Abstract
Inducible serum proteins whose concentrations oscillate between nontolerogenic and tolerogenic levels pose a particular challenge to the maintenance of self-tolerance. Temporal restrictions of intrathymic antigen supply should prevent continuous central tolerization of T cells, in analogy to the spatial limitation imposed by tissue-restricted antigen expression. Major acute-phase proteins such as human C-reactive protein (hCRP) are typical examples for such inducible self-antigens. The circulating concentration of hCRP, which is secreted by hepatocytes, is induced up to 1,000-fold during an acute-phase reaction. We have analyzed tolerance to hCRP expressed in transgenic mice under its autologous regulatory regions. Physiological regulation of basal levels (<10−9 M) and inducibility (>500-fold) are preserved in female transgenics, whereas male transgenics constitutively display induced levels. Surprisingly, crossing of hCRP transgenic mice to two lines of T cell receptor transgenic mice (specific for either a dominant or a subdominant epitope) showed that tolerance is mediated by intrathymic deletion of immature thymocytes, irrespective of widely differing serum levels. In the absence of induction, hCRP expressed by thymic medullary epithelial cells rather than liver-derived hCRP is necessary and sufficient to induce tolerance. Importantly, medullary epithelial cells also express two homologous mouse acute-phase proteins. These results support a physiological role of “ectopic” thymic expression in tolerance induction to acute-phase proteins and possibly other inducible self-antigens and have implications for delineating the relative contributions of central versus peripheral tolerance.
Keywords: self-tolerance, inducible self-antigens, acute-phase proteins, thymic medullary epithelium, deletion
Tolerance to self is a fundamental property of the immune system. Several mechanisms, including physical elimination (clonal deletion), receptor downregulation, and functional inactivation (clonal anergy), acting both on immature and mature T lymphocytes, have been shown to contribute to tolerance induction in T cells. The individual contribution of each single mechanism to the complex phenomenon of T cell tolerance is difficult to assess. Negative selection of developing T cells apparently constitutes the major mechanism of T cell tolerance. It is estimated that about one half of all positively selectable T cells are removed from the repertoire due to negative selection (1). This process requires constant presentation of a given self-antigen on MHC molecules of thymic APCs, and thus can operate only for those antigens that either are expressed intrathymically (2) or are transported from the tissue of origin into the thymus (3, 4). In addition to central tolerance, peripheral tolerance has been implicated in the case of “tissue-restricted” self-antigens that are neither expressed nor cross-presented in the thymus. A number of model systems have been designed, in most cases with mice expressing an MHC class I–restricted T cell receptor transgene and a tissue-restricted neo–self-antigen, in which distinct mechanisms of peripheral tolerance induction have been described (5–8).
At face value, the prerequisites for tolerance induction to MHC class II–restricted soluble proteins should be less complex. Expression of MHC class II molecules is restricted to professional APCs and thymic epithelium, and as a rule presentation of epitopes on MHC class II requires internalization of a given antigen from the extracellular space. For CD4 T cells, tolerance should be determined primarily by the concentration of the circulating protein rather than the cell type of origin. Constitutively secreted self-antigens equilibrate between the vascular and interstitial compartments of lymphoid organs and will be presented by peripheral, as well as thymic, MHC class II–positive APCs (9). Epitopes that are presented above a critical density within the thymus will thus purge the developing T cell repertoire of high avidity self-reactive T cells, whereas epitopes presented below a certain threshold will be ignored both in the thymus and periphery, and thus T cells expressing such TCRs should not pose a danger to self-tolerance (4, 8). An additional safeguard in this delicate balance is provided by the higher sensitivity of central tolerance induction in immature thymocytes versus activation of mature T cells in the periphery (10).
The above considerations apply to MHC class II–restricted epitopes of proteins that are produced constitutively at constant levels either in the thymus or the periphery. However, in the case of inducibly secreted proteins with serum levels that can vary over a wide range, blood-borne antigen may not be sufficient to ensure tolerance imposition on the developing T cell repertoire. Under such circumstances, temporal limitations of intrathymic antigen supply should prevent continuous central tolerization of CD4 T cells, in analogy to the spatial limitations imposed by tissue-restricted antigen expression that prevent central tolerization of specific CD8 T cells. Acute-phase proteins (APPs)1 are a prototypic example of such inducible self-antigens. APPs are a class of liver-derived serum proteins with circulating concentrations that rapidly oscillate between trace amounts in healthy individuals and up to 1,000-fold increased levels in the course of induction under pathological conditions (11).
Several particular features would predict strong immunogenicity of APPs upon induction: (a) the presence of very high serum levels (up to 2 mg/ml); (b) as part of the innate immune response, APPs accumulate at the site of infection and antigen processing; and (c) the uptake of APPs by professional APCs may be facilitated by specific surface receptors (12, 13). How is tolerance maintained under such conditions? To address this issue, we have studied transgenic mice expressing human C-reactive protein (hCRP), the major human APP, under control of all known autologous regulatory cis-acting elements (14, 15). The physiological regulation of hCRP is preserved in female transgenic mice, with basal levels of <10−9 M, which upon experimental elicitation of an acute phase rise up to 500-fold within a few hours (16). Due to hormonal influences, basal levels in male hCRP transgenic mice are elevated (5 × 10−7 M, which incidentally equals the concentration in induced females) and can be induced 20-fold (17). To date, few data exist on the concentration range required for induction of intrathymic deletion of CD4 T cells specific for blood-borne antigens. CD4 T cells specific for the constitutive serum component C5 are deleted at a physiological concentration of 10−7 M (4). Clonal deletion of thymocytes specific for an immunoglobulin idiotype required experimentally induced serum levels of >10−6 M (18). In another model, Ig allotype–specific CD4 T cells were not deleted at serum concentrations of ∼10−8 M (19). Extrapolating from these observations, basal serum levels of hCRP in female mice might thus lie below the threshold requirements for central tolerance (notwithstanding parameters such as efficiency of antigen uptake, processing, and presentation, and TCR affinity). In contrast, hCRP levels observed in female mice after induction may surpass this tolerance threshold and may even be sufficient to activate peripheral T cells. We previously reported the intriguing observation that female as well as male transgenic mice, despite widely differing basal serum concentrations, are equally tolerant to a dominant T cell epitope of hCRP, but reactive to a subdominant epitope (16). In this study we have addressed the mechanism of CD4 T cell tolerance at the clonal level by crossing hCRP transgenic animals to two strains of mice expressing transgenic TCRs specific for either the dominant or the subdominant epitope of hCRP. T cells of both specificities are deleted early in the thymus of double transgenic animals. We show that ectopic expression of hCRP by medullary epithelial cells ensures central tolerance irrespective of fluctuating serum levels.
Materials and Methods
Animals.
C57BL/6 and transgenic mice were kept under specific pathogen–free conditions in the animal facilities of the German Cancer Research Center (Heidelberg, Germany). TCR and hCRP transgenic mice were bred as heterozygotes.
Generation of TCR Transgenic Mice.
To generate TCR transgenic mice, rearranged V(D)J regions of TCRs from the hCRP-specific T cell clones T1CRP6 and T3CRP2 were cloned into the cassette vectors pTαcass and pTβcass (20; provided by D. Mathis and C. Benoist, I.G.B.M.C., Strasbourg, France). Both CD4 T cell clones were derived from a C57BL/6 mouse immunized with hCRP. The TCR from clone T1CRP6 (dominant epitope [Dep] TCR) recognizes the immunodominant epitope (amino acid 89–101) of hCRP and the TCR from clone T3CRP2 (subdominant [Sep] TCR) recognizes the subdominant epitope (amino acid 80–94) of hCRP, both in the context of MHC class II I-Ab (21).
The variable region of the Dep TCR contains rearranged Vα11.2/Jα26 and Vβ5.1/D/Jβ1.6 elements. The rearranged regions were amplified by PCR from genomic DNA of the T cell clone T1CRP6 using the oligonucleotides 5′-GAG GAT CCC GGG GAT TGG ACA GGG GCC-3′ (sense) and 5′-CAG GCG GCC GCA TTG TTC AAA ATA C-3′ (antisense) for the α chain, and 5′-ATC GAC TCG AGA GGA AGC ATG TCT AAC-3′ (sense) and 5′-CCA AGA CCG CGG TCA TCC AAC ACA G-3′ (antisense) for the β chain. Both PCR fragments were digested with appropriate restriction enzymes (α chain: PspAI/ NotI; β chain: XhoI/SstII) and cloned into pBluescript SK+ (Stratagene, Heidelberg, Germany). After verification of the correct sequence, the fragments were subcloned into the cassette vectors pTαcass and pTβcass resulting in constructs termed pTαT1CRP6 and pTβT1CRP6.
