Abstract
Symptomatic primary Epstein-Barr virus (EBV) infection and elevated humoral immune responses to EBV are associated with an increased risk of developing multiple sclerosis (MS). We explored mechanisms leading to this change in EBV-specific immunity in untreated patients with MS and healthy virus carriers matched for MS-associated HLA alleles. MS patients showed selective increase of T cell responses to the EBV nuclear antigen 1 (EBNA1), the most consistently recognized EBV-derived CD4+ T cell antigen in healthy virus carriers, but not to other EBV-encoded proteins. In contrast, influenza and human cytomegalovirus–specific immune control was unchanged in MS. The enhanced response to EBNA1 was mediated by an expanded reservoir of EBNA1-specific central memory CD4+ T helper 1 (Th1) precursors and Th1 (but not Th17) polarized effector memory cells. In addition, EBNA1-specific T cells recognized myelin antigens more frequently than other autoantigens that are not associated with MS. Myelin cross-reactive T cells produced IFN-γ, but differed from EBNA1-monospecific cells in their capability to produce interleukin-2, indicative of a polyfunctional phenotype as found in controlled chronic viral infections. Our data support the concept that clonally expanded EBNA1-specific CD4+ T cells potentially contribute to the development of MS by cross-recognition of myelin antigens.
Multiple sclerosis (MS) is an inflammatory, demyelinating disease of the central nervous system, believed to be initiated and mediated by autoreactive T cells directed against myelin antigens. It develops in young adults with a complex genetic predisposition and is thought to require an inciting environmental insult such as a viral infection to trigger the disease (1). So far, EBV stands out as the infectious agent for which there is the most compelling evidence for an association with MS (2–7).
The evidence implicating EBV in MS development includes the observation that individuals with a history of symptomatic primary EBV infection or infectious mononucleosis have a more than twofold increased risk of developing MS compared with subjects who acquired EBV without symptoms (4, 5). Adults and children with MS are universally, i.e., nearly 100%, seropositive for EBV compared with seropositivity rates of 90–95% in adults (2, 6) and <85% in age-matched pediatric cohorts (3). In addition, a marked increase in serum antibody titers to EBV nuclear antigens (EBNAs) several years before the onset of first symptoms suggests an involvement of EBV early in the pathogenesis of MS (2, 6). Finally, EBV-infected B cells have recently been found to be significantly enriched in tertiary lymphoid tissues in postmortem brain samples from patients with MS, but not in other inflammatory central nervous system diseases (8).
EBV-specific immunity in MS and the mechanisms by which EBV infection increases the risk for MS are not well characterized and are poorly understood. We investigated cellular and humoral immune responses to a broad panel of EBV-encoded proteins, as well as to other ubiquitous viruses, i.e., human cytomegalovirus (HCMV) and influenza A virus in 24 untreated patients with MS and 24 healthy EBV carriers. Our data indicate that EBNA1-specific CD4+ Th1 cells are selectively expanded in MS and that some of these have the ability to cross-recognize MS-associated myelin antigens.
RESULTS AND DISCUSSION
Selective increase of EBNA1-specific T cell responses in MS
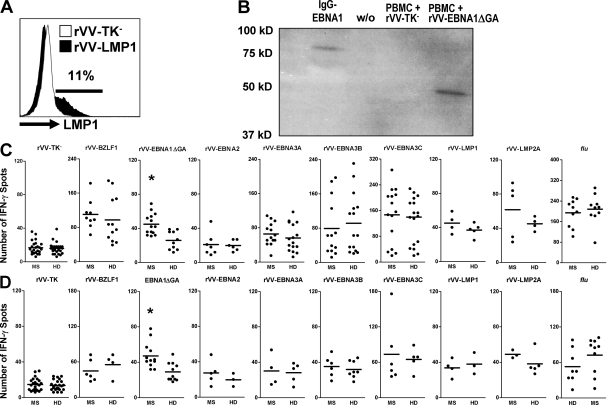
To evaluate T cell responsiveness to EBV latency gene products in untreated patients with MS versus healthy virus carriers (Table S1, available at http://www.jem.org/cgi/content/full/jem.20072397/DC1), PBMCs were separately infected with recombinant vaccinia virus (rVV) constructs expressing the latent EBV nuclear antigens EBNA1, 2, 3A, 3B, 3C, latent membrane protein 1 (LMP1), LMP2A, and the lytic EBV-encoded protein BZLF1. Responses were assessed by IFN-γ–specific ELISPOT assays and compared with the virus vector backbone (rVV-TK−) to influenza A virus (A/Aichi/68; H3N2) infection and to superantigen stimulation (SEB).
Among all rVV-EBV antigen constructs and influenza products tested in 24 MS patients and 24 healthy EBV carriers, EBNA1 was the only differentially recognized T cell antigen (Fig. 1 B). rVV-EBV nuclear antigens were consistently recognized by both MS patients (20/24 for EBNA3 and 14/24 for EBNA1) and healthy virus carriers (18/24 for EBNA3 and 10/24 for EBNA1). The hierarchy of T cell–mediated latent EBV antigen recognition was preserved in patients and controls. EBNA3C-, EBNA3B-, and EBNA3A-specific T cells showed estimated precursor frequencies of (means ± SD) 0.023 ± 0.01%, 0.014 ± 0.01%, and 0.011 ± 0.004% of PBMCs, respectively. EBNA1-specific IFN-γ–producing T cells were detected at frequencies of 0.008 ± 0.002% of PBMC in patients with MS and 0.005 ± 0.001% of PBMC in healthy virus carriers (P = 0.005). Depletion of CD8+ T cells by magnetic cell separation resulted in a substantial decrease or abrogation of most rVV-EBNA3 antigen-specific IFN-γ responses, but had only a minimal effect on rVV-EBNA1–specific IFN-γ responses in both patients and controls (P = 0.003; Fig. 1 C and Table S2, available at http://www.jem.org/cgi/content/full/jem.20072397/DC1). In line with this, rVV-EBNA1–specific IFN-γ responses were substantially decreased after CD4+ T cell depletion in a subgroup of 12 patients and 12 controls, whereas rVV-EBNA3C–specific responses were still detectable in most donors that showed reactivity to rVV-EBNA3C in PBMCs (Fig. S1). Altogether, these data indicate a predominant expansion of the EBNA1-specific CD4+ T cell compartment in MS.
Figure 1.
Selective increase in rVV-EBNA1 recognition in MS. PBMCs from 24 MS patients and 24 healthy virus carriers were separately infected with rVV constructs expressing the EBV latent antigens EBNA1, 2, 3A, 3B, 3C, LMP1, and LMP2A, as well as the lytic EBV-encoded protein BZLF1. (A) Approximately 10–15% of PMBCs infected with the LMP1-encoding rVV consistently showed intracellular expression of LMP1 by flow cytometry, indicating that rVVs achieve EBV antigen expression in PBMCs. (B) EBNA1 protein expression in rVV-EBNA1ΔGA infected PBMCs compared with a fusion protein consisting of the C terminus of EBNA1 (aa 400–641) coupled to the heavy chains of an antibody (IgG-EBNA1). (C) IFN-γ specific ELISPOT responses in PBMCs to the indicated EBV antigens, to the virus vector backbone (rVV-TK−), and to influenza A virus (A/Aichi/68; H3N2) infection. Bars represent means. *, P = 0.005. (D) IFN-γ –specific ELISPOT responses to the indicated antigens, but in CD8-depleted PBMCs. *, P = 0.003.
