The thymus is organized into discrete subcapsular, cortical, and medullary environments that have been defined by the distribution of morphologically and phenotypically distinct populations of epithelial cells and other stromal cell constituents (1–5). T cell progenitor cells entering the thymus move in a centripetal fashion from the subcapsular region through the cortex to the medullary area during their maturation process. Proximal stages of thymocyte development thought to occur in the subcapsular and cortical compartments include expansion of the progenitor pool and expression of pre-TCR, which in turn selects thymocytes with a functional TCR-β chain to undergo further maturation and to become CD4+8+ (double positive, DP) thymocytes expressing low levels of TCR-α/β. Further development of DP thymocytes is dependent on recognition of MHC–peptide ligands expressed by cortical thymic epithelial cells. DP thymocytes expressing receptors of appropriate specificity for these ligands exhibit some phenotypic characteristics of activation and downregulate expression of either CD4 or CD8 to yield mature CD4+ or CD8+ single positive thymocytes (6).
Concurrent with this process of positive selection, self-reactive thymocytes are eliminated. The anatomic setting for this process within the thymus is variable and appears to be dependent on the qualitative and quantitative parameters of ligand expression. Extensive cortical deletion has been observed in some TCR transgenic mouse models, whereas in others deletion was more evident in corticomedullary and medullary compartments (7). Hematogenously derived dendritic cells localized to the inner cortex and corticomedullary interface represent one major cell type able to mediate clonal deletion in the thymus. Several transgenic model systems have also pointed to a role for medullary thymic epithelium in this process (8–10), although the extent of functional overlap of these different cell types in their ability to mediate negative selection remains unclear.
A significant constraint on the process of thymic negative selection for a given self-protein is a requirement for expression of the cognate peptide–MHC complex by thymic APCs, either as a consequence of expression of this particular protein by the thymic APCs or due to adequate levels in the circulation. Although such a requirement would be compatible with negative selection of T cells specific for some self molecules, it poses a challenge for thymic elimination of T cells specific for self-proteins expressed in developmentally, temporally, or spatially regulated patterns, such as inducible tissue–specific proteins, hormones that exhibit oscillating levels in the circulation, or proteins whose pattern of expression are under tight developmental control. It has been proposed that T cells reactive to such autoantigens are inactivated in the periphery (11).
An alternative mechanism of central tolerance to one such autoantigen is reported in this issue (12). Klein et al. examined the mechanism of CD4 T cell tolerance to C-reactive protein, a prototype inducible serum protein produced in the liver during acute phase reaction. In this study, mice expressing human C-reactive protein (hCRP) transgene under autologous regulatory elements were crossed with two strains of mice expressing transgenic TCRs specific for dominant and subdominant hCRP epitopes. Based on their initial observation of a profound deletion of CD4+ thymocytes in these mice irrespective of hCRP serum levels, the authors examined tissue specificity of transgene expression and found hCRP mRNA in medullary thymic epithelial cells in addition to hepatocytes. The physiological relevance of hCPR expression by medullary thymic epithelium (TE) was supported by similarities in the regulation of hCRP expression by medullary TE and hepatocytes and by the demonstration of CRP message in human medullary thymic epithelium and homologous acute phase proteins in medullary thymic epithelium in mice. The demonstration by Klein et al. that the murine homologue of human CRP is expressed by medullary TE in a physiologically regulated manner, together with previous studies indicating thymic expression of a wide spectrum of molecules expressed by other organs and tissues of ectodermal or endodermal origin, indicate that expression of extrathymic “tissue-specific” genes in the thymus is more prevalent than previously appreciated and is of importance for the central tolerance induction.