The variable regions of the Sep TCR are encoded by Vα4/ Jα17 and Vβ8.3./D/Jβ1.6 elements. The following oligonucleotides were used to amplify these rearrangements: 5′-GAG GAC CCG GGA ATA CCA CTC TGA AC-3′ (sense) and 5′-TCA TCC GCG GCC GCC AAA ATA ACC CAC ACA C-3′ (antisense) for the α chain, and 5′-GCA TAC TCG AGT CGC GAG ATG GGC TCC-3′ (sense) and 5′-CCA AGA CCG CGG TCA TCC AAC ACA G-3′ (antisense) for the β chain. The amplification products were cloned into pSK+ and then into the respective TCR expression cassettes as described above, yielding the vectors pTαT3CRP2 and pTβT3CRP2.
The constructs were functionally tested in vitro after electroporation into the TCR-negative T cell hybridoma BW58 (see reference 22). Recognition of the specific peptide–MHC complex by the transfected TCR was assessed by IL-2 production upon stimulation with BL/6 splenocytes and the respective peptide.
Before microinjection, the pTαcass and the pTβcass vectors were digested with SalI and KpnI, respectively, to remove prokaryotic regions. The corresponding α and β chain constructs were coinjected into (C57BL/6 × C3H)F2 zygotes. Transgenic founders were backcrossed to C57BL/6 for at least 5 generations. The resulting mouse lines were termed Sep TCR-tg and Dep TCR-tg according to expression of a TCR specific either for the subdominant or dominant epitope of hCRP.
Thymus Organ Culture.
Fetal thymic lobes were removed from embryos on day 14 of gestation (E14) and cultured on polycarbonate filters (Costar, Bodenheim, Germany) supported by sponges (Upjohn, Erlangen, Germany) in 12-well plates containing 2 ml cell culture medium (Iscove's medium, 10% FCS). Cultures were kept in a humidified chamber at 37°C and 7% CO2 for 8 d before analysis.
Thymus Transplantation and Bone Marrow Reconstitution.
Fetal thymic lobes (E15) were cultured in vitro (as described above) for 24 h to allow for genotyping of the donor embryos for the hCRP transgene by slot blot. Two to four irradiated (5 Gy) lobes were implanted under the kidney capsule of thymectomized host animals. 4 wk later, recipients were lethally irradiated (9.5 Gy) and reconstituted with T cell–depleted Dep-tg bone marrow (5 × 106 cells/animal). Immunization or phenotype analysis of grafted animals was carried out after another 6–8 wk.
Immunization and T Cell Proliferation Assays.
Immunizations were performed as previously described (16). In brief, mice were immunized in the foot pads of the hind legs with 100 μg of peptide emulsified in CFA (PBS/CFA vol/vol 1:1). 8–9 d later, popliteal and inguinal lymph nodes were removed. Cells were cultured for 72 h in triplicates at 4 × 105 cells/well in flat-bottomed 96-well plates in serum-free medium (HL-1; Boehringer Ingelheim, Ingelheim, Germany) in the presence or absence of peptide. Proliferation was measured by incorporation of [3H]Tdr, which was added for the last 12 h of culture (1 μCi/well).
Flow Cytometric Analysis.
Lymphocytes were stained with the following antibodies in various combinations according to standard procedures: FITC-coupled anti-Vβ5.1 (MR9-4; PharMingen, San Diego, CA); RR8-1 (reference 23); FITC-coupled anti-Vβ8.3 (1B3.3; PharMingen); FITC-coupled anti-pan TCR-β (H57-579; reference 24); Red-613– or biotin-coupled H129.19 (anti-CD4; Gibco-BRL, Eggenstein, Germany); and Red-613– or PE-coupled anti-CD8 (53-6.7; Gibco-BRL). mAb anti-Vα11 was detected with an anti–rat F(ab)2 fragment coupled to PE (Tagoimmunologicals, Biosource, Fleuris, Belgium). Biotinylated antibodies were detected using Red-670–coupled streptavidin (Gibco-BRL) or Cy5 (Dianova, Hamburg, Germany). Three-color fluorescence was analyzed on a FACScan® (Becton Dickinson, Heidelberg, Germany) and four-color fluorescence on a FACSvantage® (Becton Dickinson). Data were collected from viable lymphocytes by appropriate forward and side scatter gating. Data analysis was performed with Cellquest software (Becton Dickinson).
Enrichment of Thymic Stromal Cells.
Thymi of young adult mice were finely minced and then slowly stirred in medium for 5 min at room temperature to release the bulk of free thymocytes. The tissue fragments were subsequently slowly stirred in a collagenase/dispase mixture (25) for two rounds of 20 min at 37°C. Tissue fragments remaining after the second round were vigorously pipetted to further facilitate release of epithelial cells. Both collagenase/dispase fractions were pooled, washed, and filtered for further cell enrichment. The majority of thymocytes was removed by depletion of CD4-positive cells using mAb GK1.5 (26) and sheep anti–rat IgG magnetic beads (Dynal, Oslo, Norway). Subsequently, Fc receptors were blocked by a brief incubation with Ab 2.4G2. Isolation of stromal cells from this CD4-depleted cell suspension was performed in two ways. Three successive rounds of positive selection by magnetic beads (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) were applied using a modification of a previously described protocol (27). First, macrophages and dendritic cells (DCs) were enriched using Abs F4/80 (28) and N418 (29); second, cortical epithelial cells were enriched using Ab CDR1 (30); and third, medullary epithelial cells were enriched using Ab G8.8 (31). Alternatively, CD4-depleted cells were divided into three aliquots and each was subjected to a single step of positive selection by magnetic beads using the same surface markers. The second protocol routinely yielded higher cell numbers. Hemopoietic cells were separately enriched before complement lysis using an anti-CD45 Ab (T-200; reference 32). All Abs except for anti-CD45 were biotinylated and streptavidin beads or goat anti–rat beads were used as second step reagents. MiniMACS separation columns (type MS) were used for positive selection according to the manufacturer's manual. The purity of cortical and medullary epithelial cells was assessed by costaining of cytospins for cytokeratin, using mAb Lu-5 (Boehringer Mannheim, Mannheim, Germany) detected by anti–mouse IgG1-FITC (Dianova) and an independent marker for medullary epithelial cells TR-5 (33), coupled to Cy3 (Amersham, Braunschweig, Germany). In the G8.8-selected fraction, >90% of keratin-positive cells coexpressed TR-5.
Reverse Transcriptase PCR Analysis of Acute-phase Gene Expression.
RNA was isolated from tissues using TRIZOL (Gibco-BRL) followed by DNase treatment to remove genomic DNA or from single cell suspensions using the High Pure RNA Isolation Kit (Boehringer Mannheim). 2 μg of total RNA were reversely transcribed into cDNA using Superscript II Reverse Transcriptase (Gibco-BRL). PCR analyses were performed using 1/10 of this reaction. The following oligonucleotides were used: for hCRP: hCRP 2 (sense) 5′-CCA TGG AGA AGC TGT TGT G-3′ (−2 to +16), hCRP 3 (antisense) 5′-CTG TGA CTT CAG GAA CCT C-3′ (306 to 324), and hCRP 1 (antisense) 5′-CAA ATG TGT ACT GGA GCT AC-3′ (324 to 344); for mouse C-reactive protein: mCRP 1 (sense) 5′-CCA TGG AGA AGC TAC TCT G-3′ (−2 to +17), mCRP 2 (antisense) 5′-GTG TAG CCC TTG TGC AG (418 to 434) and mCRP 3 (antisense) 5′-CCC AAG ATG ATG CTT GC-3′ (448 to 464); for mouse serum amyloid P component (mSAP): mSAP 1 (sense) 5′-CAA GCA TGG ACA AGC TG-3′ (-5 to 12), mSAP 2 (antisense) 5′-CCC AAG TGG TAC ATA GG-3′ (338 to 355), and mSAP 3 (antisense) 5′-CAA CAA TGC CAG AGG AG-3′ (360 to 376). Figures given in brackets refer to the position of the primers relative to the translation start site. The positions of all sense and respective antisense primers are located in different exons, so that PCR products originating from contaminating genomic DNA could be excluded. Efficiency of cDNA synthesis was controlled using the β-actin–specific oligonucleotides 5′-TGG AAT CCT GTG GCA TCC ATG AAA C-3′ (sense) and 5′-TAA AACC GCA GCT CAG TAA CAG TCC G-3′ (antisense) under nonsaturating conditions (<24 cycles).