We previously applied a flow cytometry-based intracellular IFN-γ staining assay to determine ex vivo frequencies of T cells specific for EBNA1 and a limited set of MHC class I–restricted EBV epitopes in different cohorts of MS patients and healthy controls. We found the frequencies of EBNA1-specific CD4+ T cells to be twofold higher in MS patients than healthy virus carriers (0.149 and 0.073% of total CD4+ T cells, respectively) (9). Similar to other groups (10), we did not detect significant differences in EBV-specific CD8+ T cell frequencies. EBNA1 is the most consistently recognized CD4+ T cell antigen in healthy virus carriers (11, 12) and elevated IgG titers to EBNA1, but not to EBNA2, EBV-encoded early antigens or the viral capsid antigen, are the strongest predictor of MS risk (2, 6). In this study, we extended our previous finding to a broad panel of EBV-encoded antigens, showing that EBNA1-specific CD4+ T cells are indeed selectively expanded in MS.
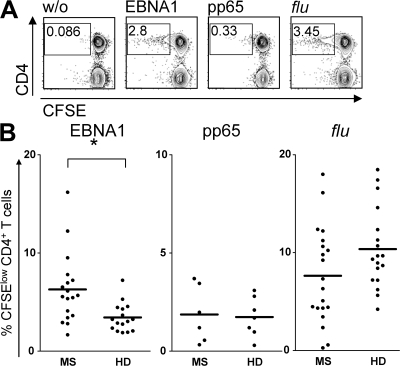
To further characterize if enhanced T cell responses selectively target EBNA1 in MS, we compared these to reactivities against other ubiquitous viral antigens. Using a flow cytometry-based CFSE-dilution assay, we analyzed EBNA1, HCMV-encoded phosphoprotein 65 (pp65)-antigen– and influenza A virus-specific CD4+ and CD8+ T cell responses in patients and healthy virus carriers matched for expression of MS-associated HLA-DR alleles. Both HCMV and influenza are ubiquitous pathogens; HCMV induces a latent infection with continuous virus production, and both HCMV-pp65 stimulation and influenza infection are known to elicit robust CD4+ T cell responses (13). Consistent with our data on IFN-γ secretion to rVV-EBNA1 infection, patients with MS showed significantly higher proliferative responses to the C-terminal domain of EBNA1 (aa 400–641), toward which most of the T cell responses of healthy virus carriers are directed (Fig. 2 B) (12). EBNA1-specific CD4+ T cell responses were detectable in 75% (18/24) of MS and 66% (16/24) of healthy donors, with mean proliferating T cell frequencies of 6.12 ± 3.2% (MS) and 3.38 ± 1.2% (HD; P = 0.004). CD4+ T cell responses to an overlapping peptide library covering the entire sequence of the HCMV-encoded pp65 protein (aa 1–561) were detectable in 25% (6/24) of MS and 29% (7/24) of healthy donors with similar frequencies of 1.87 ± 1.1% (MS) and 1.76 ± 0.7% (HD). Influenza-specific responses were detectable in 83% (20/24) of MS and 75% (18/24) of HD with frequencies of CFSE-diluted CD4+ T cells of 7.33 ± 5.1% (MS) and 10.43 ± 4.4% (HD). CD3+CD4−, presumably CD8+ T cell proliferation upon stimulation with any of the tested antigens did not significantly differ between patients and controls (Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20072397/DC1). These data further indicate that EBNA1-specific CD4+ T cell responses are selectively increased in MS.
Figure 2.
Increased CD4+ T cell proliferation to EBNA1, but not HCMV-pp65 or influenza, in MS. PBMCs from 24 MS patients and 24 healthy virus carriers were stimulated with overlapping peptide libraries spanning the C-terminal domain of EBNA1 (aa 400–641), with the entire sequence of the HCMV-encoded pp65 protein (aa 1–561), and by influenza A virus infection. Antigen-specific proliferative responses were determined in a flow cytometry–based CFSE-dilution assay. (A) An analysis of a representative blood donor. (B) Summary of all positive responses. *, P = 0.004. Bars represent means.
EBNA1-specific T cell subsets reflect frequent antigen recognition in MS
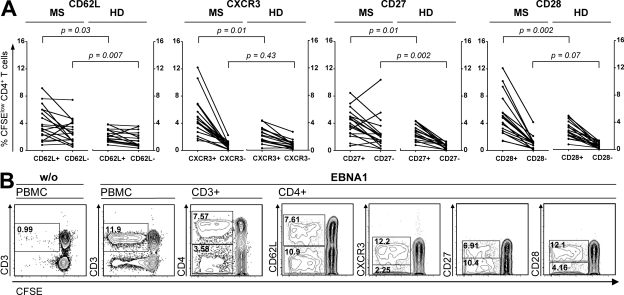
To identify which CD4+ T cell subsets contribute to the increased response to EBNA1, we determined the phenotype of CFSE-diluted T cells using markers indicative for central and effector memory compartments (CD45RO, CD62L), co-stimulation (CD27, CD28), Th1 commitment (CXCR3), and homing capacity and recruitment to secondary lymphoid organs and inflamed tissue (CD62L, CXCR3) (14). Nearly all proliferating EBNA1-specific CD4+ T cells in healthy virus carriers originated from the memory pool (CD45RO+) and consisted of both CD62L+ central memory (TCM) and CD62L− effector memory (TEM) T cell subsets (9, 15, 14). We have previously shown that EBNA1-specific T cells maintain their CD62L surface expression during a 6-d CFSE assay (14). As shown in Fig. 3 A, both memory compartments contributed to increased T cell response to EBNA1 (P = 0.007 for TEM and P = 0.03 for TCM) and the TEM expansion (2.1-fold) appeared to be slightly higher than TCM expansion (1.7-fold), indicating frequent antigen recognition in vivo.
Figure 3.
Phenotype of EBNA1-specific T cell subsets is indicative of frequent antigen recognition in MS. The phenotype of EBNA1-specific T cells was assessed on CFSElow cells proliferating in response to stimulation with the EBNA1 peptide library by CD27, CD28, CD62L, and CXCR3 flow cytometric staining in 24 MS patients and 24 healthy virus carriers. (A) Frequencies of the indicated subsets in MS patients (MS) and healthy controls (HD). (B) Analysis of a representative patient with MS.
CXCR3, the chemokine receptor for CXCL9/MIG, CXCL10/IP-10, and CXCL11/I-TAC, is preferentially expressed on activated Th1 T cells (15) and has been shown to play an important role in T cell trafficking to inflamed tissues (16). CXCR3 expression is consistently increased on CSF versus blood CD4+ memory T cells and CXCR3+ T cells and its ligand CXCL10/IP-10 are readily detectable in active MS lesions (17). We observed that both proliferating EBNA1-specific TCM and TEM cells were predominantly CXCR3+ (Fig. 3 A). The increased response in MS resulted from an exclusive expansion of CXCR3+, but not CXCR3−, CD4+ T cells (P = 0.01).
The majority of latent EBV antigen-specific CD4+ and CD8+ memory T cells accumulate within a CD27+CD28+ differentiation compartment during primary infection and remain enriched within this compartment throughout the persistent phase of infection (18). CD8+ T cells specific for lytic cycle antigens accumulate within both CD27+CD28+ and CD27−CD28+ compartments, indicating differing differentiation states and/or co-stimulatory requirements (18). The majority of EBNA1-specific T cells in both patients and controls were CD27+ CD28+ (Fig. 2 C). However, we observed that EBNA1-specific CD4+ T cells of some MS patients were enriched in the CD27−CD28+ compartment (Fig. 3 B), which was not detectable in healthy virus carriers. Initial up-regulation of CD27 expression upon TCR engagement of CD4+ T cells is followed by its irreversible loss after repeated antigenic stimulation, as observed in terminally differentiated memory T cells (19). Our data, therefore, suggest that the increased effector and central memory T cell response to EBNA1 in MS might generate a minor population of presumably further differentiated CD4+ T cells.