The association of numerous neuroendocrine peptides such as vasopressin and oxytocin with thymic epithelial cells in situ (13), and the production of some of these hormones by thymic epithelial cell lines in vitro (14), has led to the consideration of the thymus as an endocrine organ. Additional proteins that are normally associated with other tissues/cell types and have been localized to the thymus at the mRNA level include insulin (15), interphotoreceptor binding protein (16), and myelin basic protein (17), as well as the precursor of peptide amidating enzymes necessary to generate biologically active regulatory peptide hormones (peptidyl-glycine a-amidating monooxygenase [PAM]; reference 14). Because of the diversity of these “tissue-specific” molecules detected within the thymus and the observation that thymic and hepatic CRP expression appears to be similarly regulated (12), thymic expression of these genes may reflect a general physiological property of medullary thymic epithelium. Thus, as a consequence, medullary thymic epithelium displays within the thymus a mosaic of epithelial molecules also expressed by other epithelial tissues and organs.
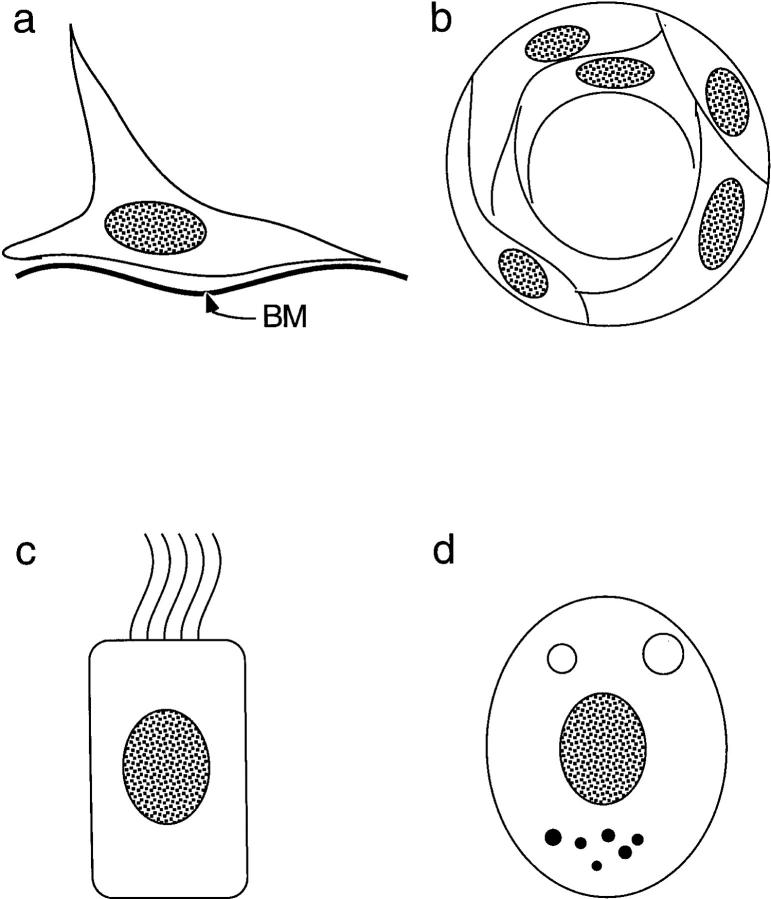
Epithelial cells comprising the medullary compartment exhibit considerably more morphological heterogeneity than do their cortical counterparts. Several distinctive epithelial cell types found in the medulla are depicted in Fig. 1. One subset is associated with basal laminae of major medullary blood vessels, but continue out through the cortex along connective tissue septae and are continuous with subcapsular epithelial cells that share the basal lamina of the thymic capsule. These cells can be identified by their reactivity with the 8.1.1 mAb, which detects a 36-KD cell surface molecule (18). Additional medullary epithelial subsets ultrastructurally resemble other types of terminally differentiated epithelium, including whorls of stratifying epithelium reminiscent of ectodermally derived epidermal epithelium (Hassal's bodies), ciliated epithelium, similar to that observed in proximal segments of the respiratory system and in the reproductive system derived from endoderm (19), and epithelial cells containing small electron-dense vesicles resembling neurosecretory cells found in endocrine organs and the diffuse neuroendocrine system (4). Phenotypic heterogeneity of medullary TE has also been well documented, with shared antigenicity with a variety of cell types, including keratinocytes (20), neuroendocrine cells (21), and neural crest cells (22). Medullary epithelium has recently been shown to express class Ib HLA-G molecules, which are also expressed by fetal trophoblasts (23).
