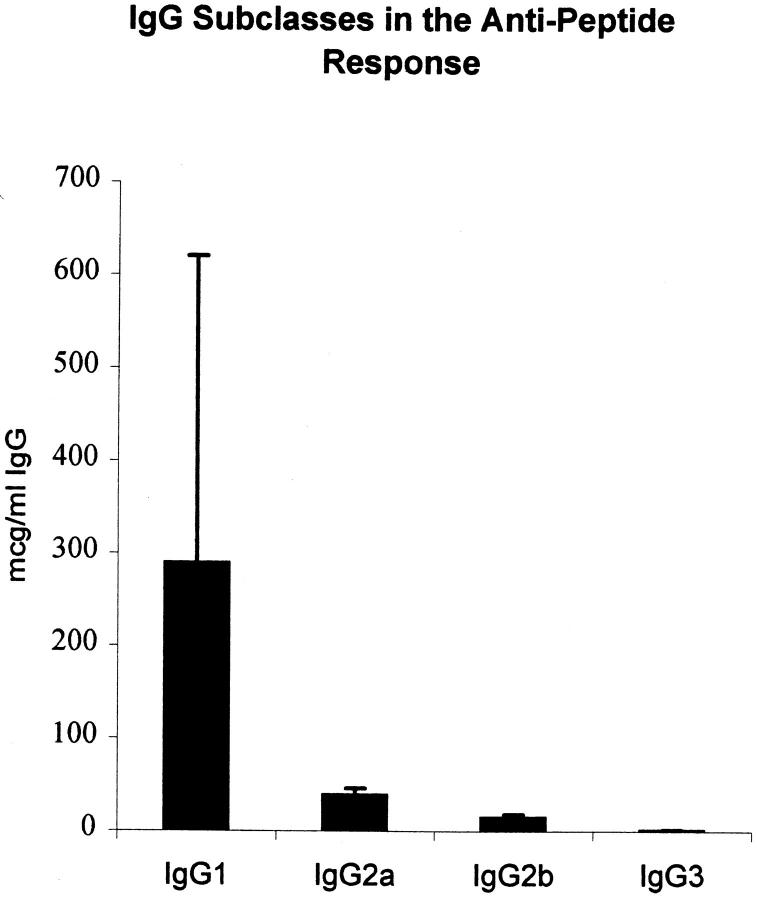
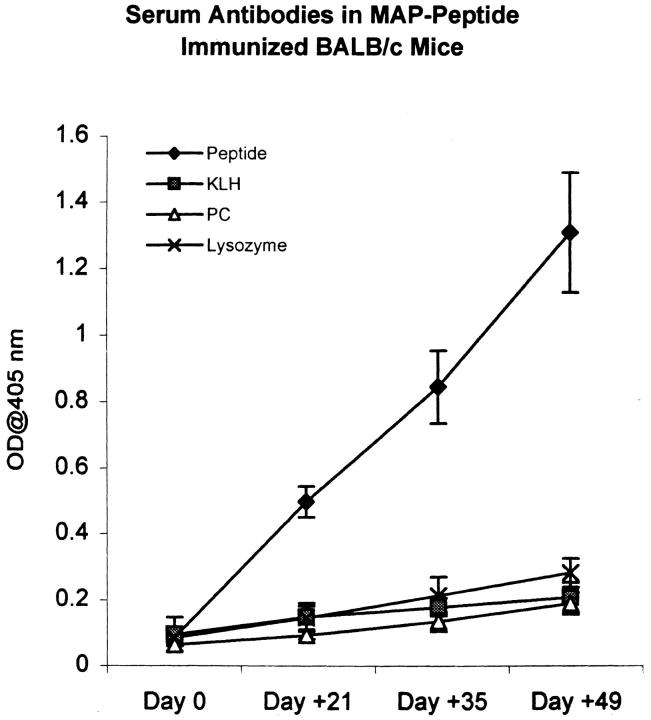
Immunization of BALB/c mice with the octameric peptide MAP–DWEYSVWLSN in CFA, followed by two booster injections of MAP–peptide in IFA, resulted in production of antipeptide antibodies. There was a small IgM antipeptide response in both peptide-immunized mice and in mice immunized with MAP core alone. The IgG antipeptide titer in MAP–peptide-immunized mice rose by day 14 and continued to increase until day 49 when the mice were killed (Fig. 1). The IgG1 subclass dominated the IgG antipeptide response. Although IgG2a, IgG2b, and IgG3 antipeptide antibodies were also detectable, these were present at lower levels (Fig. 2). The anti-DWEYSVWLSN response was specific: IgG antibody titers to phosphorylcholine, KLH, and lysozyme did not increase significantly (Fig. 3).
Figure 1.
Antipeptide antibodies in MAP–peptide-immunized mice. BALB/c mice were immunized with 100 μg of MAP–DWEYSVWLSN in CFA on day 0, and boosted with MAP–peptide in IFA on days 7 and 14. Control mice were immunized with MAP core in adjuvant. Sera from the different time points were diluted 1:500, and assayed for IgG and IgM antipeptide antibodies by ELISA. Data represent averages of five mice receiving MAP–peptide in adjuvant and five mice receiving MAP core in adjuvant.
Figure 2.
IgG antipeptide antibodies are primarily of the IgG1 subclass. The subclasses of the IgG antipeptide antibodies in day +49 sera from five MAP–DWEYSVWLSN-immunized mice were measured by ELISA.
Figure 3.
Antipeptide reactivity in MAP–peptide-immunized mice is antigen specific. Sera from MAP–DWEYSVWLSN-immunized mice was assayed for reactivity with DWEYSVWLSN and three additional irrelevant antigens (KLH, phosphorylcholine [PC], and lysozyme) by ELISA. A significant antibody response was demonstrated only against the immunizing peptide. Data represent averages of five immunized mice for each antigen.
Peptide Immunization Induces Anti-dsDNA Antibodies.