We had previously reported that EBNA1-specific Th1-polarized cells predominate the CD4+ T cell response (20). To additionally determine the Th1 versus Th17 lineage commitment of EBNA1-specific T cells, we applied an intracellular cytokine staining assay in CFSE-diluted T cells after antigen-specific proliferation. PBMCs from six patients with MS and six healthy virus carriers responding to EBNA1, were stimulated with purified Candida albicans antigens (21), EBNA1, or SEB. After 7 d, cells were restimulated with PMA/ionomycin for intracellular IL-17 detection. C. albicans–stimulated CD4+ T cells produced IL-17 at mean frequencies of 6.7% of CFSE-diluted cells (Fig. S3, available at http://www.jem.org/cgi/content/full/jem.20072397/DC1). In contrast, IL-17 could not be detected in any EBNA1-stimulated T cells, neither in patients with MS nor in healthy virus carriers, indicating that deregulated T cell response to EBNA1 in MS is mediated by Th1, and not by Th17, cells.
Collectively, these data suggest that patients with MS show an expanded reservoir of EBNA1-specific TCM CD4+ Th1 precursors, which might continuously fuel Th1-polarized effector cells that display a phenotype indicative of frequent antigen recognition and increased homing capacity to sites of inflammation.
Elevated titers and IgG1 polarization of EBNA1-specific antibodies in MS
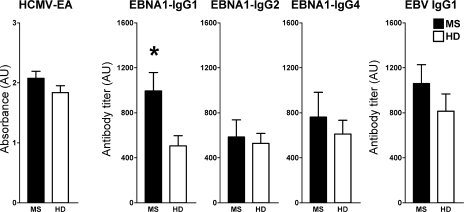
To further assess the relative activity of EBNA1-specific Th1 cells in vivo, we analyzed EBNA1-targeting IgG isotype-specific responses by ELISA assays with recombinant EBNA1 (20). Th1 cytokines are considered to skew antibody responses toward the IgG1 opsonizing and complement-fixing immunoglobulin subclass, and Th2 toward the allergic IgG4 subclass (20). As shown in Fig. 4, patients with MS showed significantly increased titers of EBNA1-specific IgG1 (P = 0.02), but not IgG2 or IgG4. EBNA1-specific IgG3 antibodies could neither be detected in HD, nor in MS patients (unpublished data). IgG1 was the most frequently detected isotype in all individuals tested, reflecting the Th1 polarization of EBNA1-specific T cell immunity. IgG2 and IgG4 responses to EBNA1 were detected in a minor subgroup of patients and controls with no statistically significant differences between both cohorts. There were also no differences in the IgG-response to HCMV. To further determine whether the increased antibody response is selectively skewed toward EBNA1 or reflects a broadly enhanced humoral immune response to EBV, we compared IgG1 responses to a standardized infected B cell lysate that predominantly contains lytic EBV antigens (18). Antibody reactivities to EBV-infected cell lysates tended to be higher in MS patients, but did not differ significantly between both cohorts, indicating a prominent increase of EBNA1-specific, but not generally of EBV-specific IgG responses in MS. Although EBNA1-specific IgG responses have previously been described to be prominently elevated in MS (2, 6), we show that this increase in humoral immunity to EBNA1 is mediated by IgG1 antibodies reflecting the Th1 commitment of EBNA1-specific T cell immunity in MS.
Figure 4.
Elevated titers of EBNA1-specific IgG1 antibodies in MS. EBNA1-specific IgG isotype-specific titers were determined by ELISA and compared with HCMV-EA IgG responses. The presented data summarize 20 MS patients and 16 healthy virus carriers. *, P = 0.02. Error bars represent the SD.
Efficient regression of EBV-infected B cell outgrowth in MS
Selectively deregulated immune responses to EBNA1 in MS could result from an increased availability of the viral antigen caused by heightened viral replication. Although there is evidence for an association between clinical disease activity and EBV reactivation in MS (7), we detected levels of cell-bound viral genomes in circulating blood cells that were not statistically different from healthy EBV carriers in a cross-sectional analysis (9). To assess if EBV immune control was intact in MS patients, resulting in an unchanged EBV load, we compared the B cell transformation rate and the inhibition of EBV-infected B cell outgrowth in a modified regression assay. CD19+ selected B cells from 16 patients with MS and 16 healthy virus carriers were infected with EBV supernatant overnight and incubated for 12 d with increasing concentration of CD19− effector cells. As shown in Fig. S4 (available at http://www.jem.org/cgi/content/full/jem.20072397/DC1), patients with MS did not differ from healthy EBV carriers in the rate of EBV-induced B cell transformation or in their ability to control EBV-infected B cell outgrowth in vitro. Thus, although high viral loads that occur during symptomatic primary infection are associated with an increased risk of developing MS (4, 5), our data do not provide evidence for an increased viral replication or impaired immune control of EBV infection in MS during chronic infection.
EBNA1-specific CD4+ T cells recognize myelin antigens
EBV-derived epitopes have previously been identified as molecular mimics of immunodominant myelin T cell antigens. Wucherpfennig and Strominger (22) and Lang et al. (23) characterized an epitope derived from the DNA-polymerase protein of EBV (BALF5), which showed strong agonistic activity in myelin basic protein (MBP)–specific (aa 83–99) T cells in the context of MS-associated restriction elements. However, it is not known whether BALF5-specific immune responses are part of the EBV-specific immune control in humans, and whether they qualitatively or quantitatively differ in patients with MS. Because we found that EBNA1-specific T cells are expanded in MS, we determined the frequency of myelin-recognizing EBNA1-specific T cells using a limiting dilution approach as outlined in Fig. S5 (available at http://www.jem.org/cgi/content/full/jem.20072397/DC1). Based on a precursor frequency of EBNA1-specific CD4+ T cells of 5–10 per 100,000 circulating mononuclear cells (9, 14), PBMCs were seeded at cell concentrations of 12,500, 25,000, and 50,000 per well in 96-well plates and stimulated with peptides covering the C-terminal domain of EBNA1 (aa 400–641) to obtain single-cell precursors in plates with <30% positive cultures (24). Single-cell precursor plates were restimulated after 10 d and expanded for an additional 12 d in the presence of low-dose IL-2 (10 IU/ml) and IL-7 (10 ng/ml). At day 22 of culture, EBNA1-specific T cells were transferred to a new plate and stimulated either with EBNA1, myelin antigen-derived peptides (n = 15), or peptides (n = 30) covering the entire sequence of the human proinsulin precursor protein (PIP, aa 1–110) at equimolar concentrations (1 μM). Myelin antigens were derived from 4 major myelin proteins, MBP, proteolipid protein, myelin oligodendrocyte glycoprotein, and 2′,3′-cyclic nucleotide 3′ phosphodiesterase (Table S3), and selected based on careful literature review as immunodominant in humans and/or encephalitogenic in animals (25). PIP was chosen because it is a prominent autoantigen expressed in the target tissue of type 1 diabetes (T1D) and CD4+ T cell responses to PIP have been implicated in the pathogenesis of T1D (26).