Figure 1.
Distinctive epithelial components of the medullary thymic environment. (a) Epithelial cells lacking ultrastructural features of terminally differentiated epithelium and associated with basal laminae associated with major blood vessels in the medulla. These cells morphologically and phenotypically resemble subcapsular epithelium. (b) Hassal's bodies consisting of concentric whorls of stratified keratinizing epithelium. Well developed in human thymus, they are small and relatively sparse in the murine thymus. (c) Ciliated columnar epithelium is often associated with cystic structures in the medulla. Occasional cells possess large ciliated vacuoles. (d) Epithelial cells considered to be neuroendocrine, based on ultrastructural similarities of small electron-dense cytoplasmic granules with those observed in bona fide neurosecretory cells. These cells often have distinctive cytoplasmic vacuoles with microvilli projecting into the lumen of the vacuoles.
What is the mechanism underlying the expression of “extrathymic” gene products by thymic epithelial cells and how is this expression regulated? Although a generalized derepression or “loose” transcriptional control of genes normally expressed by differentiated epithelial cells of different lineages cannot be excluded, the regulated nature of CRP expression by medullary TE as reported by Klein et al. (12) would argue against such a mechanism. We favor the hypothesis that expression of these molecules reflects differentiation of medullary TE along different epithelial lineages. If expression of differentiated epithelial gene products reflects differentiation of medullary TE along different lineage-restricted pathways, these proteins should be heterogeneously expressed with different gene products associated with distinct subsets of medullary thymic epithelium. Consistent with this hypothesis, thymic expression of PAM is restricted to small scattered foci of medullary TE (14), as are cells expressing high molecular weight keratins associated with terminally differentiated keratinocytes (24).
Expression of these molecules may represent the intrinsic developmental potential of medullary thymic epithelium, perhaps reflecting their embryological derivation. A widespread distribution of endocrine cells throughout other tissues and organs has become increasingly apparent with the development of appropriate antibody and nucleic acid reagents, although the embryological origins of these cells, their developmental history, and function remain poorly understood. Another nonexclusive possibility is that local stimuli generated within the thymus play an important role in this process. Previous studies have demonstrated that interactions between thymocytes and thymic stromal cells control not only T cell development and selection but also medullary epithelial cell development and the organization of the medullary compartment. The medullary compartment is present but fails to organize properly in mutant mice unable to productively generate CD3+ thymocytes (25) and is also disrupted in some lines of TCR transgenic mice (26). One aspect of medullary epithelial development affected by these interactions may be the generation of medullary TE expressing phenotypic, morphologic, and/or functional properties usually associated with other epithelial tissues or organs. In this case, the generation of CD3+ T cells required for the organization and differentiation of medullary TE and the resulting expression of “extrathymic” epithelial molecules by these cells could provide positive feedback to facilitate the clonal elimination of thymocytes with reactivity to the epithelial antigens expressed with the thymus. Circumstantial evidence that supports this possibility is the observation that a small population of medullary epithelial cell identified with the 10.1.1 mAb in normal murine thymi (27) is lacking in the poorly organized medullary thymic compartment of SCID or RAG−/− mice (Farr, A., unpublished observations). The high levels of PAM expressed by 10.1.1+ thymic epithelial cells and the ability of these cells to secrete an amidated peptide hormone, oxytocin, which is classically associated with the hypothalamohypophyseal tract (14), would indicate that these cells represent a subset of epithelial cells that have differentiated along a neuroendocrine pathway. A more extensive analysis of the cellular composition of the medullary TE in these mutant mice should indicate the extent to which normal T cell development affects medullary expression of “extrathymic” epithelial gene products.