A first round of PCR was performed using the primer pairs hCRP 1/2, mCRP 1/3, or mSAP 1/3. PCR conditions were 94°C for 3 min, 30 cycles of 94°C for 1 min, 54°C for 1 min, 72°C for 1 min, and a final elongation step of 72°C for 5 min. For nested reamplification of 1/20 of the PCR products, the primer combinations hCRP 2/3, mCRP 1/2, or mSAP 1/2 were used. The number of PCR cycles in the reamplification was adjusted to a minimum to ensure nonsaturating conditions in order to allow for semiquantitative estimates of RNA expression. All PCR reactions were carried out in a volume of 50 μl, using 1 U of Taq Polymerase (MBI Fermentas, Vilnius, Lithuania). Amplification products (15 μl) were analyzed on 1.5% agarose gels.
Results
Transgenic Expression of α/β-TCRs Recognizing Two Epitopes of hCRP: Development and Selection.
CD4 T cells of C57BL/6 mice respond against two epitopes of hCRP when immunized with complete protein or peptides corresponding to either epitope (16, 21). Transgenic mice expressing hCRP as a neo–self-antigen under control of its autologous regulatory elements are tolerant to the immunodominant and reactive to the subdominant epitope of hCRP. Responsiveness to the subdominant epitope is only revealed by peptide but not by protein immunization. This differential tolerance is independent of basal serum levels that can vary by three orders of magnitude. To address the underlying tolerance mechanisms at the clonal level, we generated two strains of TCR transgenic mice.
The transgenic line termed Dep expresses a TCR that is specific for the dominant epitope of hCRP-spanning residues 89–101 and encodes a Vα11 and a Vβ5.1 region. Expression and selection of the Dep TCR during development was followed by costaining for the transgenic Vα and Vβ chains. Selection of double positive (DP) thymocytes into the CD4 lineage in H-2b mice is highly efficient with >90% of CD4 single positive (SP) thymocytes expressing the Dep TCR and a ratio of CD4/CD8 SP thymocytes of at least 8:1 (Fig. 1). Selection of the Dep TCR is lost in mice expressing a reduced diversity of self-peptides, i.e., invariant chaino/o mice (34), EpAbIio/o transgenic mice (reference 35 and our unpublished data), and H-2Mao/o mice (36). The surface density of the Dep TCR is fivefold increased on DP thymocytes when compared with wild-type levels, but does not exceed physiological levels on mature SP thymocytes, as judged by expression of CD3ε (data not shown). The efficient positive selection is also reflected in the periphery with a CD4/CD8 ratio of 10:1 and ∼85% of peripheral CD4 T cells expressing both transgenic TCR chains at high levels. These cells are activated in vivo upon intravenous injection of peptide hCRP 89–101, as reflected by their phenotype 24 h thereafter (CD69+, HSAhi, CD62Llo; data not shown).
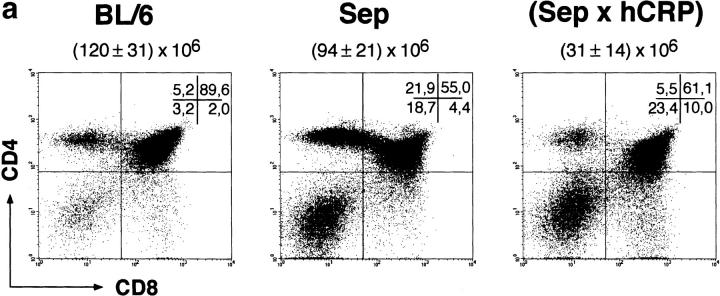
Figure 1.
Positive and negative selection of CD4 thymocytes specific for the dominant epitope of hCRP. Thymocytes of BL/6, TCR single transgenic Dep, and Dep × hCRP female mice were stained for coexpression of CD4 and CD8 (a) and the transgenic TCR chains Vα11 and Vβ5.1 (b), and were analyzed by four-color fluorescence. Note the efficient positive selection of TCR transgenic CD4 T cells in single transgenic mice and their deletion at the DP stage in noninduced Dep × hCRP mice. The total number of thymocytes (mean ± SD of at least three animals) and the percentages of subsets per quadrant are indicated. Male mice displayed an identical phenotype. Fluorescence intensity is shown on a four-decade logarithmic scale. 6–10-wk-old mice were analyzed.
The second TCR transgenic line, termed Sep, expresses a TCR that is specific for the subdominant epitope of hCRP-spanning residues 80–94 and encodes a Vα4 and a Vβ8.3 region. Based on the analysis of the Vβ8.3 epitope, the Sep TCR shows a similar expression pattern as the Dep TCR, i.e., early expression on double negative (DN) thymocytes, a physiological density of CD3 complexes on SP thymocytes, and a strong CD4 skewing among SP thymocytes and peripheral T cells (Fig. 2). How is the developmental fate and immunocompetence of hCRP-specific T cells influenced by various levels of this APP depending on gender or induction status?
Figure 2.
Positive and negative selection of CD4 thymocytes specific for the subdominant epitope of hCRP. Thymocytes of BL/6, TCR single transgenic Sep, and Sep × hCRP female mice were stained for coexpression of the transgenic TCR chain Vβ8.3, CD4, and CD8, and were analyzed by three-color fluorescence. CD4 versus CD8 expression (a) and Vβ8.3 expression gated on the CD4 SP subset (b) are shown. Note the strong bias towards CD4 SP T cells in TCR single transgenic mice and its reversion in noninduced Sep × hCRP mice. The total number of thymocytes (mean ± SD of at least three animals) and the percentages of subsets per quadrant are indicated. Male mice displayed an identical phenotype. 6–10-wk-old mice were analyzed.
Early Negative Selection of hCRP-specific Thymocytes in TCR × hCRP Mice: Tolerance Is Epitope-dependent, but Independent of hCRP Serum Levels.
Surprisingly, in both male and female Dep × hCRP mice the size of the thymus was at least 10-fold reduced despite widely differing basal serum levels. This correlated with a relative and absolute reduction of DP and CD4 SP thymocytes. Among the remaining DP and CD4 SP cells the frequency of Dep TCR-positive cells was markedly reduced, and thymocytes expressing the Dep TCR at high levels (TCRhi) were completely missing (Fig. 1). T cells expressing the Dep TCR were rare in the periphery (<3%; data not shown). This pattern was already apparent in newborn mice (data not shown). Thus, deletion of hCRP-reactive thymocytes specific for the dominant epitope starts early during ontogeny and at an early stage of their intrathymic development, namely the DP stage. Likewise, several observations indicate that expression of the Sep TCR specific for the subdominant epitope also results in intrathymic deletion. The thymus of Sep × hCRP double transgenic mice is reduced in size by 75% and so is the absolute and relative number of CD4 SP thymocytes when compared with Sep single transgenic mice (Fig. 2). Moreover, peripheral Vβ8.3+ CD4 T cells express endogenous TCR Vα elements at higher frequencies (i.e., Vα2 + 8 + 11, ∼18%) than do Sep single transgenic mice (Vα2 + 8 + 11, <2%) indicating selection for Sep-negative or Sep-low CD4 T cells (data not shown). Taken together, the observed phenotypes of the TCR × hCRP strains indicate that both T cell specificities are subject to intrathymic deletion. How do these results comply with responsiveness to the subdominant epitope of the polyclonal repertoire in hCRP single transgenic mice?
The observation that only 15% of the CD4 T cells emerging in the Dep × hCRP mice express the transgenic β chain suggests that coexpression of an endogenous α chain in addition to the transgenic chains is not sufficient to rescue Dep-expressing cells from deletion. In contrast, >70% of peripheral CD4 SP cells in the Sep × hCRP mice still do express the transgenic β chain. Thus, negative selection of the transgenic TCR for the subdominant epitope seems to be less stringent, allowing for exit of low affinity/ avidity T cells from the thymus. However, we cannot exclude that subtle differences in the precise timing or level of expression of these two transgenic TCRs influence the observed differences in β chain allelic exclusion.
The notion of differential tolerance is supported by observations at the functional level. Immunization with peptides corresponding to the antigenic epitope of each TCR revealed that tolerance by intrathymic deletion is complete only in Dep × hCRP mice, whereas Sep × hCRP mice, despite deletion of the transgenic TCR, show a vigorous proliferative response (Fig. 3). Again these differences were sex independent within each transgenic line. Thus, T cell tolerance to hCRP is dictated by epitope hierarchy rather than circulating levels of antigen.
Figure 3.