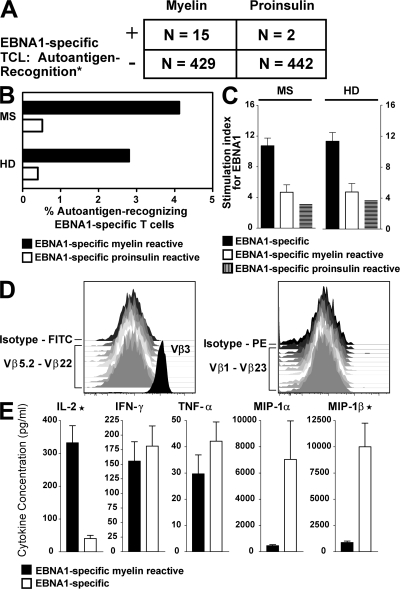
Under these conditions, the number of EBNA1-specific T cells recognizing myelin autoantigens was significantly higher compared with recognition of the control autoantigen (P = 0.01). Of a total of 444 EBNA1-specific T cell lines (TCLs) generated from 6 patients with MS and 6 healthy EBV carriers, 15 showed cross-recognition of myelin antigens corresponding to a precursor frequency of 3.37% myelin antigen-recognizing EBNA1-specific CD4+ T cells (Fig. 5, A and B). Cross-recognition of PIP could be detected in 2 TCLs only corresponding to a frequency of 0.45% of EBNA1-specific CD4+ T cells. Stimulation indices (SI) after EBNA1 stimulation of EBNA1-monospecific (mean ± SEM in MS vs. HD: 10.75 ± 0.9 vs. 11.4 ± 1.1; P = 0.09), EBNA1-specific myelin-reactive (mean ± SEM in MS vs. HD: 4.69 ± 0.9 vs. 4.7 ± 1.1; P = 0.85), and EBNA1-specific insulin-reactive (mean in MS vs. HD: 3.12 vs. 3.7) T cells did not differ between patients and controls (Fig. 5 C). SI after myelin antigen stimulation of EBNA1-specific T cells were moderately increased in patients (mean ± SEM: 2.93 ± 0.19) compared with controls (2.17 ± 0.08; P = 0.003). SI after insulin peptide stimulation was 2.9 and 3.4 in 2 TCLs derived form 1 patient and 1 healthy donor, respectively. Flow cytometric analysis of TCR Vβ chain expression demonstrated that five representative cross-reactive TCLs did not contain more than one Vβ clonotype and were homogenously CD4+CD8−, identifying the cross-reactive TCLs as EBNA1- and myelin-specific CD4+ T cell clones. Flow cytometric analysis for one Vβ3+ CD4+ T cell clone is shown in Fig. 5 D, and other clones were positive for Vβ2 or Vβ12 or did not stain with any of the TCR Vβ antibodies used (unpublished data). The frequency of autoantigen recognition within the EBNA1-specific T cell compartment did not differ significantly between patients and controls (Fig. 5 B).
Figure 5.
Autoantigen-recognition of EBNA1-specific T cells. Cross-reactivity of EBNA1-specific T cells to myelin antigens or proinsulin was analyzed. (A) Frequencies of EBNA1-specific TCLs generated from 6 patients with MS and 6 healthy EBV carriers recognizing peptides of myelin antigens or proinsulin. *, P = 0.01. (B) Frequencies of autoantigen-recognizing EBNA1-specific T cells in MS patients compared with healthy virus carriers. (C) Stimulation indices (SI) after EBNA1 stimulation of EBNA1-monospecific, EBNA1-specific myelin-reactive, and EBNA1-specific insulin-reactive T cells in patients and controls. (D) Serological clonality analysis by flow cytometric staining for TCR-Vβ3, 1, 5S1, 2, 5S2, 5S3, 6S7, 7, 8, 9, 11, 13S1, 12, 13S6, 16, 14, 17, 18, 1S3, 20, 22, and 23. Depicted are the results for one representative TCR Vβ3+ CD4+ T cell clone. (E) Cytokine profile of cross-reactive versus monospecific EBNA1-specific T cells. IL-2: *, P < 0.0001. MIP-1β: *, P = 0.02. Error bars represent the SD.
We next determined the cytokine profile of 10 myelin cross-recognizing and 30 EBNA1-monospecific T cell clones from individuals in whom we could detect cross-recognition. Among all cytokines tested (n = 25), myelin cross-recognizing T cells differed substantially from their monospecific counterparts in their ability to produce high amounts of IL-2 (P < 0.0001) and in their inability to produce certain effector cytokines implicated in antiviral defense, such as the antiviral C-C (β) chemokine macrophage inflammatory protein-1β (MIP-1β; P = 0.02; Fig. 5 D). IFN-γ was detected in both subsets. These data indicate that the expanded EBNA1-specific CD4+ T cell repertoire in MS shows two distinct functional signatures, and that the myelin-reactive population can be distinguished by its ability to produce large amounts of IL-2 and less MIP-1α and -1β.
Concluding remarks
In healthy individuals, elevated IgG titers to EBNA1, but not to EBNA2, EBV-encoded early antigens, the viral capsid antigen, or other lytic antigens, are the strongest predictor of MS risk (2, 6). The presence of oligoclonal IgG in the cerebrospinal fluid is a hallmark of MS, and the two most frequent MS-specific and high-affinity epitopes recognized by oligoclonal CSF IgG are both derived from EBV, i.e., EBNA1 and a lytic, less well characterized structural EBV protein (BRRF2) (27), suggesting that EBNA1-specific antibodies are not only systemically elevated in MS, but also enriched in the CSF. The functional relevance of EBNA1-specific antibodies is, however, unclear because EBNA antigens are only expressed intracellularly in infected proliferating B cells. In contrast, T cells specific for EBNA1 are considered to be a crucial component of EBV-specific immune control. CD4+ T cells of healthy EBV carriers consistently respond to EBNA1, are Th1 in function, recognize autologous EBV-transformed B cell lines (B-LCL), and have the capacity to kill EBNA1-expressing targets via CD95/CD95L (11, 12, 20). In this study, we provide evidence that EBNA1-specific CD4+ Th1 cells are selectively expanded in MS patients, and that these have the ability to cross-recognize MS-associated myelin antigens. Although the frequency of cross-reactive T cells within the EBNA1-specific compartment is similar in MS patients and controls, we found that the overall number of EBNA1-specific T cells is increased in MS. Because both patients and controls were matched for expression of MS-associated HLA alleles, it is tempting to speculate that risk alleles such as HLA-DRB1*1501 predispose for selection of EBNA1 cross-reactive epitopes and that an increased total number of cross-reactive EBNA1-specific T cells, generated in a susceptible HLA background, might contribute to the development of MS.
The distinct cytokine signature of cross-reactive CD4+ T cell clones is indicative of polyfunctional T cells, which are invariably found in individuals with effective immune control of chronic HIV-1 or EBV infections (28, 29). Polyfunctional T cells, which probably constitute recent recruits from the central memory compartment, differ from further differentiated effector T cells in their ability to produce IL-2, to retain their antigen-specific proliferation capacity and their lower functional antigen avidities (28, 30). Being equipped with a low-avidity TCR, it has been assumed that they are particularly important under conditions of antigen persistence and high antigen load because they are less susceptible to exhaustion or activation-induced cell death (28). Consistent with these considerations, a more extensive priming of polyfunctional and lower avidity T cells during symptomatic primary EBV infection with high levels of viral load, and continuous restimulation caused by antigen presence in MS brains (8), might establish a larger cross-reactive T cell compartment, which could predispose for MS. Such mechanisms could explain the observation that individuals with a history of infectious mononucleosis are at greater risk to develop MS compared with subjects who acquired the virus without symptoms (4, 5). This scenario might not be restricted to, but become most evident, in the context of MS and EBV as a prototypic pathogen that requires strong and sustained T cell–mediated immune control to prevent the occurrence of EBV-associated malignancies.