Expression of the “extrathymic” gene products by thymic medullary epithelium alone does not warrant presentation of peptides derived from these proteins by MHC class II molecules. As suggested by Klein et al., secreted proteins such as CRP can be taken up and presented by professional APCs, namely thymic dendritic cells (12). However, cellular proteins which are not secreted or are secreted at a very low level by thymic medullary cells are unlikely to be presented by thymic DC. Can medullary epithelial cells themselves induce tolerance in developing thymocytes? Experiments using chimeric transgenic mice with a restricted expression of MHC ligands in thymic medulla revealed tolerance induction by both deletional and nondeletional mechanisms with a variety of different antigens (8–10). Furthermore, medullary epithelial cell lines induced with γ-IFN are able to process and present exogenous protein antigens in an MHC class II–restricted fashion (28, 29). A closer examination of the antigen-presenting properties of medullary epithelial elements in situ and analyses of ex vivo isolated cells revealed significant degree of heterogeneity. Only a subset of medullary epithelial cells express MHC class II molecules (1, 30). These cells are apparently capable of processing and presenting at least some endogenous proteins as suggested by staining with mAbs specific for a major self peptide–MHC class II complex (31). However, class II–positive medullary epithelial cells differ significantly in their antigen-presenting properties as evidenced by highly variable expression of MHC class II bound invariant chain peptide CLIP, an essential intermediate of MHC class II processing (31). Finally, only a subset of medullary epithelial cells expresses the CD28 ligand B7 (9, 32), although the functional significance of CD28–B7 interaction in negative selection of thymocytes remains unclear.
Thus, thymic medullary epithelium is composed of diverse epithelial elements with different antigen-presenting potential and that display a mosaic of “peripheral” self-proteins. The functional importance of thymic expression of such proteins in tolerance induction is indicated by recent observations linking the level of expression of such autoantigens in the thymus and the predisposition to related autoimmune diseases (16, 32, 33). Further experimentation is needed to understand the role of specific subsets of thymic medullary epithelial cells in establishment of the central tolerance to peripheral autoantigens with restricted tissue distribution or inducible expression.
References
- 1.Kingston R, Jenkinson EJ, Owen JJT. Characterization of stromal cell populations in the developing thymus of normal and nude mice. Eur J Immunol. 1984;14:1052–1056. doi: 10.1002/eji.1830141117. [DOI] [PubMed] [Google Scholar]
- 2.Rouse RV, Bolin LM, Bender JR, Kyewski BA. Monoclonal antibodies reactive with subsets of mouse and human thymic epithelial cells. J Histochem Cytochem. 1988;36:1511–1517. doi: 10.1177/36.12.2461413. [DOI] [PubMed] [Google Scholar]
- 3.Kaneshima H, Ito M, Asai J, Taguchi O. Thymic epithelial reticular cell subpopulations in mice defined by monoclonal antibodies. Lab Invest. 1987;56:372–380. [PubMed] [Google Scholar]
- 4.Mandel T. Differentiation of epithelial cells in the mouse thymus. Z Zellforsch Mikrosk Anat. 1970;106:498–515. doi: 10.1007/BF00340288. [DOI] [PubMed] [Google Scholar]
- 5.van Vliet E, Melis M, van Ewijk W. Monoclonal antibodies to stromal cell types in the mouse thymus. Eur J Immuol. 1984;14:524–529. doi: 10.1002/eji.1830140608. [DOI] [PubMed] [Google Scholar]
- 6.Fink PJ, Bevan MJ. Positive selection of thymocytes. Adv Immunol. 1995;59:99–133. doi: 10.1016/s0065-2776(08)60630-6. [DOI] [PubMed] [Google Scholar]