Epitope-specific T cell tolerance in hCRP and hCRP × TCR transgenic mice. (a) BL/6, hCRP single transgenic, and Dep × hCRP male and female mice were immunized with a peptide corresponding to the dominant epitope of hCRP. (b) BL/6, hCRP single transgenic, and Sep × hCRP female mice were immunized with a peptide corresponding to the subdominant epitope of hCRP. 8–10 d later, draining lymph node cells were assessed for their proliferative response to the respective peptide. Irrespective of gender, hCRP single and Dep × hCRP mice are tolerant to the dominant epitope, whereas hCRP single and Sep × hCRP mice respond to the subdominant epitope.
Central Tolerance Is Due to Ectopic Expression of hCRP in the Thymus.
Based on previous reports, the basal levels of hCRP in male mice of ∼5 × 10−7 M are compatible with intrathymic deletion due to entry and presentation of blood-borne antigen (4, 18). The at least 500-fold lower levels in female mice (≤10−9 M) are more difficult to reconcile with the observed phenotype of early and complete intrathymic deletion. Moreover, pronounced deletion occurred in fetal thymus organ cultures (FTOC) of Dep × hCRP thymi in the absence of blood-borne antigen supply (data not shown). We thus considered an additional intrathymic source of hCRP leading to continuous and sufficient presentation of hCRP epitopes in the thymic microenvironment irrespective of circulating levels. Two major cell populations, hemopoietic and radio-resistant epithelial cells, were assessed as a putative source of ectopically expressed hCRP.
In view of previous reports on a membrane-bound form of CRP being expressed by subsets of macrophages in human and rat (13, 37, 38), we first assessed the influence of hCRP transgenic hemopoietic cells on the development of Dep cells. When lethally irradiated B6 mice were reconstituted with bone marrow cells from Dep × hCRP mice, thereby generating animals in which only hemopoietic cells carried the hCRP transgene, no deletion of the Dep TCR was observed. The central and peripheral compartments of these mice were identical to those of B6 mice reconstituted with Dep single transgenic bone marrow, i.e., mice lacking the hCRP transgene (Fig. 4). Bone marrow–derived cells (macrophages, DCs, and T and B cells) thus do not provide a source of thymic hCRP that would lead to deletion of specific T cells. This conclusion is also supported by the observation that hCRP mice reconstituted with Dep TCR bone marrow, mice in which all cells except hemopoietic cells carry the hCRP transgene, exhibit the same degree of deletion as Dep × hCRP mice (Fig. 4).
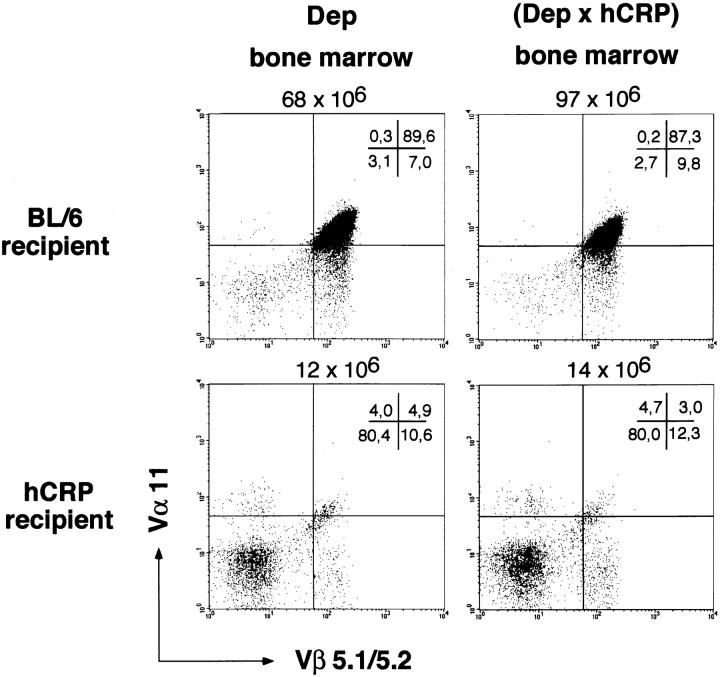
Figure 4.
Thymic deletion is not due to hemopoietic cell–derived hCRP. BL/6 and hCRP transgenic mice were lethally irradiated and reconstituted either with bone marrow cells from Dep or Dep × hCRP donors. 8 wk later, intrathymic selection of Dep T cells was analyzed by four-color fluorescence (see Fig. 1). Coexpression of the transgenic TCR chains Vα11 and Vβ5.1 on CD4 SP thymocytes is shown. Deletion is independent of the presence of the hCRP transgene in the donor bone marrow.
To assess the role of thymic epithelial cells in tolerance to hCRP, we constructed chimeric animals in which only radioresistant thymic stromal cells, i.e., epithelial cells, carry the hCRP transgene. Thymectomized B6 mice were grafted with an irradiated (5 Gy) hCRP transgenic thymus and reconstituted with Dep TCR bone marrow. In these animals, early and profound deletion of the Dep TCR was observed (Fig. 5 a) indicating that thymic epithelial cells do produce hCRP and that this source is sufficient to induce negative selection of specific T cells in the absence of liver-derived antigen. We next asked whether epithelium-derived hCRP is also necessary for negative selection. Endogenous hCRP transgenic thymus in male and female hCRP transgenic mice was replaced by fetal B6 thymi and, after reconstitution with Dep bone marrow cells, the differentiation of Dep TCR T cells was followed. In male hCRP transgenic mice deletion was seen within the B6 thymus graft (Fig. 5 a). Interestingly, the onset and extent of this deletion was indistinguishable from that observed in double transgenic mice or in a B6 animal grafted with an hCRP transgenic thymus (see Figs. 1 and 4). Thymus-derived antigen and high levels of blood-borne antigen thus cause the same phenotype of intrathymic deletion. In contrast, in female hCRP mice carrying a B6 thymus, no intragraft deletion was observed (Fig. 5 a). The cellular composition of these grafts was identical to B6 grafts carried by nontransgenic recipients. Immunization of these chimeras resulted in a strong proliferative response of peripheral Dep T cells (Fig. 5 b) and simultaneous central deletion of Dep thymocytes (data not shown). The latter is most likely caused by increased serum levels of hCRP due to concomitant induction of an acute phase.
Figure 5.
Thymus-derived hCRP is necessary and sufficient for tolerance in female hCRP transgenic mice. Thymectomized male and female hCRP transgenic mice were grafted with fetal BL/6 thymi, and thymectomized female BL/6 mice were grafted with hCRP transgenic thymi. 4 wk later, animals were lethally irradiated and reconstituted with Dep transgenic bone marrow. After another 6 wk intrathymic selection of Dep T cells was analyzed by four-color fluorescence (see Fig. 1). Coexpression of the transgenic TCR chains Vα11 and Vβ5.1 on CD4 SP thymocytes is shown (a). The same type of animals were immunized with peptide hCRP 89–100 (see Fig. 3) to assess their tolerance status (b). Thymus-derived hCRP is necessary in female, but not in male, hCRP transgenic mice for central deletion of hCRP-specific T cells and tolerance.
In summary, these data show that ectopic expression of hCRP by thymic epithelial cells is (a) sufficient to cause deletion of a T cell repertoire largely consisting of hCRP-specific cells and (b) necessary to ensure tolerance at low basal hCRP serum levels in female mice, which correspond to the levels found in healthy humans.
Transgenic hCRP Mimics Expression of Endogenous Murine APPs and of hCRP in Humans.
The thymus transplantation experiments did not formally rule out the possibility of antigen carry-over by intrathymic APCs. Liver-derived antigen might have been taken up by MHC class II–positive cells from the circulation of the fetal donor and presented at tolerogenic levels during the experimental period of ∼10 wk. Given the turnover of peptide–MHC complexes on thymic APCs (39), and the turnover of thymic DCs (40), this explanation is unlikely. To directly demonstrate the intrathymic origin of hCRP, the expression pattern of hCRP was analyzed by reverse transcriptase PCR. As expected, hCRP mRNA was readily detectable in the liver of hCRP transgenic male mice (Fig. 6 a). A weaker signal was reproducibly obtained with RNA extracts from the thymus of these mice. Under the same conditions all other organs tested (spleen, brain, heart, kidney, and lung) did not yield a signal. Further amplification of the PCR products with nested primers revealed additional signals in brain, kidney, and lung (data not shown). The signal in liver and thymus of noninduced female hCRP transgenic animals was weaker and sometimes undetectable, but upon induction of an acute-phase response, the hCRP-specific signals strongly increased in liver and thymus (data not shown).