Although our study provides evidence for a selective deregulation of EBNA1-specific T cell responses in MS and a potential pathogenic significance of polyfunctional EBNA1-specific CD4+ T cells, we are still far from understanding the mechanisms leading to disease development and progression. Further investigations will be necessary to clarify whether targeting EBV and EBV-specific immune compartments may become therapeutic options in MS.
MATERIALS AND METHODS
Patients and healthy donors.
24 untreated MS patients (23 with clinically definite, and 1 with clinically isolated demyelinating syndrome fulfilling the revised “McDonald” criteria for diagnosing MS) and 24 age-, sex-, and MHC class II–matched healthy donors were enrolled to study EBV-specific T cell responses (Table S1). Patients and controls were recruited from the New York blood bank and the Department of Neurology and Institute for Neuroimmunology and Clinical MS research, University Medical Center Hamburg-Eppendorf, Germany. Additional patients and controls were recruited form the Department of Neurology at the Phillips University of Marburg, Germany. All individuals were infected by EBV as assessed by positive IgG antibody titers against EBV-encoded viral capsid antigen. The study was approved by the local Institutional Review Boards (Ethics Committees of the Medical Faculties of the Universities of Hamburg and Marburg and the Institutional Review Board of the Rockefeller University), and all subjects provided informed consent.
rVVs.
rVVs were expanded in rabbit RK13 and titrated on monkey BSC40 kidney cells, as previously described (11). PBMCs were infected at a multiplicity of infection (MOI) of 10, incubated in ELISPOT plates for 48 h at 37°C, and washed three times. The efficiency of infection was checked after 24 h by intracellular staining with a monoclonal PE-coupled antibody specific for LMP1 (BD Biosciences) by flow cytometry. We observed that 10–15% of PBMCs became infected with rVV. If the light scatter gate was set on the monocyte fraction, 60–85% of the cells stained positively for LMP-1. LMP-1 staining was done in more than half of the experiments (the others were limited by small cell numbers) to verify that differences in the number of IFN-γ spots were not caused by variable rates of rVV infection. Samples derived from patients and healthy donors were consistently tested on the same plate to minimize the bias of plate to plate variations. ELISPOT assays were reproducible because the same results were obtained, if cryopreserved samples from healthy donors were tested on more than one occasion. The following rVVs expressing EBV latent antigens were used: rVV-TK− (negative control), rVV-BZLF1, rVV-EBNA3A, rVV-EBNA3B, rVV-EBNA 3C, rVV-LMP1, rVV-LMP2a, and rVV-EBNA1ΔGA (deleted of Gly-Ala repeat). Influenza A virus (A/Aichi/68; H3N2) was used at 50,000 HAU/ml and purchased from Charles River Laboratories. Staphylococcus enterotoxin B (Sigma-Aldrich) was additionally used as a positive control at a final concentration of 125 ng/ml.
ELISPOT for IFN-γ release.
96-well plates (Millititer; Millipore) were coated overnight at 4°C with 10 mg/ml of the primary anti-IFN-γ mAb (Mabtech). The antibody-coated plates were washed four times with PBS and blocked with RPMI containing 5% PHS for 1 h at 37°C. 2 × 105 PBMCs in 100 μl culture medium containing 1% human AB serum were added per well and infected with rVV in triplicates. Cells were incubated for 24 h at 37°C in 5% CO2. Wells were washed four times with PBS containing 0.05% Tween-20 (Sigma-Aldrich), followed by a 2-h incubation with 50 μl of the secondary antibody (1 mg/ml biotin-conjugated anti-IFN-γ mAb; Mabtech). Plates were washed four times in PBS with 0.1% Tween-20. Avidin-bound biotinylated horseradish peroxidase H (Vectastain Elite kit; Vector Laboratories, Inc.) was added to the wells for 1 h at room temperature. The plates were washed four times in PBS with 0.1% Tween-20, followed by a 5-min incubation in stable diaminobenzene (Research Genetics) to develop the reaction; tap water was added to stop the reaction. The spots were counted with an ELISPOT reader (AID Autoimmun Diagnostika GmbH). Only spots with a fuzzy border and a brown color were counted. A response was considered positive if there was at least twice the number of spots compared with rVV-TK−. Frequencies of T cells specific for rVV were calculated by subtracting the mean spot number of rVV-TK−–stimulated cells from the EBV antigen-rVV–stimulated wells.
Western blot.
To determine EBNA1 protein expression in rVV-EBNA1ΔGA–infected PBMCs, 5 × 106 PBMCs were infected with rVV-EBNA1ΔGA or mock infected at a MOI of 10 for 16 h. Samples were boiled for 30 min in Laemmli sample buffer (BioRad Laboratories). A recombinantly expressed fusion protein consisting of the C terminus of EBNA1 (aa 400–641) coupled to the heavy chains of an antibody, which was available in our laboratory, was used as a positive control. Proteins from 1.25 × 106 PBMCs were separated on 7.5% SDS-PAGE gels and, after blotting, stained with the EBNA1-specific mouse monoclonal Ab 5F12. HRP-coupled goat IgG (BioRad Laboratories) was used as secondary antibody. Western blots were developed using the ECLplus kit (GE Healthcare).
Antigens.
51 peptides covering the C-terminal of EBV nuclear antigen 1 (aa 400–641), 147 peptides covering the entire sequence of the HCMV-encoded pp65 protein (aa 1–561), and 28 peptides covering the entire sequence of the human proinsulin precursor protein (aa 1–110) were purchased from the Proteomics Resource Center of the Rockefeller University (Table S3) (9, 14). In addition, we included 15 peptides derived from 4 major myelin proteins. MBP, proteolipid protein, myelin oligodendrocyte glycoprotein, and 2′,3′-cyclic nucleotide 3′ phosphodiesterase were selected based on careful literature review as immunodominant in humans and/or encephalitogenic in animals (Table S3) (25). Influenza A virus (A/Aichi/68, H3N2; Charles River Laboratories) infection and C. albicans (Greer Laboratories) served as additional controls.
Flow cytometry.