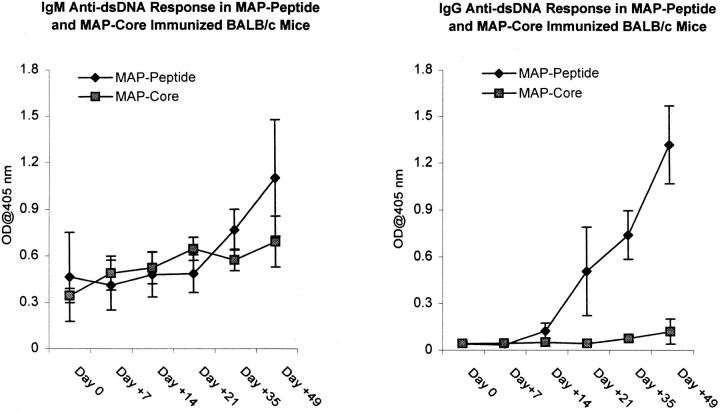
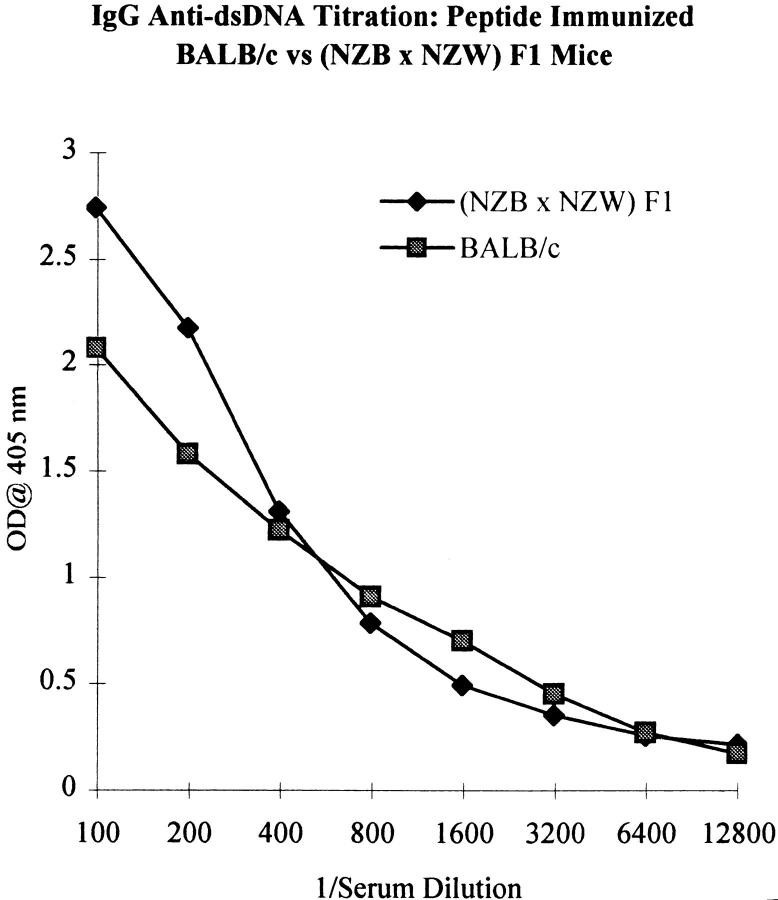
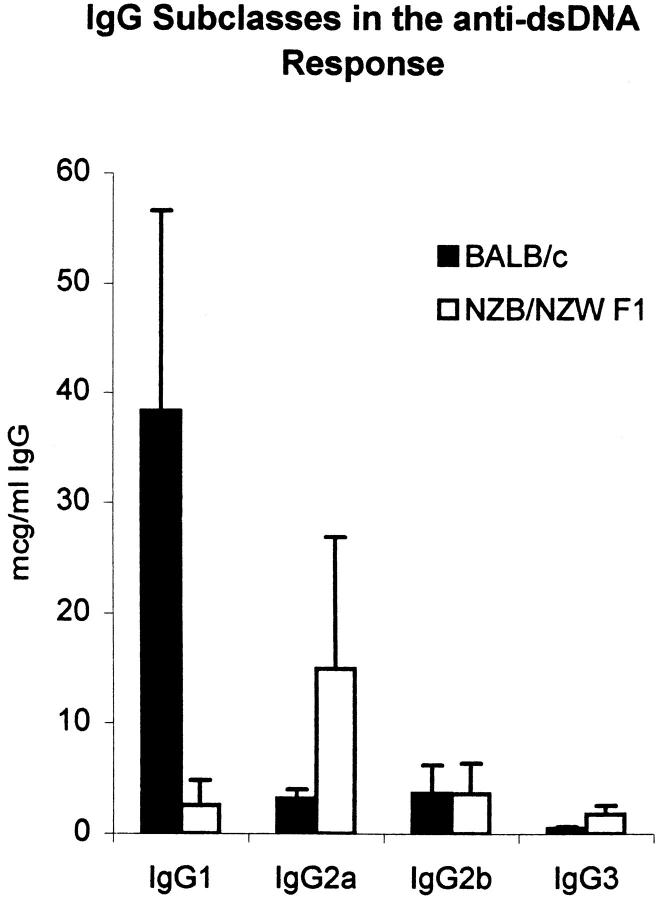
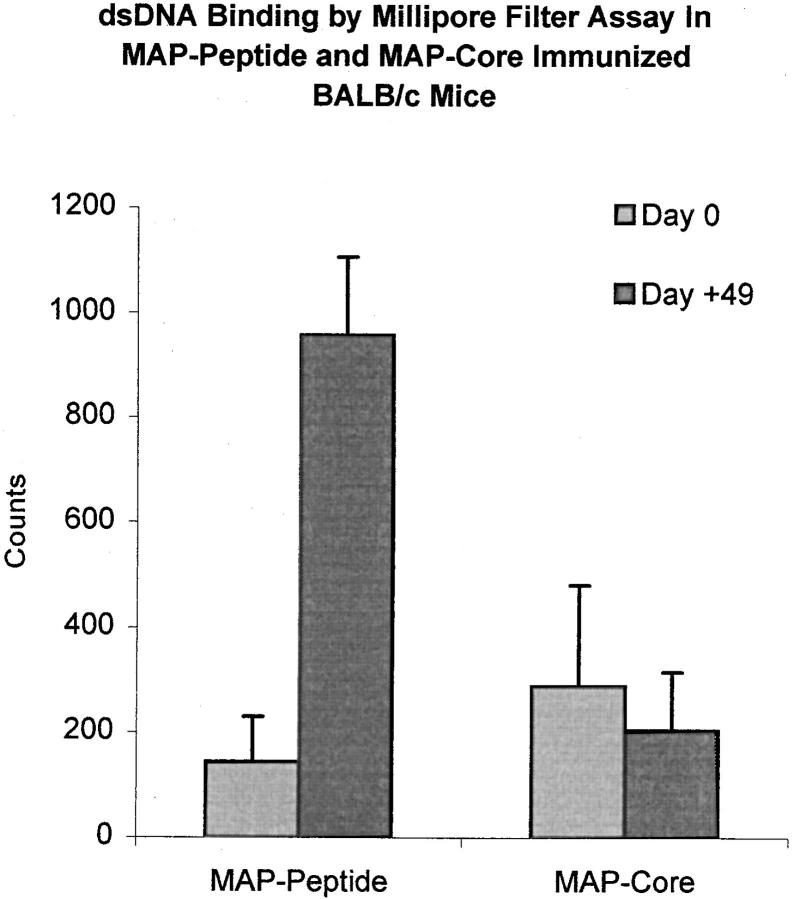
To determine whether the peptide was a true mimotope of dsDNA, we measured the anti-dsDNA antibody titer in peptide-immunized mice. Although only a small increase in the titer of IgM anti-dsDNA antibodies was found in peptide-immunized mice compared with mice immunized with MAP core (Fig. 4, left), a marked rise in the IgG anti-dsDNA antibody titer was observed (Fig. 4, right). Dilution studies demonstrated that the titer of IgG anti-dsDNA antibodies in peptide-immunized mice at 3 mo of age was only slightly lower than the titer in 6-mo-old nonimmunized (NZB × NZW) F1 mice (Fig. 5). Spontaneously arising anti-dsDNA antibodies from the lupus-prone (NZB × NZW) F1 mouse are mostly of the IgG2a and IgG2b isotype (reference 17, and Fig. 6). In peptide-immunized mice, the IgG1 isotype dominated the anti-dsDNA response (Fig. 6), although both IgG2a and IgG2b anti-dsDNA antibodies were also detectable. The presence of anti-dsDNA activity in MAP–peptide-immunized mice was verified by Millipore filter assay. Peptide-immunized mice developed significant dsDNA binding at day +49 compared with baseline levels, whereas no such increase was detected in MAP core immunized mice (Fig. 7).
Figure 4.
Anti-dsDNA antibodies in MAP–peptide-immunized mice. BALB/c mice were immunized with 100 μg of MAP–DWEYSVWLSN in CFA on day 0, and boosted with MAP–peptide in IFA on days 7 and 14. Control mice received MAP core in adjuvant. Sera from the different time points were diluted 1:500, and assayed for IgM (left) and IgG (right) anti-dsDNA antibodies by ELISA. Data represent averages of five mice receiving MAP–peptide and five mice receiving MAP core.
Figure 5.
The titer of IgG anti-dsDNA antibodies in MAP–peptide-immunized mice is comparable to the titer of IgG anti-dsDNA antibodies in (NZB × NZW) F1 mice. Serial dilutions of day +49 sera (starting from 1:100) from MAP–peptide-immunized mice were assayed by ELISA for IgG anti-dsDNA antibodies. Sera from lupus-prone 6-mo-old (NZB × NZW) F1 mice were used for comparison. Graph depicts the average OD at different dilutions of 10 MAP–peptide-immunized mice versus 9 (NZB × NZW) F1 mice.
Figure 6.
IgG anti-dsDNA antibodies in MAP–peptide-immunized mice are primarily IgG1. The subclasses of the IgG anti-dsDNA antibodies in day +49 sera from five MAP–peptide-immunized mice were measured by ELISA. Controls for this assay were five sera from 6-mo-old (NZB × NZW) F1 mice.