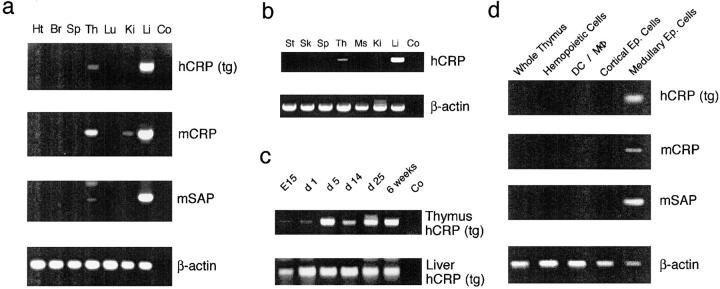
Figure 6.
Expression pattern of transgenic hCRP and endogenous murine APPs. (a) The organ-specific expression pattern of the hCRP transgene and the homologous mouse APPs (mCRP and mSAP) was analyzed by reverse transcriptase PCR (35 cycles) in a young adult, noninduced, male hCRP transgenic mouse. Expression of the hCRP transgene parallels that of endogenous APPs and is confined to liver and thymus. An identical pattern was identified for induced female hCRP animals under the same PCR conditions. Signals for hCRP and mSAP, but not of mCRP, in liver and thymus of noninduced females were consistently lower (detection of thymic expression required reamplification with nested primers). The weak mCRP band in the kidney was not consistently detected. Ht, heart; Br, brain; Sp, spleen; Th, thymus; Lu, lung; Ki, kidney; Li, liver; Co, control. (b) The organ-specific expression pattern of hCRP was analyzed in various human tissues. Expression is confined to liver and thymus. St, stomach; Sk, skin; Ms, muscle. (c) Expression of hCRP in thymus and liver was analyzed during ontogeny in noninduced hCRP transgenic females. Expression in both organs was detected from day E15 onwards to adulthood. Thymus samples were reamplified with nested primers. (d) Expression of hCRP, mCRP, and mSAP was analyzed in whole thymocytes and in highly enriched thymic subsets of a noninduced female hCRP mouse (reverse transcriptase PCR; 30 cycles). Expression of the hCRP transgene and endogenous APP is confined to medullary epithelial cells. Note that detection of APP expression in enriched medullary epithelial cells of noninduced female mice does not require nested reamplification.
Since ectopic expression of transgenes is often attributed to positional effects, we asked whether intrathymic expression of the hCRP transgene mimics the tissue-specificity of endogenous APPs. The expression analysis was extended to two endogenous murine APPs with homology to hCRP, namely mouse CRP (mCRP) and mSAP. Messenger RNA of mCRP could be detected by reverse transcriptase PCR in liver and thymus of male and female mice irrespective of experimental induction of an acute phase (Fig. 6 a). This constitutive expression is in accordance with CRP being a minor APP in mice, which is only weakly induced during the course of an acute-phase response. Similarly, expression of mSAP, the major murine APP, was detectable in the liver and thymus of noninduced male and female animals (Fig. 6 a). Upon induction, these signals increased in strength in the liver, as expected for a major APP, and also in the thymus (data not shown). Analysis of various human tissues also revealed ectopic expression of hCRP in the postnatal human thymus (age 3 mo; Fig. 6 b). Thus, the organ-specific expression and the induction pattern of the hCRP transgene closely resemble those of its functional murine homologue mSAP and of hCRP in humans.
Prenatal Onset of Ectopic Expression.
Since central deletion of Dep TCR transgenic thymocytes already occurred during the fetal period (data not shown), intrathymic expression of hCRP during ontogeny was assessed. In accordance with the prenatal onset of tolerance induction, hCRP mRNA was detectable by reverse transcriptase PCR in fetal thymi of E15 embryos (earliest time point tested; Fig. 6 c). Likewise, mSAP and mCRP were expressed in thymi without any gender difference (data not shown). Thus, both murine APP and the human transgene are expressed in the thymus throughout the pre- and early postnatal period, when the bulk of the T cell repertoire is generated.
Medullary Epithelial Cells Express APPs.
As shown above, an hCRP source in radioresistant thymic stromal cells rather than in bone marrow–derived cells was responsible for deletion of Dep T cells in the absence of liver-derived hCRP (Figs. 4 and 5). To delineate this cell type more precisely, we enriched hemopoietic cells (mostly thymocytes), monocytes (macrophages and DCs), cortical epithelial cells, or medullary epithelial cells using appropriate cell surface markers. These cell subsets were enriched either sequentially or separately (see Materials and Methods). Since the marker used for medullary epithelial cells (G8.8) is also weakly expressed on cortical epithelial cells (31), it was important to deplete cortical epithelial cells before selection of G8.8-positive cells. Irrespective of the enrichment procedure, reverse transcriptase PCR analyses on cDNA prepared from these thymic cell fractions revealed expression of transgenic hCRP as well as endogenous mCRP and mSAP exclusively in medullary epithelial cells (Fig. 6 d).
Discussion
The surprising finding of early and profound central deletion of T cells specific for both epitopes of hCRP even at very low levels of circulating protein, and the striking similarity of intrathymic T cell fate and peripheral reactivity irrespective of 500-fold differing serum levels prompted us to reanalyze the tissue specificity of hCRP expression. Transplantation experiments as well as reverse transcriptase PCR analyses revealed expression of hCRP in medullary epithelial cells of the thymus. We consider this “ectopic” expression of the neo–self-antigen to be physiological for several reasons. (a) The hCRP transgene spans a region of 31 kb containing all known autologous 5′ and 3′ cis-acting elements (15). Liver-specificity (as formerly assessed by northern blotting) and inducibility of hepatic gene expression indicate that the trans-acting factors are conserved in mouse and humans (14). (b) We show that ectopic expression of hCRP is exclusively restricted to medullary epithelial cells of the thymus. Gene-regulation in this cellular subset of the thymus is comparable to that in hepatocytes with regard to onset during ontogeny, and, surprisingly, inducibility and sexual dimorphism. (c) Importantly, we demonstrate that hCRP is also expressed in the human thymus and that the homologous APPs of the mouse (mSAP and mCRP) show a cell type–specific expression pattern, identical to the hCRP transgene. It is noteworthy that hCRP and mSAP, two species-specific major APPs, are inducible in medullary cells of the thymus (data not shown), indicating the presence of all components of the signaling cascade (cytokine receptors, second messengers, and transcription factors) that hitherto were thought to be confined to hepatocytes and a macrophage subset (38).
Several neo–self-antigens under direction of putatively tissue-specific promoters have been found to be expressed in the thymus of transgenic mice. These include the promoters of rat insulin II (41, 42), rat elastase I (43), guinea pig α-lactalbumin, human beta globin (44, 45), keratin-IV (46), and metallothionein (47). Thymic expression of different model antigens driven by these heterologous regulatory elements was often variable and usually found in some but not all transgenic lines, consistent with the notion that the integration site and/or the copy number influence expression. In this context it is noteworthy that a truncated version of the hCRP promoter directing expression of an MHC class I alloantigen was active in hepatocytes but not in the thymus (as assessed by selection of specific T cells in chimeras and PCR analysis; reference 48). In view of the variability and unpredictability of the thymic activity of these hybrid transgenes, it was initially difficult to assess the biological significance of this ectopic gene expression. However, recent studies revealed a diverse group of endogenous “peripheral” antigens to be expressed in the thymus of rodents or primates, including pancreas-specific genes (41), components of the myelin sheath (myelin basic protein and myelin proteolipid protein; references 49–51), S-100β (52), acetylcholine receptor (53), retinal proteins (arrestin and interphotoreceptor retinoid–binding protein; reference 54), and neuro-endocrine hormones (55). Thus, thymic expression of “tissue-specific” genes seems to be a common occurrence and to be part of physiological expression patterns. This should result in a more diverse presentation of “self” within the thymus than has been appreciated previously. Although a role of intrathymic expression of “nonthymic” proteins in the establishment of self-tolerance has been proposed, it has not been formally demonstrated. Nevertheless, intriguing correlations between the thymic expression level of self-antigens and the propensity to spontaneously develop or succumb to experimental induction of autoimmune disease have been recently reported for insulin (56, 57) and two retinal proteins (54). Our analysis demonstrates for the first time that thymic expression of a secreted neo–self-antigen under its autologous regulatory elements confers tolerance upon the developing T cell repertoire by deletion of specific T cells with remarkable efficiency.