PBMCs were isolated from blood samples via density centrifugation. PBMCs were washed in PBS and incubated at 37°C in 0.3 μM CFSE (Invitrogen) in PBS at a concentration of 107 cells per ml for 10 min. Cells were washed in PBS and resuspended in 5% PHS with 1 μg/ml co-stimulatory monoclonal antibodies to CD28 and CD49d (BD Biosciences) at a concentration of 1.7 × 106 cells per ml. PBMCs were distributed at 1 ml or 1.7 × 106 cells per well into 48-well plates. Cells were stimulated with the respective pools of peptides with a final concentration of 3.5 μmol per EBNA1-peptide and 5 μmol of the HCMV-pp65–derived antigens. Staphylococcus enterotoxin B (SEB; Sigma-Aldrich) was additionally used as a positive control at a final concentration of 125 ng/ml. At the conclusion of a 6-d incubation at 37°C and 5% CO2, cells were harvested and washed once in PBS and stained with a combination of directly fluorochrome-labeled antibodies against CD3, CD4, CD62L, CD27, CD28, and CXCR3 (BD Biosciences) for 30 min at 4°C. The cells were washed once with PBS and resuspended in 200 μl FACS buffer (0.01% sodium azide in PBS) before FACS analysis. The samples were analyzed on an LSR II flow cytometer gating on lymphocytes based on size and on being CD3+. Responses were considered positive if the frequency of CFSElow T cells in peptide-stimulated cultures exceeded those from unstimulated cultures by at least twofold. Frequencies were calculated by subtracting background events (without antigen) from events in antigen-stimulated samples. Gating and calculations for precursor frequencies were performed with FlowJo (Tree Star, Inc.) software. To determine the frequency of IL-17–producing antigen-specific T cells, CFSE-stained PBMC were stimulated with pooled C. albicans antigens (Greer Laboratories) at a concentration of 40 μg/ml, or with EBNA1 or with SEB. After 7 d, the cells were stimulated with PMA (10 ng/ml) and ionomycin (10 mg/ml) for 24 h, whereas monensin (2 μM) was added for the last 12 h of incubation. The frequency of antigen-specific Th17 cells was determined by gating on the CD3+CD4+ CFSElow population and using a monoclonal PE-coupled IL-17 antibody (eBioscience). To determine the clonality of myelin cross-reactive, EBNA1-specific T cells, growing T cell cultures were characterized by staining with a panel of 22 anti-TCR Vβ fluorescein-conjugated antibodies recognizing TCR-Vβ3, -Vβ1, -Vβ5S1, -Vβ2, -Vβ5S2, -Vβ5S3, -Vβ6S7, -Vβ7, -Vβ8, -Vβ9, -Vβ11, -Vβ13S1, -Vβ12, -Vβ13S6, -Vβ16, -Vβ14, -Vβ17, -Vβ18, -Vβ21S3, -Vβ20, -Vβ22, and -Vβ23 (Immunotech), as well as CD4 and CD8 monoclonal antibodies (Beckman Coulter). Fluorescence intensity was measured on an LSR II flow cytometer and analyzed with FlowJo (Tree Star, Inc.) software.
ELISA and expression and purification of recombinant EBNA1.
HCMV-EA (early antigen)–specific antibodies were detected with a commercial ELISA kit following the manufacturer's recommendations using predetermined cut-off values based on the manufacturer's criteria (Bio-Quant, Inc.). The C-terminal domain of EBNA1 (aa 458–641) inserted in the expression vector pET15b (Novagen) was transfected into Escherichia coli BL21 (DE3) pLysS cells and expression was induced with 1 mM IPTG (Invitrogen). The protein was purified, and the identity was determined by Western blot analysis with EBNA1-specific antibody (MAB8173; Chemicon International). Standardized lysates of EBV-infected B cells and noninfected control B cells were obtained from EastCoast Bio. 96-well polystyrene plates (Thermo Fisher Scientific) were coated with 1 μg/well of rEBNA1 protein in PBS, or infected and noninfected cell lysates, or PBS alone overnight at 4°C. Plates were blocked with 200 μl/well 5% nonfat milk powder for 30 min, followed by 30 min in PBS containing 5% BSA. Test plasma samples, diluted 1:10, 1:100, 1:200, 1:500, 1:1,000, and 1:2,000 in 3% BSA, were added for 30 min at room temperature. Plates were washed three times with TBST (10 mM Tris, 140 mM NaCl, and 0.05% Tween 20). Biotin mouse anti–human IgG1, IgG2, and IgG3 antibodies (BD Biosciences) were added at 1:1,000, and the anti–human IgG4 antibody was added at 1:5,000 in TBST for 30 min at room temperature. After the plates were washed three times in TBST, avidin-bound biotinylated HRP was added for 20 min at room temperature, followed by TMB substrate (R&D Systems) to develop the reaction for 10 min at room temperature and 1 M H2SO4 to stop the reaction. Plates were read in a microplate reader (Dynex Technologies). Samples were processed blinded to the clinical diagnosis. Sample ODs from noninfected control cell lysates were subtracted from ODs from EBV-infected cell lysates. Titers were defined by the 10% effect concentration (EC10) value of individual titration curves.
Regression assay.
B cells were isolated by positive selection using CD19-Microbeads (Miltenyi Biotec). The negative fraction (CD19−) was plated in 48-well plates in increasing cell concentrations (0, 1 × 105, 0.5 × 105, 105, and 5 × 105) in duplicates. 5 × 106 CD19+ cells per donor were infected overnight at 37°C and in 5% CO2 with supernatant of the EBV+ marmoset cell line B95-8 cultured for 12 d in RPMI-1640 containing 10% FCS and gentamycin without refeeding. Virus-containing supernatant was centrifuged at 2,000 rpm for 10 min and passed through a 0.45-μm filter. The same supernatant was used to infect B cells from both patients with MS and healthy blood donors. After 24 h, EBV-infected B cells were washed twice and added to CD19- cells at effector to target ratios of 1:1, 1:5, 1:10, and 1:50. After 12 d, transformation and regression of transformed B cells were quantified by determining the ratio of CD19+ CD23+ cells to live CD19+ B cells by flow cytometry. Samples were analyzed on an LSR II flow cytometer. Gating and calculations were performed with FlowJo (Tree Star, Ashland, OR) software.
T cell cross-recognition.
To obtain single-cell, EBNA1-specific T cell precursors, we adapted a modified limiting dilution protocol that has initially been described for mixed lymphocyte cultures (24). The experimental setup for this assay is outlined in Fig. S5. PBMCs were isolated from blood samples via density centrifugation and seeded in increasing cell concentrations of 0.125 × 105, 0.25 × 105, and 0.5 × 105 cells per well together with autologous irradiated (3,000 rad) feeder cells resulting in a total cell number of 105 per well in 96-well U-bottom microtiter plates in T cell medium (RPMI; Invitrogen) containing 2 mM l-glutamine, 50 μg/ml gentamicin, and 100 U/ml penicillin/streptomycin (all from Whittacker Bioproducts), and 5% pooled human AB serum, and enriched with 10 ng/ml IL-7 (PeproTech). 50 wells were stimulated with EBNA1 peptides (aa 400–641), and 10 wells did not receive any antigens. All peptides were used at a final concentration of 1 μM. After 7 d of incubation at 37°C and in 5% CO2, each culture was resuspended and half of the total volume (100 μl) was transferred into new plates, which were pulsed with 3H-thymidine (GE Healthcare) for 16 h at 1 μCi/well. T cell medium enriched with 10 IU/ml IL-2 was added to the remaining nonpulsed wells to propagate the cultures. The incorporated radioactivity (cpm) in “daughter” plates was measured by scintillation counting (1450 Microbeta; Wallac/PerkinElmer Life Sciences). Individual wells were considered positive if their stimulation index (SI = cpm of wells with antigen/mean cpm of 10 negative control wells) was >2. Positive cultures were identified on the corresponding “mother” plates and restimulated on day 10 with 105 autologous irradiated antigen-pulsed (1 μM per peptide) PBMCs. IL-2 (10 U/ml) and IL-7 (10 ng/ml)-enriched medium was added on day 11, and again 5 d later. After 12 d of expansion, 4 × 40 μl of a total of 200 μl were transferred to a new daughter plate and stimulated with EBNA1, myelin antigens, and PIP-derived antigens (1 μM per peptide) or without antigen (negative control). 3H-thymidine was added 40 h later at 1 μCi/well, and cells were harvested 60 h after stimulation (day 25). Again, individual wells were considered positive if SI was >2. T cell cultures identified as cross-reactive were restimulated once with EBNA1 peptides in the presence of autologous feeders and 20 IU/ml IL-2 and analyzed for TCR Vβ expression after 8–14 d. Precursor frequencies of cross-reactive and non–cross-reactive TCLs were determined by the number of positive wells compared with number of seeded cells in single precursor plates, which were restimulated after primary proliferation. Corresponding positive cultures on the mother plate were restimulated with autologous feeders and further expanded in the presence of 20 IU/ml IL-2.