Figure 7.
Anti-dsDNA antibodies in the Millipore filter assay. BALB/c mice were immunized with 100 μg of MAP–DWEYSVWLSN in CFA on day 0, and boosted with MAP–peptide in IFA on day 7 and day 14. Control mice received MAP core in adjuvant. Sera from five mice in each group at baseline (day 0) and at day +49 were diluted 1:5,000 and incubated with radiolabeled dsDNA. The solution was filtered through a nitrocellulose membrane, and the retained radioactivity was counted.
As R4A is encoded by the S107 V11 H chain gene, we wanted to determine whether anti-dsDNA antibodies induced by immunization with a R4A-selected peptide would also be encoded by S107 VH genes. The mAb TC54 recognizes both S107 V1 and V11 H chains, without respect to L chain (14); therefore, we tested the anti-dsDNA antibodies for expression of the TC54 idiotype. TC54+ anti-dsDNA antibodies were present in peptide-immunized mice, suggesting that at least some of the anti-DNA antibodies elicited by peptide immunization express the same VH gene family as R4A (data not shown). Therefore, the peptide induces activation of B cells, making antibodies similar to the parental R4A antibody. However, not all the anti-DNA response is TC54 reactive, suggesting involvement of other VH genes, or somatic mutation affecting idiotypic specificity.
DWEYSVWLSN Immunization Induces Antibodies Reactive with Other Autoantigens.
As antibodies against additional autoantigens occur commonly in systemic lupus, we studied whether peptide-immunized BALB/c mice produced antibodies against other antigens. Using Hep-2 cells as a substrate, positive ANAs were detected in all DWEYSVWLSN-immunized mice tested. The serum ANA titers ranged from 1:80 (7/10 mice) to 1:160 (3/10 mice). Pre- and postimmune sera from mice receiving adjuvant alone, and preimmune sera from the peptide-immunized mice, were all negative for ANA by immunofluorescence at a dilution of 1:40 (data not shown).
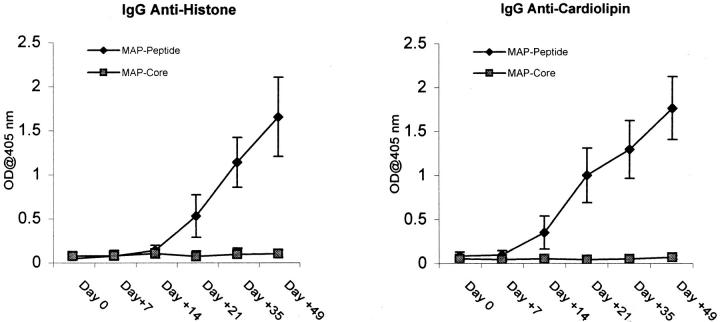
To further characterize the antigenic specificities present, assays were performed to detect reactivity to specific nuclear antigens. IgG anticardiolipin and antihistone titers increased in MAP–peptide-immunized mice (Fig. 8), whereas these antibodies were not present in mice immunized with the MAP core. To confirm that the binding to histone was specific and not due to contaminating DNA, we repeated the assay using DNase-treated antigen. There was no loss of reactivity (data not shown).