Expression of several “peripheral” antigens has been assigned to the thymic medulla by histological analysis (50, 52, 58), although delineation of the precise cell type has been difficult. Expression of hCRP and mouse APP is clearly confined to radio-resistant stromal cells as shown by transplantation experiments, and more precisely to medullary epithelial cells as shown by cell separation and subsequent reverse transcriptase PCR. Medullary epithelial cells have been previously implicated in tolerance induction both by deletion or anergy induction (46, 59–61). How does the restriction of hCRP expression to the medulla comply with the profound deletion phenotype, in particular the lack of immature DP thymocytes, most of which reside in the cortex? We suggest that hCRP is secreted by medullary epithelial cells and subsequently presented by MHC class II–positive APCs, including DCs and medullary and cortical epithelial cells. Such intercellular antigen transfer and “cross presentation” within the thymic microenvironment has been described for an MHC class II– restricted membrane protein (62). Recognition of specific peptide–MHC complexes on cortical epithelial cells would result in deletion of hCRP-specific cells at the transition from the DN to DP stage as soon as they become susceptible to apoptotic signals via the TCR (63; note that DN thymocytes prematurely express the transgenic TCR at high levels [Fig. 1]). Interestingly, an identical phenotype of early intrathymic deletion is observed when hCRP is derived either exclusively from epithelial cells (as in hCRP grafts in B6 mice; Fig. 5 a) or from the circulation (as in B6 grafts in male hCRP transgenic mice; Fig. 5 a), arguing for efficient antigen presentation on cortical cells in both instances. In the latter case, hCRP may gain access to the cortex via blood vessels or the capsule (64). Indeed, cortical epithelial cells have been shown to be accessible to blood-borne proteins (9) and to mediate deletion of immature thymocytes in vitro and in fetal thymus organ cultures (25, 27). In support of this notion, hCRP protein can be directly visualized within the cortical parenchyma by immunohistology in hCRP transgenic mice after experimental induction (data not shown).
The proposed intrathymic “antigen spread” may explain why deletion of hCRP-specific thymocytes is remarkably efficient when compared with other experimental models in which ectopic expression of heterologous transgene-constructs was observed. Ectopic expression in the thymus of various model-antigens under control of the rat insulin promoter (RIP), for instance, yielded divergent results. No central deletion of TCR transgenic CD4 cells specific for the large T antigen (Tag) of SV 40 was observed in RIP-Tag × TCR mice (22), yet tolerance of CD4 and CD8 T cells could be demonstrated at the polyclonal level in RIP-Tag single transgenic mice (58). Ectopic expression of the nuclear protein of LCMV or the allo-MHC antigen Kb driven by the RIP lead to partial tolerance among CD8 T cells (42, 65). In the latter study, deletion of only those Kb-specific T cells that express a transgenic TCR at high density was described. Deletion of CD8 T cells was also incomplete when ectopic Kb expression was directed by the promoters of guinea pig α-lactalbumin or human beta globin (45). Intrathymic expression of Tag under the rat elastase I promoter or MHC class I-Kb under the keratin IV promoter resulted in anergy induction of specific CD8 T cells rather than deletion (43, 46). Several reasons may account for these different outcomes, namely antigen availability (secreted versus intracellular antigens), the type of APC (bone marrow–derived versus epithelial cell), and the affinity/avidity of the TCR–MHC interaction (66, 67). Given this variability, it will be important to test the contribution of the thymic activity of each of these promoters to tolerance induction in their native genetic context.
Tolerance to a major murine APP has recently been documented (68). Is ectopic expression of APPs necessary for tolerance induction? Serum levels of hCRP in female transgenic animals closely mimic hCRP levels in humans. These basal levels of circulating hCRP are insufficient to induce intragraft deletion and tolerance in transgenic female mice grafted with a B6 thymus. Upon induction of an acute phase, hCRP levels in females rise 500-fold, and are now sufficient to activate peripheral T cells (naive Dep and Sep T cells, when transferred into hCRP transgenic animals expand vigorously in male, but not in female recipients; our unpublished results). Tolerance induction in the absence of a thymic antigen source would thus be confined to irregular intervals of acute phases and likely to be insufficient to impose tolerance on a continuously developing T cell repertoire. In contrast, expression of APPs in the pre- and postnatal thymus results in presentation of both dominant and subdominant epitopes at levels sufficient to confer functional tolerance irrespective of an acute phase. Ectopic expression by thymic medullary epithelial cells thus seems to be a physiological device to safeguard tolerance to APPs.
Sensitive detection methods reveal the expression of a growing number of “peripheral” antigens in the thymus, yet based on the available number of MHC molecules on thymic APCs, the number of self-peptides presented at tolerogenic levels has to be limited. It will be important to identify common biological and possibly genetic features that have led to ectopic expression of certain “peripheral” proteins during evolution.
Acknowledgments
We would like to thank Bernd Arnold and Jacqueline Trotter for helpful comments on the manuscript; Sonja Höflinger for expert technical assistance; Judith Alferink for advice in animal experiments; and Klaus Hexel for competent support in flow cytometry. We are indebted to Diane Mathis and Cristoph Benoist and Mark B. Pepys (Hammersmith Hospital, London, UK) for generous supply of reagents; Siegfried Hagl (Department of Cardiac Surgery), Peter Galle (Department of Gastroenterology), and Gunhild Mechtersheimer (Department of Pathology) of the Heidelberg Medical School for supplying human tissues; and the core facilities of the German Cancer Research Center for peptide and primer synthesis.
Abbreviations used in this paper
- APP
acute-phase protein
- DC
dendritic cell
- Dep
dominant epitope
- DN
double negative
- DP
double positive
- hCRP
human C-reactive protein
- RIP
rat insulin promoter
- mSAP
mouse serum amyloid P component
- Sep
subdominant epitope
- SP
single positive
- Tag
large T antigen
Footnotes
This study has been supported by the DFG (Ky 7/7-1), the German Cancer Research Center (B. Kyewski) and the Volkswagenstiftung (U. Rüther).
References
- 1.van Meerwijk JPM, Marguerat S, Lees RK, Germain RN, Fowlkes BJ, MacDonald HR. Quantitative impact of thymic clonal deletion on the T cell repertoire. J Exp Med. 1997;185:377–383. doi: 10.1084/jem.185.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kappler JW, Roehm N, Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987;49:273–280. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- 3.Kyewski BA, Fathman CG, Kaplan HS. Intrathymic presentation of circulating non–major histocompatibility complex antigens. Nature. 1984;308:196–199. doi: 10.1038/308196a0. [DOI] [PubMed] [Google Scholar]
- 4.Zal T, Volkmann A, Stockinger B. Mechanisms of tolerance induction in major histocompatibility complex class II–restricted T cells specific for a blood-borne self-antigen. J Exp Med. 1994;180:2089–2099. doi: 10.1084/jem.180.6.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnold, B., G. Schönrich, I. Ferber, J. Alferink, and G.J. Hämmerling. 1994. Peripheral T cell tolerance: distinct levels and multistep mechanisms. In Transgenesis and Targeted Mutagenesis in Immunology. H. Bluethmann and P.S. Ohashi, editors. Academic Press, San Diego, CA. 135–140.
- 6.Ohashi, P.S., H. Hengartner, M. Battegay, R.M. Zinkernagel, and H. Pircher. 1994. Thymocyte selection and peripheral tolerance using the lymphocytic choriomeningitis virus as a model antigen. In Transgenesis and Targeted Mutagenesis in Immunology. H. Bluethmann, H., and P.S. Ohashi, editors. Academic Press, San Diego, CA. 114–130.