Luminex assay.
Cell supernatants from cultures obtained from myelin antigen-reactive and only EBNA1-specific T cells were analyzed at day 1 after the second restimulation for cytokines and using the human cytokine 25-plex antibody bead kit (Invitrogen/Biosource) as per the manufacturer's protocol. This kit comprises analyte specific components for the quantification of the following 25 cytokines and chemokines: IL-1β, IL-1ra, IL-2, IL-2R, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12p40, IL-13, IL-15, IL-17, TNF-α, IFN-α, IFN-γ, GM-CSF, MIP-1α, MIP-1β, IP-10, MIG, Eotaxin, RANTES, and MCP-1. Multiplex beads were vortexed and sonicated for 30 s, and 25 μl was added to each well and washed two times with wash buffer. The samples were diluted 1:1 with assay diluent and loaded onto a Multiscreen BV 96-well filter plate (Millipore) with 50 μl of incubation buffer already added to each well. Serial dilutions of cytokine standards were prepared in parallel and added to the plate. Samples were then incubated on a plate shaker at 600 rpm in the dark at room temperature for 2 h. The plate was applied to a Multiscreen Vacuum Manifold (Millipore) and washed twice with 200 μl of wash buffer. 100 μl of biotinylated anti–human Multi-Cytokine Reporter (Biosource International) was added to each well. The plate was incubated on a plate shaker at 600 rpm in the dark at room temperature for 1 h. The plate was applied to a Multiscreen Vacuum Manifold and washed twice with 200 μl of wash buffer. Streptavidin-phycoerythrin was diluted 1:10 in wash buffer, and 100 μl was added directly to each well. The plate was incubated on a plate shaker at 600 rpm in the dark at room temperature for 30 min. The plate was then applied to the vacuum manifold, washed twice, and each well was resuspended in 100 μl wash buffer and shaken for 1 min. The assay plate was then transferred to the Bio-Plex Luminex 100 XYP instrument (Millipore) for analysis. Cytokine concentrations were calculated using Bio-Plex Manager 3.0 software with a 25-parameter curve-fitting algorithm applied for standard curve calculations.
Statistical analysis.
Statistics were performed using commercial software (PRISM 4; GraphPad Software). Comparisons between MS patients and healthy donors are based on the nonparametric Mann-Whitney U rank sum test. Categorial analyses were performed using Fisher's exact test. To correct for multiple testing, i.e., IFN-γ responses to BZLF1, EBNA1ΔGA, EBNA2, EBNA3A, EBNA3B, EBNA3C, LMP1, LMP2A, and influenza infection, the conservative Bonferroni correction was 0.05/9 = 0.0055. Consequently, P < 0.0055 was considered significant and 0.05 > P > 0.0055 was a statistical trend.
Online supplemental material.
Fig. S1 shows rVV-EBV antigen-specific IFN-γ–responses in CD4+ T cell–depleted PBMCs. Fig. S2 shows CD3+CD4− T cell proliferation to EBNA1, HCMV-pp65, and influenza in MS patients and healthy controls. Fig. S3 shows that C. albicans antigen-specific, but not EBNA1-specific, CD4+ T cells produce IL-17. Fig. S4 shows regression of EBV-infected B cell outgrowth in MS patients and healthy donors. Fig. S5 shows the cloning strategy to determine T cell cross-recognition. Table S1 displays characteristics of MS patients and healthy donors included in this study. Table S2 shows the number of IFN-γ spots in cell preparations derived from MS patients and healthy virus carriers in response to rVV-EBV antigens, as well as the relative changes in the number of IFN-γ spots before and after CD8+ T cell depletion. Peptides used in this study are listed in Table S3.The online version of this article is available at http://www.jem.org/cgi/content/full/jem.20072397/DC1.
Supplementary Material
Acknowledgments
We thank all our patients for their cooperation.
J.D. Lünemann is a recipient of the Dana Foundation and Irvington Institute's Human Immunology Fellowship, of a Pilot Grant from the National Multiple Sclerosis Society (PP1145), and of an Institutional Clinical and Translational Science Pilot and Collaborative Project Grant (to the Rockefeller University Hospital). I. Jelčić is supported by the Deutsche Forschungsgemeinschaft (JE 530/1-1). A. Lutterotti is a recipient of a Research Fellowship of the Humboldt Foundation. C. Münz is supported by the Dana Foundation's Neuroimmunology program, the Beckman Foundation, the Sinsheimer Foundation, the Burroughs Wellcome Fund, the Starr Foundation, the National Cancer Institute (R01CA108609 and R01CA101741), the National Institute of Allergy and Infectious Diseases (RFP-NIH-NIAID-DAIDS-BAA-06-19), the Foundation for the National Institutes of Health (Grand Challenges in Global Health), and an Institutional Clinical and Translational Science Award (to The Rockefeller University Hospital). The Institute for Neuroimmunology and Clinical Multiple Sclerosis Research is supported by the Gemeinnützige Hertie Stiftung.
The authors have no conflicting financial interests.