Figure 8.
MAP–peptide-immunized mice have increased serum titers of antihistone and anticardiolipin antibodies. Sera from different time points after immunization were diluted 1:500 and assayed for IgG antihistone and IgG anticardiolipin antibodies by ELISA.
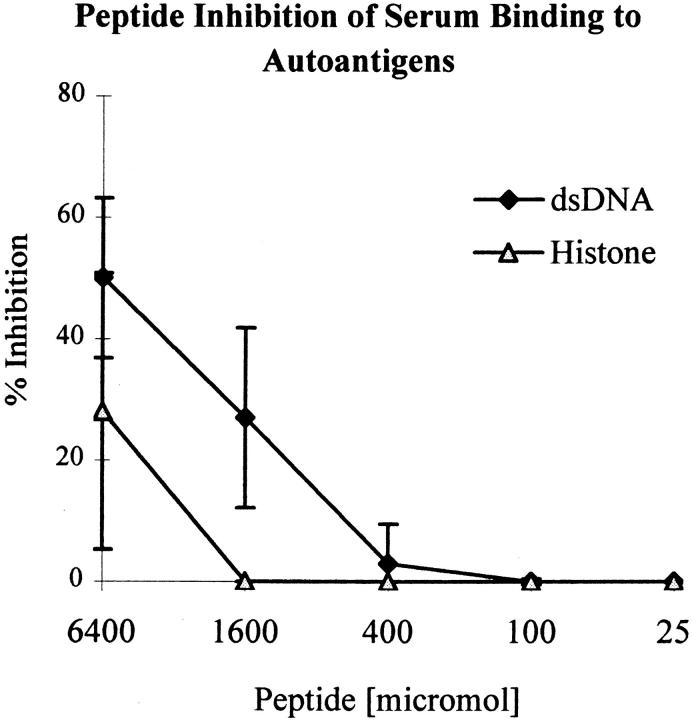
To determine if the multiple antigenic specificities observed in the DWEYSVWLSN-immunized mice represented antibodies cross-reactive with peptide, we performed inhibition experiments. Soluble DWEYS inhibited ∼50% of the binding of sera to dsDNA; binding to histone was less well inhibited by peptide (Fig. 9). As the MAP core and histone both have many lysine residues, we performed inhibition assays to see if the antihistone response cross-reacted with MAP core. MAP core alone failed to inhibit binding of sera to histone (data not shown).
Figure 9.
DWEYS inhibits binding of immunized mice sera to autoantigens. Day +49 sera diluted 1:250 from four peptide-immunized mice were incubated with serial dilutions of l-DWEYS at an initial concentration of 6.4 mM (5 mg/ml) and assayed for binding to dsDNA and histone. Graph depicts the average percentage of inhibition at different peptide concentrations. Peptide displays partial inhibition of binding to dsDNA; the effect on histone binding is less.
Peptide-immunized Mice Develop Immunoglobulin Deposition in the Kidney.
Anti-DNA antibodies have been shown to cause lupus-like nephritis in mice (18–20). To assess if peptide-induced autoantibodies were potentially pathogenic, we assayed for renal Ig deposition in five mice on day +49. Immunized mice all had moderate Ig deposition, mostly localized to glomeruli (Fig. 10). Repeating the kidney staining with goat anti–mouse IgG1 revealed a similar pattern and intensity of glomerular deposition, whereas the glomerular staining was greatly reduced or absent for IgG2a, IgG2b, and IgG3 (data not shown). No glomerular Ig deposition was present in mice immunized with MAP core alone (Fig. 10).
Figure 10.
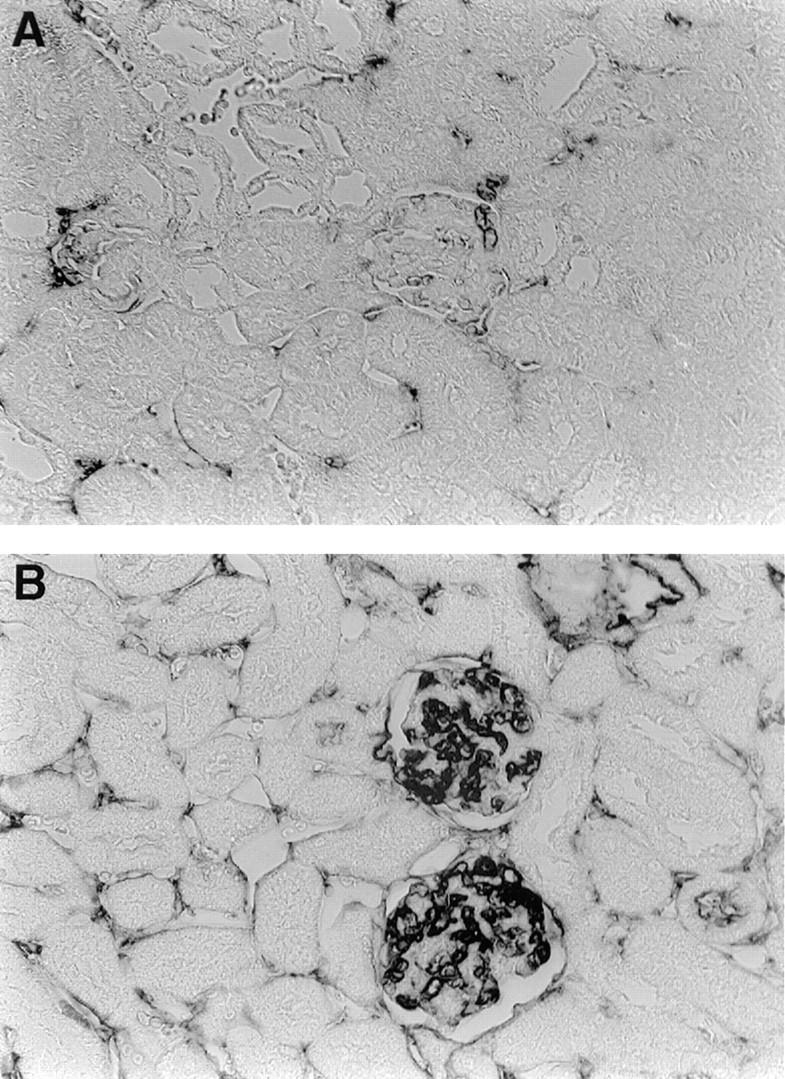
Glomerular Ig deposition is present in MAP–peptide-immunized BALB/c mice. Mice were killed on day +49 after the initial MAP– DWEYSVWLSN immunization. Kidney sections were stained with biotinylated goat anti–mouse IgG, followed by streptavidin–alkaline phosphatase and substrate. (A, top) Representative section from a mouse receiving MAP core. (B, bottom) Representative section from a MAP– peptide-immunized mouse.