- 7.Morishima C, Norby-Slycord C, McConnell KR, Finch RJ, Nelson AJ, Farr AG, Pullen AM. Expression of two structurally identical viral superantigens results in thymic elimination at distinct developmental stages. J Immunol. 1994;153:5091–5103. [PubMed] [Google Scholar]
- 8.Hoffmann MW, Allison J, Miller JFAP. Tolerance induction by thymic medullary epithelium. Proc Natl Acad Sci USA. 1992;89:2526–2530. doi: 10.1073/pnas.89.7.2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Degermann S, Surh CD, Glimcher LH, Sprent J, Lo D. B7 expression on thymic medullary epithelium correlates with epithelium-mediated deletion of Vβ5+ thymocytes. J Immunol. 1994;152:3254–3263. [PubMed] [Google Scholar]
- 10.Oukka M, Colucci-Guyton E, Tran PL, Cohen-Tannoudji M, Babinet C, Lotteau V, Kosmatopoulos K. CD4 T cell tolerance to nuclear proteins induced by medullary thymic epithelium. Immunity. 1996;4:543–553. doi: 10.1016/s1074-7613(00)80481-1. [DOI] [PubMed] [Google Scholar]
- 11.Hammerling GJ, Schonrich G, Ferber I, Arnold B. Peripheral tolerance as a multi-step mechanism. Immunol Rev. 1993;133:83–104. doi: 10.1111/j.1600-065x.1993.tb01511.x. [DOI] [PubMed] [Google Scholar]
- 12.Klein L, Klein T, Rüther U, Kyewski B. CD4 T cell tolerance to human C-reactive protein, an inducible serum protein, is mediated by medullary thymic epithelium. J Exp Med. 1998;188:5–16. doi: 10.1084/jem.188.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robert F, Geenen V, Schoenen J, Burgeon E, DeGroote D, Defresne MP, Legros JJ, Franchiment P. Colocalization of immunoreactive oxytocin, vasopressin and interleukin-1 in human thymic epithelial neuroendocrine cells. Brain Behav Immun. 1991;5:102–115. doi: 10.1016/0889-1591(91)90010-8. [DOI] [PubMed] [Google Scholar]
- 14.Martinez A, Farr A, Vos MD, Cuttitta F, Treston AM. Peptide-amidating enzymes are expressed in the stellate epithelial cells of the thymic medulla. J Histochem Cytochem. 1998;46:661–668. doi: 10.1177/002215549804600511. [DOI] [PubMed] [Google Scholar]
- 15.Jolicoeur C, Hanahan D, Smith KM. T-cell tolerance toward a transgenic B-cell antigen and transcription of endogenous pancreatic genes in thymus. Proc Natl Acad Sci USA. 1994;91:6707–6711. doi: 10.1073/pnas.91.14.6707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Egwuagu CE, Charukamnoetkanok P, Gery I. Thymic expression of autoantigens correlates with resistance to autoimmune disease. J Immunol. 1997;159:3109–3112. [PubMed] [Google Scholar]
- 17.Mathisen PM, Pease S, Garvey J, Hood L, Readhead C. Identification of an embryonic isoform of myelin basic protein that is expressed widely in the mouse embryo. Proc Natl Acad Sci USA. 1993;90:10125–10129. doi: 10.1073/pnas.90.21.10125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farr A, Nelson A, Hosier S. Characterization of an antigenic determinant preferentially expressed by type 1 epithelial cells in the murine thymus. J Histochem Cytochem. 1992;40:651–664. doi: 10.1177/40.5.1374092. [DOI] [PubMed] [Google Scholar]
- 19.Khosla S, Ovalle WK. Morphology and distribution of cystic cavities in the normal murine thymus. Cell Tissue Res. 1986;246:531–542. doi: 10.1007/BF00215193. [DOI] [PubMed] [Google Scholar]
- 20.Patel DD, Whichard LP, Radcliff G, Denning SM, Haynes BF. Characterization of human thymic epithelial cell surface antigens: phenotypic similarity of thymic epithelial cells to epidermal keratinocytes. J Clin Immunol. 1995;15:80–92. doi: 10.1007/BF01541736. [DOI] [PubMed] [Google Scholar]