- 7.Dillon SR, MacKay VL, Fink PJ. A functionally compromised intermediate in extrathymic CD8+T cell deletion. Immunity. 1995;3:321–333. doi: 10.1016/1074-7613(95)90117-5. [DOI] [PubMed] [Google Scholar]
- 8.Akkaraju S, Ho WY, Leong D, Canaan K, Davis MM, Goodnow CC. A range of CD4 T cell tolerance: partial inactivation to organ-specific antigen allows nondestructive thyroiditis or insulitis. Immunity. 1997;7:255–271. doi: 10.1016/s1074-7613(00)80528-2. [DOI] [PubMed] [Google Scholar]
- 9.Lorenz RG, Allen P. Thymic cortical epithelial cells can present self-antigens in vivo. . Nature. 1989;337:560–563. doi: 10.1038/337560a0. [DOI] [PubMed] [Google Scholar]
- 10.Pircher H, Rohrer UH, Moskophidis D, Zinkernagel RM, Hengartner H. Lower receptor avidity required for thymic clonal deletion than for effector T-cell function. Nature. 1991;351:482–485. doi: 10.1038/351482a0. [DOI] [PubMed] [Google Scholar]
- 11.Baumann H, Gauldie J. The acute phase response. Immunol Today. 1994;15:74–80. doi: 10.1016/0167-5699(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 12.Tebo JM, Mortenson RF. Characterization and isolation of a C-reactive protein receptor from the human monocytic cell line U-937. J Immunol. 1990;144:231–238. [PubMed] [Google Scholar]
- 13.Egenhofer C, Alsdorff K, Fehsel K, Kolb-Bachofen V. Membrane-associated C-reactive protein on rat liver macrophages is synthesized within the macrophages, expressed as neo-C-reactive protein and bound through a C-reactive protein-specific membrane receptor. Hepatology. 1993;18:1216–1223. [PubMed] [Google Scholar]
- 14.Ciliberto G, Arcone R, Wagner EF, Rüther U. Inducible and tissue-specific expression of human C-reactive protein in transgenic mice. EMBO (Eur Mol Biol Organ) J. 1987;6:4017–4022. doi: 10.1002/j.1460-2075.1987.tb02745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy C, Beckers J, Rüther U. Regulation of the human C-reactive protein gene in transgenic mice. J Biol Chem. 1995;270:704–708. doi: 10.1074/jbc.270.2.704. [DOI] [PubMed] [Google Scholar]
- 16.Klein TC, Döffinger R, Pepys MB, Rüther U, Kyewski B. Tolerance and immunity to the inducible self antigen C-reactive protein in transgenic mice. Eur J Immunol. 1995;25:3489–3495. doi: 10.1002/eji.1830251242. [DOI] [PubMed] [Google Scholar]
- 17.Szalai AJ, Briles DE, Volanakis JE. Human C-reactive protein is protective against fatal Streptococcus pneumoniaeinfection in transgenic mice. J Immunol. 1995;155:2557–2563. [PubMed] [Google Scholar]
- 18.Bogen B, Dembic Z, Weiss S. Clonal deletion of specific thymocytes by an immunoglobulin idiotype. EMBO (Eur Mol Biol Organ) J. 1993;12:357–363. doi: 10.1002/j.1460-2075.1993.tb05664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Granucci F, Rescigno M, Marconi G, Foti M, Ricciardi-Castagnoli P. Ig-specific T cell receptor-transgenic T cells are not deleted in the thymus and are functional in vivo. J Exp Med. 1996;183:203–213. doi: 10.1084/jem.183.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kouskoff V, Signorelli K, Benoist C, Mathis D. Cassette vectors directing expression of T cell receptor genes in transgenic mice. J Immunol Meth. 1995;180:273–280. doi: 10.1016/0022-1759(95)00002-r. [DOI] [PubMed] [Google Scholar]
- 21.Döffinger R, Klein TC, Pepys MB, Casanova J-L, Kyewski B. The MHC class II–restricted T cell response of C57BL/6 mice to human C-reactive protein: homology to self and the selection of T cell epitopes and T cell receptors. Mol Immunol. 1997;34:115–124. doi: 10.1016/s0161-5890(97)00014-x. [DOI] [PubMed] [Google Scholar]
- 22.Förster I, Hirose R, Arbeit JM, Clausen BE, Hanahan D. Limited capacity for tolerization of CD4 T cells specific for a pancreatic β cell neo-antigen. Immunity. 1995;2:573–585. doi: 10.1016/1074-7613(95)90002-0. [DOI] [PubMed] [Google Scholar]
- 23.Jameson SC, Nakajima PB, Brooks JL, Heath W, Kanagawa O, Gascoigne NR. The T cell receptor V alpha 11 family. Analysis of allelic sequence polymorphism and demonstration of J alpha region-dependent recognition by allele-specific antibodies. J Immunol. 1991;147:3185–3193. [PubMed] [Google Scholar]
- 24.Kubo RT, Born W, Kappler JW, Marrack P, Pigeon M. Characterisation of a monoclonal antibody which detects all murine alpha beta T cell receptors. J Immunol. 1989;142:2736–2742. [PubMed] [Google Scholar]
- 25.Pircher H, Brduscha K, Steinhoff U, Kasai M, Mizuochi T, Zinkernagel RM, Hengartner H, Kyewski B, Müller K-P. Tolerance induction by clonal deletion of CD4+8+ thymocytes in vitrodoes not require dedicated antigen-presenting cells. Eur J Immunol. 1993;23:669–674. doi: 10.1002/eji.1830230315. [DOI] [PubMed] [Google Scholar]
- 26.Dialynas DP, Quan ZS, Wall KA, Pierres A, Quintas J, Loken MR, Pierres M, Fitch FW. Characterization of the murine T cell surface molecule designated L3T4, identified by monoclonal antibody GK 1.5: similarity of L3T4 to the human Leu-3T4 molecule. J Immunol. 1983;131:2445–2451. [PubMed] [Google Scholar]
- 27.Volkmann A, Zal T, Stockinger B. Antigen-presenting cells in the thymus that can negatively select MHC class II–restricted T cells recognizing a circulating self antigen. J Immunol. 1997;158:693–706. [PubMed] [Google Scholar]
- 28.Austyn JM, Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol. 1981;11:805–815. doi: 10.1002/eji.1830111013. [DOI] [PubMed] [Google Scholar]
- 29.Metlay JP, Witmer-Pack MD, Agger R, Crowley MT, Lawless D, Steinman RM. The distinct leukocyte integrins of mouse spleen dendritic cells as identified with new hamster monoclonal antibodies. J Exp Med. 1990;171:1753–1771. doi: 10.1084/jem.171.5.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rouse RV, Bolin LM, Bender JR, Kyewski BA. Monoclonal antibodies reactive with subsets of mouse and human thymic epithelial cells. J Histochem Cytochem. 1988;36:1511–1517. doi: 10.1177/36.12.2461413. [DOI] [PubMed] [Google Scholar]
- 31.Nelson AJ, Dunn RJ, Peach R, Aruffo A, Farr AG. The murine homolog of human Ep-CAM, a homotypic adhesion molecule, is expressed by thymocytes and thymic epithelial cells. Eur J Immunol. 1996;26:401–408. doi: 10.1002/eji.1830260220. [DOI] [PubMed] [Google Scholar]
- 32.Ledbetter JA, Herzenberg LA. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- 33.van Vliet E, Melis M, van Ewijk W. Monoclonal antibodies to stromal cell types in the mouse thymus. Eur J Immunol. 1984;14:524–529. doi: 10.1002/eji.1830140608. [DOI] [PubMed] [Google Scholar]
- 34.Viville S, Neefjes J, Lotteau V, Dierich A, Lemeur M, Ploegh H, Benoist C, Mathis D. Mice lacking the MHC class II–associated invariant chain. Cell. 1993;72:635–648. doi: 10.1016/0092-8674(93)90081-z. [DOI] [PubMed] [Google Scholar]
- 35.Ignatowicz L, Kappler J, Marrack P. The repertoire of T cells shaped by a single MHC/peptide ligand. Cell. 1996;84:521–529. doi: 10.1016/s0092-8674(00)81028-4. [DOI] [PubMed] [Google Scholar]
- 36.Tourne S, Miyazaki T, Oxenius A, Klein L, Fehr T, Kyewski B, Benoist C, Mathis D. Selection of a broad repertoire of CD4+ T cells in H-2Mao/omice. Immunity. 1997;7:187–195. doi: 10.1016/s1074-7613(00)80522-1. [DOI] [PubMed] [Google Scholar]
- 37.Murphy TM, Baum LL, Beaman KD. Extrahepatic transcription of human C-reactive protein. J Exp Med. 1991;173:495–498. doi: 10.1084/jem.173.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dong Q, Wright JR. Expression of C-reactive protein by alveolar macrophages. J Immunol. 1996;156:4815–4820. [PubMed] [Google Scholar]
- 39.Müller K-P, Schumacher J, Kyewski BA. Half-life of antigen/major histocompatibility complex class II complexes in vivo: intra- and interorgan variations. Eur J Immunol. 1994;23:3203–3207. doi: 10.1002/eji.1830231224. [DOI] [PubMed] [Google Scholar]