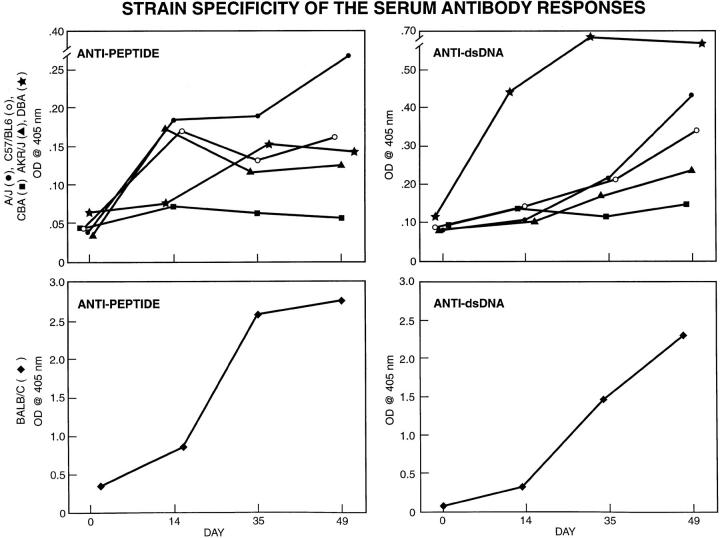
Response to Peptide Immunization in Different Inbred Mouse Strains.
To study if the breakdown in tolerance was strain specific, we immunized several additional inbred mouse strains [C57Bl/6 (H-2b), A/J (H-2a), AKR/J (H-2k), DBA/2 (H-2d), and CBA/Ca (H-2k)] with MAP–DWEYSVWLSN using the same protocol detailed above for BALB/c mice. No strain mounted either an antipeptide or an anti-DNA response comparable to that induced in BALB/c (H-2d) mice (Fig 11, bottom). Although there were some differences among the additional mouse strains examined, even the highest responder strain had a significantly lower antibody response to peptide and to dsDNA than BALB/c (Fig. 11, top). Thus, only BALB/c mice demonstrated a breakdown in tolerance when immunized with a peptide mimotope of dsDNA.
Figure 11.
(Left) Antipeptide response in nonautoimmune mouse strains. Groups of mice from the BALB/c (five mice), AKR/J (five mice), A/J (three mice), C57B1/6 (five mice), DBA/2 (five mice), and CBA (five mice) strains were immunized with MAP–peptide as described, and sera were diluted 1:500. The ELISA was developed with an anti-IgG antibody. Although some differences in the magnitude of the antipeptide response between these strains are evident, even the highest responder in this group has a markedly lower response than BALB/c mice. (Right) Anti-dsDNA autoantibody response in nonautoimmune mouse strains. Sera were diluted 1:500, and the ELISA was performed as described. The anti-DNA antibody response in BALB/c mice was higher than in any other strain.
Discussion
Although anti-dsDNA antibodies are the hallmark of the disease systemic lupus, it has been difficult to identify an antigen that will elicit this specificity on immunization. DNA immunization has routinely failed to elicit anti-dsDNA antibodies in nonautoimmune hosts (4). In an effort to find an antigen that can induce anti-DNA reactivity, we immunized BALB/c mice with a peptide surrogate for dsDNA. We have shown that the immunization of nonautoimmune BALB/c mice with a peptide that is bound by a pathogenic anti-dsDNA antibody elicits anti-dsDNA and other autoantibodies.
Peptide libraries have been very useful in identifying ligands for mAbs (21) when the antibody binds a protein antigen. Yet often the ligands are not mimotopes for antigen. Lundin et al. immunized mice with peptides derived by screening random peptide libraries with a neutralizing mAb against HIV gp120. Although immunization with peptide led to a peptide-specific response, no reactivity against native gp120 was detectable (22). Felici immunized BALB/c mice with peptides derived from screening a phage library with a neutralizing antibody against pertussis toxin. Sera from immunized mice bound to phage bearing the epitope and to MAP–peptide but did not recognize toxin or neutralize its biologic activity (23). However, Westerink et al. (24) were able to generate a protective response against meningococcal group C infection using a peptide mimic where the peptide was derived from the variable region of an antiidiotypic antibody. This peptide elicited protective immunity, but only after it was complexed to group B meningococcal outer membrane proteins. Valadon et al. screened phage peptide display libraries with 2H1, a protective antibody that binds capsular glucoronoxylomannan (GXM) of Cryptococcus neoformans to identify peptides that might be mimotopes for a polysaccharide antigen (25). One consensus peptide motif reacted with several protective anti-GXM antibodies, yet immunization with this peptide failed to elicit anti-GXM antibodies even though there was a strong antipeptide response.
Using a phage peptide display library, we were able to identify a peptide surrogate for dsDNA for the R4A antibody. Our present studies demonstrate that not only was this peptide a mimetic of the nucleic acid, as shown previously by competitive inhibition of DNA binding to an anti-dsDNA antibody (11), but also a mimotope that can elicit anti-dsDNA antibody production. Importantly, mice not only develop multiple serological autospecificities of SLE, but also demonstrate Ig deposition in the kidney. Thus, immunization with a peptide surrogate for dsDNA results in a lupus-like syndrome in a nonautoimmune mouse. We demonstrated an IgG response to dsDNA with the highest anti-dsDNA titers to be IgG1. This suggests a TH2-related mechanism for induction of autoantibodies. Further experiments are underway to confirm T cell involvement in the autoantibody response and to characterize the responding T cells.