- 21.Haynes BF, Shimizu K, Eisenbarth GS. Identification of human and rodent thymic epithelium using tetanus toxin and monoclonal antibody A2B5. J Clin Invest. 1983;71:9–14. doi: 10.1172/JCI110755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bodey B, Bodey B, Jr, Siegel SE, Kemshead JT, Kaiser HE. Identification of neural crest derived cells within the cellular microenvironment of the human thymus employing a library of monoclonal antibodies raised against neuronal tissues. In Vivo. 1996;10:39–47. [PubMed] [Google Scholar]
- 23.Crisa L, McMaster MT, Ishii JK, Fisher SJ, Salomon DR. Identification of a thymic epithelial cell subset sharing expression of the class Ib HLA-G molecule with fetal trophoblasts. J Exp Med. 1997;186:289–298. doi: 10.1084/jem.186.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nicolas JF, Savino W, Reano A, Viac J, Brochier J, Dardenne M. Heterogeneity of thymic epithelial cell (TEC) keratins. Immunohistochemical and biochemical evidence for a subset of highly differentiated TEC in the mouse. J Histochem Cytochem. 1985;33:687–694. doi: 10.1177/33.7.2409128. [DOI] [PubMed] [Google Scholar]
- 25.Shores EW, Van Ewijk W, Singer A. Disorganization and restoration of thymic medullary epithelial cells in T cell receptor–negative scid mice: evidence that receptor-bearing lymphocytes influence maturation of the thymic microenvironment. Eur J Immunol. 1991;21:1657–1661. doi: 10.1002/eji.1830210711. [DOI] [PubMed] [Google Scholar]
- 26.Goverman J, Brabb T, Huseby ES, Farr AG. TCR signaling regulates thymic organization: lessons from TCR-transgenic mice. Immunol Today. 1997;18:204–208. doi: 10.1016/s0167-5699(97)01055-4. [DOI] [PubMed] [Google Scholar]
- 27.Farr A, Nelson A, Hosier S, Kim A. A novel cytokine-responsive cell surface glycoprotein defines a subset of medullary thymic epithelium in situ. J Immunol. 1993;150:1160–1171. [PubMed] [Google Scholar]
- 28.Mizuochi T, Kasai M, Kokuho T, Kakiuchi T, Hirokawa K. Medullary but not cortical thymic epithelial cells present soluble antigens to helper T cells. J Exp Med. 1992;175:1601–1605. doi: 10.1084/jem.175.6.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oukka M, Andre P, Turmel P, Besnard N, Angevin V, Karlsson L, Trans PL, Charron D, Bihain B, Kosmatopoulos K, Lotteau V. Selectivity of the major histocompatibility complex class II presentation pathway of cortical thymic epithelial cell lines. Eur J Immunol. 1998;27:855–859. doi: 10.1002/eji.1830270408. [DOI] [PubMed] [Google Scholar]
- 30.Surh CD, Gao EK, Kosaka H, Lo D, Ahn C, Murphy DB, Karisson L, Peterson P, Sprent J. Two subsets of epithelial cells in the thymic medulla. J Exp Med. 1992;176:495–505. doi: 10.1084/jem.176.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farr A, DeRoos P, Eastman S, Rudensky A. Differential expression of CLIP:MHC class II and conventional endogenous peptide:MHC class II complexes by thymic epithelial cells and peripheral antigen presenting cells. Eur J Immunol. 1996;26:3185–3193. doi: 10.1002/eji.1830261252. [DOI] [PubMed] [Google Scholar]
- 32.Nelson AJ, Hosier S, Brady W, Linsley PS, Farr AG. Medullary thymic epithelium expresses a ligand for CTLA4 in situ and in vitro. J Immunol. 1993;151:2453–2461. [PubMed] [Google Scholar]
- 33.Vafiadis P, Bennett ST, Todd JA, Nadeau J, Grabs R, Goodyer CG, Wickramasinghe S, Colle E, Polychronakos C. Insulin expression in human thymus is modulated by INS UNTR alleles at the IDDM2 locus. Nat Genet. 1997;15:289–292. doi: 10.1038/ng0397-289. [DOI] [PubMed] [Google Scholar]
- 34.Pugliese A, Zeller M, Fernandez A, Jr, Zalcberg LJ, Bartlett RJ, Ricordi C, Pietropaolo M, Eisenbarth GS, Bennett ST, Patel DD. The insulin gene is transcribed in the human thymus and transcription levels correlated with allelic variation at the INS UNTR-IDDM2 susceptibility locus for type 1 diabetes. Nat Genet. 1997;15:293–297. doi: 10.1038/ng0397-293. [DOI] [PubMed] [Google Scholar]