- 40.Wu L, Vremec D, Ardavin C, Winkel K, Suss G, Georgiou H, Maraskovsky E, Cook W, Shortman K. Mouse thymus dendritic cells: kinetics of development and changes in surface markers during maturation. Eur J Immunol. 1995;25:418–425. doi: 10.1002/eji.1830250217. [DOI] [PubMed] [Google Scholar]
- 41.Jolicoeur C, Hanahan D, Smith KM. T-cell tolerance toward a transgenic β-cell antigen and transcription of endogenous pancreatic genes in thymus. Proc Natl Acad Sci USA. 1994;91:6707–6711. doi: 10.1073/pnas.91.14.6707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Herrath MG, Dockter D, Oldstone MBA. How virus induces a rapid or slow onset insulin-dependent diabetes mellitus in a transgenic model. Immunity. 1994;1:231–242. doi: 10.1016/1074-7613(94)90101-5. [DOI] [PubMed] [Google Scholar]
- 43.Antonia SJ, Geiger T, Miller J, Flavell RA. Mechanisms of immune tolerance induction through the thymic expression of a peripheral tissue-specific protein. Int Immunol. 1994;7:715–725. doi: 10.1093/intimm/7.5.715. [DOI] [PubMed] [Google Scholar]
- 44.Husbands SD, Schönrich G, Arnold B, Chandler PR, Simpson E, Philipott KL, Tomlinson P, O'Reilly L, Cooke A, Mellor AL. Expression of major histocompatibility complex class I antigens at low levels in the thymus induces T cell tolerance via a non-deletional mechanism. Eur J Immunol. 1994;22:2655–2661. doi: 10.1002/eji.1830221027. [DOI] [PubMed] [Google Scholar]
- 45.Sponaas AM, Tomlinson PD, Antoniou J, Auphan N, Langlet C, Malissen B, Schmitt-Verhulst A, Mellor AL. Induction of tolerance to self MHC class I molecules expressed under the control of milk protein or beta-globin gene promoters. Int Immunol. 1994;6:277–287. doi: 10.1093/intimm/6.2.277. [DOI] [PubMed] [Google Scholar]
- 46.Schönrich G, Momburg F, Hämmerling GJ, Arnold B. Anergy induced by thymic medullary epithelium. Eur J Immunol. 1992;22:1687–1691. doi: 10.1002/eji.1830220704. [DOI] [PubMed] [Google Scholar]
- 47.Morahan G, Brennan FE, Bathal PS, Allison J, Cox KO, Miller JFAP. Expression in transgenic mice of class I histocompatibility antigens controlled by the metallothionein promoter. Proc Natl Acad Sci USA. 1989;86:3782–3786. doi: 10.1073/pnas.86.10.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferber I, Schönrich G, Schenkel J, Mellor AL, Hämmerling GJ, Arnold B. Levels of peripheral T cell tolerance induced by different doses of antigen. Science. 1994;263:274–276. doi: 10.1126/science.8303275. [DOI] [PubMed] [Google Scholar]
- 49.Mathisen PM, Pease S, Garvey J, Hood L, Readhead C. Identification of an embryonic isoform of myelin basic protein that is expressed widely in the mouse embryo. Proc Natl Acad Sci USA. 1993;90:10125–10129. doi: 10.1073/pnas.90.21.10125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pribyl TM, Campagnoni C, Kampf K, Handley VW, Campagnoni AT. The major myelin protein genes are expressed in the human thymus. J Neurosci Res. 1996;45:812–819. doi: 10.1002/(SICI)1097-4547(19960915)45:6<812::AID-JNR18>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 51.Fritz RB, Zhao M-L. Thymic expression of myelin basic protein (MBP) J Immunol. 1996;157:5249–5253. [PubMed] [Google Scholar]
- 52.Kojima K, Reindl M, Lassmann H, Wekerle H. The thymus and self tolerance: co-existence of encephalitogenic S100β-specific T cells and their nominal autoantigen in the normal adult rat thymus. Int Immunol. 1997;9:897–904. doi: 10.1093/intimm/9.6.897. [DOI] [PubMed] [Google Scholar]
- 53.Kirchner T, Tzartos S, Hoppe F, Schalke B, Wekerle H, Müller-Hermelink HK. Pathogenesis of myasthenia gravis. Acetylcholine receptor-related antigenic determinants in tumor-free thymuses and thymic epithelial tumors. Am J Pathol. 1988;130:268–280. [PMC free article] [PubMed] [Google Scholar]
- 54.Egwuagu CE, Charukamnoetkanok P, Gery I. Thymic expression of autoantigens correlates with resistance to autoimmune disease. J Immunol. 1997;159:3109–3112. [PubMed] [Google Scholar]
- 55.Martens H, Goxe B, Geenen V. The thymic repertoire of neuroendocrine self-antigens: physiological implications in T-cell life and death. Immunol Today. 1996;17:312–317. doi: 10.1016/0167-5699(96)10023-2. [DOI] [PubMed] [Google Scholar]
- 56.Pugliese A, Zeller M, Fernandez A, Jr, Zalcberg LJ, Bartlett RJ, Ricordi C, Pietrapaolo M, Eisenbarth GS, Bennet ST, Patel DD. The insulin gene is transcribed in the human thymus and transcription levels correlate with allelic variation at the INS VNTR-IDDM2 susceptibility locus for type 1 diabetes. Nat Genet. 1997;15:293–297. doi: 10.1038/ng0397-293. [DOI] [PubMed] [Google Scholar]
- 57.Vafiadis P, Bennet ST, Todd JA, Nadeau J, Grabs R, Goodyer CG, Wickramasinghe S, Colle E, Polychronakos C. Insulin expression in human thymus is modulated by INS VNTR alleles at the IDDM2 locus. Nat Genet. 1997;15:289–292. doi: 10.1038/ng0397-289. [DOI] [PubMed] [Google Scholar]
- 58.Smith KM, Olson DC, Hirose R, Hanahan D. Pancreatic gene expression in rare cells of the thymic medulla: evidence for functional contribution to T cell tolerance. Int Immunol. 1997;9:1355–1365. doi: 10.1093/intimm/9.9.1355. [DOI] [PubMed] [Google Scholar]
- 59.Hoffmann MW, Allison J, Miller JFAP. Tolerance induction by thymic medullary epithelium. Proc Natl Acad Sci USA. 1992;89:2526–2530. doi: 10.1073/pnas.89.7.2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Degermann S, Surh CD, Glimcher H, Sprent J. B7 Expression on thymic medullary epithelium correlates with epithelium mediated deletion of Vβ5+thymocytes. J Immunol. 1994;152:3254–3263. [PubMed] [Google Scholar]
- 61.Oukka M, Colucci-Guyon E, Lan P, Tran, Cohen-Tannoudji M, Babinet C, Lotteau V, Kosmatopoulos K. CD4 T cell tolerance to nuclear proteins induced by medullary thymic epithelium. Immunity. 1996;4:545–553. doi: 10.1016/s1074-7613(00)80481-1. [DOI] [PubMed] [Google Scholar]
- 62.Humblet C, Rudensky AY, Kyewski BA. Presentation and intercellular transfer of self antigen within the thymic microenvironment: expression of the Eα peptide–I-Abcomplex by isolated thymic stromal cells. Int Immunol. 1994;12:1949–1958. doi: 10.1093/intimm/6.12.1949. [DOI] [PubMed] [Google Scholar]
- 63.Spain LM, Berg LJ. Developmental regulation of thymocyte susceptibility to deletion by “self”-peptide. J Exp Med. 1992;176:213–223. doi: 10.1084/jem.176.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Niewenhuis P, Set RJM, Wagenaar JPA, Wubbena AS, Kampinga J, Karrenbeld A. The transcapsular route: a new way for (self-) antigens to by-pass the blood-thymus barrier? . Immunol Today. 1988;9:372–375. doi: 10.1016/0167-5699(88)91236-4. [DOI] [PubMed] [Google Scholar]
- 65.Heath WR, Allison J, Hoffmann MW, Schönrich G, Hämmerling GJ, Arnold B, Miller JFAP. Autoimmune diabetes as a consequence of locally produced interleukin-2. Nature. 1992;359:547–550. doi: 10.1038/359547a0. [DOI] [PubMed] [Google Scholar]
- 66.Oehen S, Feng L, Xia Y, Surh CD, Hedrick SM. Antigen compartmentation and T helper cell tolerance induction. J Exp Med. 1996;183:2617–2626. doi: 10.1084/jem.183.6.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wack A, Ladyman HM, Williams O, Roderick K, Ritter MA, Kioussis D. Direct visualization of thymocyte apoptosis in neglect, acute and steady-state negative selection. Int Immunol. 1996;8:1537–1548. doi: 10.1093/intimm/8.10.1537. [DOI] [PubMed] [Google Scholar]
- 68.Botto M, Hawkins PN, Bickerstaff MCM, Herbert J, Bygrave AE, McBride A, Hutchinson WL, Tennent GA, Walport MJ, Pepys MB. Amyloid deposition is delayed in mice with targeted deletion of the serum amyloid Pcomponent gene. Nat Med. 1997;3:855–859. doi: 10.1038/nm0897-855. [DOI] [PubMed] [Google Scholar]