Peptide-immunized BALB/c mice produce multiple autoantibodies. Inhibition studies with soluble peptide demonstrate that antibodies induced by peptide immunization are broadly cross-reactive, as dsDNA and histone reactivity can be partially inhibited by peptide. It has been reported that anti-DNA antibodies that bind chromatin may be inhibited by DNA or histone (26, 27) suggesting the existence of common antigenic epitopes on these autoantigens. However, not all the reactivity to these autospecificities is peptide inhibitable. There are several mechanisms that could account for the generation of autoreactive B cells that do not react with the immunizing peptide. Some B cells may initially be specific for peptide, and then acquire novel autospecificities or lose peptide specificity through somatic mutation. Previous studies from this laboratory (28, 29) have shown that anti-dsDNA B cells can be generated by somatic mutation of antiphosphorylcholine antibodies arising during an in vivo response. If this mechanism is operative in our model, by studying genealogies of mAb from peptide-immunized mice we would expect to find cross-reactive B cells (those reacting with both peptide and dsDNA) as well as sibling B cells in which mutation has led to the loss of reactivity with peptide while maintaining reactivity with autoantigen. A second mechanism to explain the generation of novel autoantibody specificities might be B cell antigen presentation and epitope spreading. Recently, James et al. (30) demonstrated that rabbits immunized with a Sm B/B′–derived octapeptide develop antibodies that not only bind this peptide, but also bind to many other Sm B/B′ peptides; subsequently, these rabbits develop antibodies that bind to other splicesosomal proteins (Sm D, RNP 70K, A, C). Similarly, rabbits immunized with short peptides from the 60-kD Ro particle develop an autoimmune response to the entire 60-kD Ro antigen (31). Four of the nine rabbits also developed anti-La reactivity, while two of the nine rabbits were found to have anti-dsDNA antibodies. Other studies have also demonstrated that immunization with peptides derived from known self-protein molecules (Ro, and La) can lead to intra- and intermolecular epitope spreading of the immune response to molecules that exist in vivo as multimolecular complexes (32). A third possible mechanism for generation of the multiple specificities involves the idiotypic network. Antiidiotypic antibodies to antibodies generated in the antipeptide immune response might induce production of antibodies that bear a shared idiotype but lack any specificity for the immunizing peptide. Some of these might bind self-antigens. Although this mechanism has been hypothesized in both human and murine lupus (33), evidence remains inconclusive.
One unanticipated result of this study was the finding that only BALB/c mice developed an antipeptide response and significant autoimmunity after peptide immunization. That little to no antipeptide response was elicited in five other mouse strains raises two possibilities. First, given the strains studied, we may be observing an Ir gene effect in the response to the immunizing peptide. It is possible that class II molecules of the d haplotype are necessary for peptide presentation (34). Class II genes are often important susceptibility factors for autoimmune disease (35, 36). Studies are underway in other strains, including congenic strains, to address this question. However, preliminary studies suggest that Ir genes do not control this response, as DBA/2 (H-2d) mice do not mount an antipeptide or an anti-DNA response. A similar finding was reported recently by James and Harley (37), showing a non–H-2–dependent, strain-specific response to an Sm-derived peptide in mice. Alternatively, it may be that peptide induces few antibodies that do not cross-react with DNA or another self-specificity. The BALB/c genetic background may predispose these mice to a breakdown of self-tolerance. In strains that maintain self-tolerance, no antipeptide response can be induced. The genetic factors that might cause BALB/c mice to be susceptible to breaking self-tolerance in this model are not known. However, it is known that thresholds for maintaining self-tolerance differ in different strains of mice, and are determined by the interplay of many genes. For example, homozygosity for the lpr defect on a C57Bl/6 genetic background results in a greatly attenuated phenotype, relative to the same gene defect on the MRL background (38). Similarly, the bc1-2 transgene expressed in the B cell lineage causes autoantibody production in some strains but not others (39). Further studies will allow us to dissect the full complement of genetic determinants responsible for the autoimmune response to the DWEYSVWLSN peptide.
Immunization of nonautoimmune mice with unmodified mammalian or bacterial dsDNA does not lead to the production of high affinity IgG anti-DNA antibodies that bind eukaryotic DNA. The elicited antibodies are specific for bacterial DNA and so are dissimilar to the anti-DNA antibodies found in lupus (5, 6). In recent years, it has been reported that DNA may become more immunogenic when complexed to various DNA-binding proteins. In these models, a foreign protein functions as a carrier and DNA as a hapten. For example, immunization of nonautoimmune BALB/c mice with dsDNA complexed with DNase I elicited IgG anti-dsDNA antibodies in about one third of immunized mice, whereas no such antibodies were generated by DNA immunization alone (40). Rekvig et al. (42) found that mice given polyoma BK virus developed anti-dsDNA and antihistone antibodies. This autoantibody response could be replicated by immunization with the intact polyoma BK virus large T antigen, a DNA-binding protein. In this model, a single induced mutation in T antigen that resulted in loss of its DNA-binding property almost completely abrogated the anti-DNA response to immunization (41, 42). This suggests that the anti-DNA response was dependent on the DNA-binding property of T antigen. Similarly, Marion et al. (43) have described induction of anti-dsDNA antibodies by complexes of DNA and a DNA-binding protein, the Fus 1 peptide derived from Trypanosoma cruzi. The conclusion from all of these studies is that DNA complexed to a DNA-binding protein has immunogenic potential in several nonautoimmune models, whereas eukaryotic DNA alone is relatively nonimmunogenic.
In all of these models, a DNA-binding protein that functions as a carrier protein is required to be recognized by T cells, and to help elicit the B cell response to dsDNA. These studies, as well as studies by Pisetsky and coworkers (5, 6, 44) addressing immunogenicity of bacterial DNA, focus on DNA as a crucial eliciting antigen for B cells in the autoimmune process in SLE. In the model reported here, peptide is a DNA surrogate, not a DNA-binding protein. Thus, in this model, anti-DNA antibodies are elicited directly by a protein antigen, demonstrating that nucleic acid is not essential for driving production of anti-DNA antibodies, and that a protein that mimics DNA as an antigen is sufficient to generate autoantibody production in nonautoimmune animals. We would like to suggest that the triggering antigen in SLE could be a protein antigen without DNA-binding properties, and that the search for triggering antigens in SLE must be significantly expanded.
Acknowledgments
These studies were supported by grants from the National Institutes of Health to B. Diamond and C. Putterman, and from the S.L.E. Foundation, Inc. to C. Putterman. C. Putterman is the recipient of the Robert Wood Johnson, Jr. Charitable Trust SLE Young Scholar Award from the New York Chapter of the Arthritis Foundation.
Abbreviations used in this paper
- ANA
antinuclear antibody
- dsDNA
double-stranded DNA
- GXM
glucoronoxylomannan
- MAP
multiple antigenic peptide
- RNP
ribonucleoprotein
References
- 1.Diamond B, Katz JB, Paul E, Aranow C, Lustgarten D, Scharff MD. The role of somatic mutation in the pathogenic anti-DNA response. Annu Rev Immunol. 1992;10:731–757. doi: 10.1146/annurev.iy.10.040192.003503. [DOI] [PubMed] [Google Scholar]
- 2.Marion TN, Tillman DM, Jou NT, Hill RJ. Selection of immunoglobulin variable regions in autoimmunity to DNA. Immunol Rev. 1992;128:123–149. doi: 10.1111/j.1600-065x.1992.tb00835.x. [DOI] [PubMed] [Google Scholar]
- 3.Radic MZ, Weigert M. Genetic and structural evidence for antigen selection of anti-DNA antibodies. Annu Rev Immunol. 1994;12:487–520. doi: 10.1146/annurev.iy.12.040194.002415. [DOI] [PubMed] [Google Scholar]
- 4.Madaio MP, Hodder S, Schwartz RS, Stollar BD. Responsiveness of autoimmune and normal mice to nucleic acid antigens. J Immunol. 1984;132:872–876. [PubMed] [Google Scholar]
- 5.Pisetsky D, Grudier JP, Gilkeson GS. A role for immunogenic DNA in the pathogenesis of systemic lupus erythematosus. Arthritis Rheum. 1990;33:153–159. doi: 10.1002/art.1780330202. [DOI] [PubMed] [Google Scholar]
- 6.Gilkeson GS, Pippen AM, Pisetsky DS. Induction of cross-reactive anti-dsDNA antibodies in preautoimmune NZB/NZW mice by immunization with bacterial DNA. J Clin Invest. 1995;95:1398–1402. doi: 10.1172/JCI117793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grayzel A, Solomon A, Aranow C, Diamond B. Antibodies elicited by pneumococcal antigens bear an anti-DNA–associated idiotype. J Clin Invest. 1991;87:842–846. doi: 10.1172/JCI115088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El-Roiey A, Sela O, Isenberg DA, Feldman R, Colaco BC, Kennedy RC, Shoenfeld Y. The sera of patients with Klebsiella infections contain an anti-DNA idiotype (16/6) Id and anti-polynucleotide activity. Clin Exp Immunol. 1987;67:507–515. [PMC free article] [PubMed] [Google Scholar]
- 9.Izui S, Zaldivar IM, Scher I, Lambert PH. Mechanisms of induction of anti-DNA antibodies by bacterial lipopolysaccharide in mice. I. Anti-DNA induction by LPS without significant release of DNA in circulating blood. J Immunol. 1977;119:2151–2156. [PubMed] [Google Scholar]
- 10.Shefner R, Kleiner G, Turken A, Papazian L, Diamond B. A novel class of anti-DNA antibodies identified in BALB/c mice. J Exp Med. 1991;173:287–296. doi: 10.1084/jem.173.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaynor B, Putterman C, Valadon P, Spatz L, Scharff MD, Diamond B. Peptide inhibition of glomerular deposition of an anti-DNA antibody. Proc Natl Acad Sci USA. 1997;94:1955–1960. doi: 10.1073/pnas.94.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tam JP. Synthetic peptide vaccine design: synthesis and properties of a high density, multiple antigenic peptide system. Proc Natl Acad Sci USA. 1988;85:5409–5413. doi: 10.1073/pnas.85.15.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tam JP. Recent advances in multiple antigenic peptides. J Immunol Methods. 1996;196:17–32. doi: 10.1016/0022-1759(96)00066-x. [DOI] [PubMed] [Google Scholar]
- 14.Desaymard CA, Giusti M, Scharff MD. Rat anti-T15 monoclonal antibodies with specificity for VH and VH-VL epitopes. Mol Immunol. 1984;21:961–967. doi: 10.1016/0161-5890(84)90154-8. [DOI] [PubMed] [Google Scholar]
- 15.Ginsberg B, Keiser H. A Millipore filter assay for antibodies to native DNA in sera of patients with systemic lupus erythematosus. Arthritis Rheum. 1973;16:199–207. doi: 10.1002/art.1780160210. [DOI] [PubMed] [Google Scholar]
- 16.Putterman C, Limpanasithikul W, Edelman M, Diamond B. The double edged sword of the immune response: mutational analysis of a murine anti-pneumococcal, anti-DNA antibody. J Clin Invest. 1996;97:2251–2259. doi: 10.1172/JCI118666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steward MW, Hay FC. Changes in immunoglobulin class and subclass of anti-DNA antibodies with increasing age in NZB/NZW F1 hybrid mice. Clin Exp Immunol. 1976;26:363–370. [PMC free article] [PubMed] [Google Scholar]
- 18.Ohnishi K, Ebling FM, Mitchell B, Singh RR, Hahn BH, Tasao BP. Comparison of pathogenic and non-pathogenic murine antibodies to DNA: antigen binding and structural characteristics. Int Immunol. 1994;6:817–830. doi: 10.1093/intimm/6.6.817. [DOI] [PubMed] [Google Scholar]
- 19.Foster MH, Cizman B, Madaio MP. Nephritogenic autoantibodies in systemic lupus erythematosus: immunochemical properties, mechanisms of immune deposition, and genetic origins. Lab Invest. 1993;69:494–507. [PubMed] [Google Scholar]
- 20.Raz E, Brezis M, Rosenmann E, Eilat D. Anti-DNA antibodies bind directly to renal antigens and induce kidney dysfunction in the isolated perfused rat kidney. J Immunol. 1989;142:3076–3082. [PubMed] [Google Scholar]
- 21.Scott JK. Discovering peptide ligands using epitope libraries. Trends Biochem Sci. 1992;17:241–245. doi: 10.1016/0968-0004(92)90401-t. [DOI] [PubMed] [Google Scholar]
- 22.Lundin K, Samuelsson A, Jansson M, Hinkula J, Wharen B, Wigzell H, Persson MA. Peptides isolated from random peptide libraries on phage elicit a neutralizing anti-HIV-1 response: analysis of immunological mimicry. Immunology. 1996;89:579–586. doi: 10.1046/j.1365-2567.1996.d01-772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Felici F, Luzzago A, Folgori A, Cortese R. Mimicking of discontinuous epitopes by phage-displayed peptides. II. Selection of clones recognized by monoclonal antibody against the Bordetella pertussistoxin from phage peptide libraries. Gene. 1993;128:21–27. doi: 10.1016/0378-1119(93)90148-v. [DOI] [PubMed] [Google Scholar]
- 24.Westerink MA, Giardina PC, Apicella MA, Kieber-Emmons T. Peptide mimicry of the meningococcal group C capsular polysaccharide. Proc Natl Acad Sci USA. 1995;92:4021–4025. doi: 10.1073/pnas.92.9.4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valadon P, Nussbaum G, Boyd LF, Margulies DH, Scharff MD. Peptide libraries define the fine specificity of anti-polysaccharide antibodies to Cryptococcus neoformans. . J Mol Biol. 1996;261:11–22. doi: 10.1006/jmbi.1996.0438. [DOI] [PubMed] [Google Scholar]
- 26.Pisetsky DS, Hoch SO, Klatt SL, O'Donnell MA, Keene JD. Specificity and idiotypic analysis of a monoclonal anti-Sm antibody with anti-DNA activity. J Immunol. 1985;135:4080–4085. [PubMed] [Google Scholar]
- 27.Losman MJ, Fasy TM, Novick KE, Monestier M. Relationship among antinuclear antibodies from autoimmune MRL mice reacting with histone H2A-H2B dimers and DNA. Int Immunol. 1993;5:513–523. doi: 10.1093/intimm/5.5.513. [DOI] [PubMed] [Google Scholar]
- 28.Ray SK, Putterman C, Diamond B. Pathogenic antibodies are routinely generated during the response to foreign antigen: a paradigm for autoimmunity. Proc Natl Acad Sci USA. 1996;93:2019–2024. doi: 10.1073/pnas.93.5.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Limpanasithikul W, Ray S, Diamond B. Cross reactive antibodies have both protective and pathogenic potential. J Immunol. 1995;155:967–973. [PubMed] [Google Scholar]
- 30.James JA, Gross T, Scofield RH, Harley JB. Immunoglobulin epitope spreading and autoimmune disease after peptide immunization: Sm B/B′–derived PPPGMRPP and PPPGIRGP induce spliceosome autoimmunity. J Exp Med. 1995;181:453–461. doi: 10.1084/jem.181.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scofield RH, Henry WE, Kurien BT, James JA, Harley JB. Immunization with short peptides from the sequence of the systemic lupus erythematosus-associated 60-kD Ro autoantigen results in anti-Ro ribonucleoprotein autoimmunity. J Immunol. 1996;156:4059–4066. [PubMed] [Google Scholar]
- 32.Topfer F, Gordon T, McCluskey J. Intra- and intermolecular spreading of autoimmunity involving the nuclear self-antigens La (SS-B) and Ro (SS-A) Proc Natl Acad Sci USA. 1995;92:875–879. doi: 10.1073/pnas.92.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mendelovic S, Brocke S, Shoenfeld Y, Ben-Bassat M, Meshorer A, Bakimer R, Mozes E. Induction of a systemic lupus erythematosus-like disease in mice by a common human anti-DNA idiotype. Proc Natl Acad Sci USA. 1988;85:2260–2264. doi: 10.1073/pnas.85.7.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McDevitt HO, Tyan ML. Genetic control of the antibody response in inbred mice: transfer of the response by spleen cells and linkage to the major histocompatibility (H-2) locus. J Exp Med. 1968;128:1–11. [PMC free article] [PubMed] [Google Scholar]
- 35.Arnett FC, Goldstein R, Duvic M, Reveille JD. Major histocompatibility complex genes in systemic lupus erythematosus, Sjogren's syndrome, and polymyositis. Am J Med. 1988;85:38–41. doi: 10.1016/0002-9343(88)90381-6. [DOI] [PubMed] [Google Scholar]
- 36.Fronek Z, Timmerman LA, Alper CA, Hahn BH, Kalunian K, Peterlin BM, McDevitt HO. Major histocompatibility complex genes and susceptibility to systemic lupus erythematosus. Arthritis Rheum. 1990;33:1542–1553. doi: 10.1002/art.1780331012. [DOI] [PubMed] [Google Scholar]
- 37.James JA, Harley JB. A model of peptide-induced lupus autoimmune B cell epitope spreading is strain specific and is not H-2 restricted in mice. J Immunol. 1998;160:502–508. [PubMed] [Google Scholar]
- 38.Izui S, Kelley VE, Masuda K, Yoshida H, Roths JB, Murphy ED. Induction of various autoantibodies by mutant gene lpr in several strains of mice. J Immunol. 1984;133:227–233. [PubMed] [Google Scholar]
- 39.Strasser A, Harris AW, Cory S. The role of bc1-2 in lymphoid differentiation and neoplastic transformation. Curr Top Microbiol Immunol. 1992;182:299–302. doi: 10.1007/978-3-642-77633-5_37. [DOI] [PubMed] [Google Scholar]
- 40.Marchini B, Puccetti A, Dolcher MP, Madaio MP. Induction of anti-DNA antibodies in non-autoimmune mice by immunization with a DNA-DNAse I complex. Clin Exp Rheumatol. 1995;13:7–10. [PubMed] [Google Scholar]
- 41.Rekvig OP, Moens U, Sundsfjord A, Bredholt G, Osei A, Haaeim H, Traavik-Arensen E, Haga HJ. Experimental expression in mice and spontaneous expression in human SLE of polyomavirus T-antigen. A molecular basis for induction of antibodies to DNA and eukaryotic transcription factors. J Clin Invest. 1997;99:2045–2054. doi: 10.1172/JCI119373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rekvig OP, Moens U, Fredrikson K, Traavik T. Human polyomavirus and immunogenicty of mammalian DNA: a conceptual framework. Methods. 1997;11:44–54. doi: 10.1006/meth.1996.0386. [DOI] [PubMed] [Google Scholar]
- 43.Desai DD, Krishnan MR, Swindle JT, Marion TN. Antigen-specific induction of antibodies against native mammalian DNA in nonautoimmune mice. J Immunol. 1993;151:1614–1626. [PubMed] [Google Scholar]
- 44.Pisetsky DS. DNA and the immune system. Ann Intern Med. 1997;126:169–171. doi: 10.7326/0003-4819-126-2-199701150-00015. [DOI] [PubMed] [Google Scholar]