References
- 1.Hafler, D.A. 2004. Multiple sclerosis. J. Clin. Invest. 113:788–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sundstrom, P., P. Juto, G. Wadell, G. Hallmans, A. Svenningsson, L. Nystrom, J. Dillner, and L. Forsgren. 2004. An altered immune response to Epstein-Barr virus in multiple sclerosis: a prospective study. Neurology. 62:2277–2282. [DOI] [PubMed] [Google Scholar]
- 3.Banwell, B., L. Krupp, J. Kennedy, R. Tellier, S. Tenembaum, J. Ness, A. Belman, A. Boiko, O. Bykova, E. Waubant, et al. 2007. Clinical features and viral serologies in children with multiple sclerosis: a multinational observational study. Lancet Neurol. 6:773–781. [DOI] [PubMed] [Google Scholar]
- 4.Thacker, E.L., F. Mirzaei, and A. Ascherio. 2006. Infectious mononucleosis and risk for multiple sclerosis: a meta-analysis. Ann. Neurol. 59:499–503. [DOI] [PubMed] [Google Scholar]
- 5.Nielsen, T.R., K. Rostgaard, N.M. Nielsen, N. Koch-Henriksen, S. Haahr, P.S. Sorensen, and H. Hjalgrim. 2007. Multiple sclerosis after infectious mononucleosis. Arch. Neurol. 64:72–75. [DOI] [PubMed] [Google Scholar]
- 6.Levin, L.I., K.L. Munger, M.V. Rubertone, C.A. Peck, E.T. Lennette, D. Spiegelman, and A. Ascherio. 2005. Temporal relationship between elevation of Epstein-Barr virus antibody titers and initial onset of neurological symptoms in multiple sclerosis. JAMA. 293:2496–2500. [DOI] [PubMed] [Google Scholar]
- 7.Wandinger, K., W. Jabs, A. Siekhaus, S. Bubel, P. Trillenberg, H. Wagner, K. Wessel, H. Kirchner, and H. Hennig. 2000. Association between clinical disease activity and Epstein-Barr virus reactivation in MS. Neurology. 55:178–184. [DOI] [PubMed] [Google Scholar]
- 8.Serafini, B., B. Rosicarelli, D. Franciotta, R. Magliozzi, R. Reynolds, P. Cinque, L. Andreoni, P. Trivedi, M. Salvetti, A. Faggioni, and F. Aloisi. 2007. Dysregulated Epstein-Barr virus infection in the multiple sclerosis brain. J. Exp. Med. 204:2899–2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lünemann, J.D., N. Edwards, P.A. Muraro, S. Hayashi, J.I. Cohen, C. Münz, and R. Martin. 2006. Increased frequency and broadened specificity of latent EBV nuclear antigen-1-specific T cells in multiple sclerosis. Brain. 129:1493–1506. [DOI] [PubMed] [Google Scholar]
- 10.Gronen, F., K. Ruprecht, B. Weissbrich, E. Klinker, A. Kroner, H.H. Hofstetter, and P. Rieckmann. 2006. Frequency analysis of HLA-B7-restricted Epstein-Barr virus-specific cytotoxic T lymphocytes in patients with multiple sclerosis and healthy controls. J. Neuroimmunol. 180:185–192. [DOI] [PubMed] [Google Scholar]
- 11.Münz, C., K.L. Bickham, M. Subklewe, M.L. Tsang, A. Chahroudi, M.G. Kurilla, D. Zhang, M. O'Donnell, and R.M. Steinman. 2000. Human CD4+ T lymphocytes consistently respond to the latent Epstein-Barr virus nuclear antigen EBNA1. J. Exp. Med. 191:1649–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hislop, A.D., G.S. Taylor, D. Sauce, and A.B. Rickinson. 2007. Cellular responses to viral infection in humans: lessons from Epstein-Barr virus. Annu. Rev. Immunol. 25:587–617. [DOI] [PubMed] [Google Scholar]
- 13.Bunde, T., A. Kirchner, B. Hoffmeister, D. Habedank, R. Hetzer, G. Cherepnev, S. Proesch, P. Reinke, H.D. Volk, H. Lehmkuhl, and F. Kern. 2005. Protection from cytomegalovirus after transplantation is correlated with immediate early 1–specific CD8 T cells. J. Exp. Med. 201:1031–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heller, K.N., J. Upshaw, B. Seyoum, H. Zebroski, and C. Münz. 2007. Distinct memory CD4+ T-cell subsets mediate immune recognition of Epstein Barr virus nuclear antigen 1 in healthy virus carriers. Blood. 109:1138–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sallusto, F., J. Geginat, and A. Lanzavecchia. 2004. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu. Rev. Immunol. 22:745–763. [DOI] [PubMed] [Google Scholar]
- 16.Qin, S., J.B. Rottman, P. Myers, N. Kassam, M. Weinblatt, M. Loetscher, A.E. Koch, B. Moser, and C.R. Mackay. 1998. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J. Clin. Invest. 101:746–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sorensen, T.L., M. Tani, J. Jensen, V. Pierce, C. Lucchinetti, V.A. Folcik, S. Qin, J. Rottman, F. Sellebjerg, R.M. Strieter, et al. 1999. Expression of specific chemokines and chemokine receptors in the central nervous system of multiple sclerosis patients. J. Clin. Invest. 103:807–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amyes, E., C. Hatton, D. Montamat-Sicotte, N. Gudgeon, A.B. Rickinson, A.J. McMichael, and M.F. Callan. 2003. Characterization of the CD4+ T cell response to Epstein-Barr virus during primary and persistent infection. J. Exp. Med. 198:903–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fritsch, R.D., X. Shen, G.P. Sims, K.S. Hathcock, R.J. Hodes, and P.E. Lipsky. 2005. Stepwise differentiation of CD4 memory T cells defined by expression of CCR7 and CD27. J. Immunol. 175:6489–6497. [DOI] [PubMed] [Google Scholar]
- 20.Bickham, K., C. Münz, M.L. Tsang, M. Larsson, J.F. Fonteneau, N. Bhardwaj, and R. Steinman. 2001. EBNA1-specific CD4+ T cells in healthy carriers of Epstein-Barr virus are primarily Th1 in function. J. Clin. Invest. 107:121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Acosta-Rodriguez, E.V., L. Rivino, J. Geginat, D. Jarrossay, M. Gattorno, A. Lanzavecchia, F. Sallusto, and G. Napolitani. 2007. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat. Immunol. 8:639–646. [DOI] [PubMed] [Google Scholar]
- 22.Wucherpfennig, K.W., and J.L. Strominger. 1995. Molecular mimicry in T cell-mediated autoimmunity: viral peptides activate human T cell clones specific for myelin basic protein. Cell. 80:695–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lang, H.L., H. Jacobsen, S. Ikemizu, C. Andersson, K. Harlos, L. Madsen, P. Hjorth, L. Sondergaard, A. Svejgaard, K. Wucherpfennig, et al. 2002. A functional and structural basis for TCR cross-reactivity in multiple sclerosis. Nat. Immunol. 3:940–943. [DOI] [PubMed] [Google Scholar]
- 24.Taswell, C., H.R. MacDonald, and J.C. Cerottini. 1980. Clonal analysis of cytolytic T lymphocyte specificity. I. Phenotypically distinct sets of clones as the cellular basis of cross-reactivity to alloantigens. J. Exp. Med. 151:1372–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bielekova, B., M.H. Sung, N. Kadom, R. Simon, H. McFarland, and R. Martin. 2004. Expansion and functional relevance of high-avidity myelin-specific CD4+ T cells in multiple sclerosis. J. Immunol. 172:3893–3904. [DOI] [PubMed] [Google Scholar]
- 26.Kent, S.C., Y. Chen, L. Bregoli, S.M. Clemmings, N.S. Kenyon, C. Ricordi, B.J. Hering, and D.A. Hafler. 2005. Expanded T cells from pancreatic lymph nodes of type 1 diabetic subjects recognize an insulin epitope. Nature. 435:224–228. [DOI] [PubMed] [Google Scholar]
- 27.Cepok, S., D. Zhou, R. Srivastava, S. Nessler, S. Stei, K. Bussow, N. Sommer, and B. Hemmer. 2005. Identification of Epstein-Barr virus proteins as putative targets of the immune response in multiple sclerosis. J. Clin. Invest. 115:1352–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harari, A., C. Cellerai, F.B. Enders, J. Kostler, L. Codarri, G. Tapia, O. Boyman, E. Castro, S. Gaudieri, I. James, et al. 2007. Skewed association of polyfunctional antigen-specific CD8 T cell populations with HLA-B genotype. Proc. Natl. Acad. Sci. USA. 104:16233–16238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Younes, S.A., B. Yassine-Diab, A.R. Dumont, M.R. Boulassel, Z. Grossman, J.P. Routy, and R.P. Sekaly. 2003. HIV-1 viremia prevents the establishment of interleukin 2-producing HIV-specific memory CD4+ T cells endowed with proliferative capacity. J. Exp. Med. 198:1909–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Betts, M.R., M.C. Nason, S.M. West, S.C. De Rosa, S.A. Migueles, J. Abraham, M.M. Lederman, J.M. Benito, P.A. Goepfert, M. Connors, et al. 2006. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 107:4781–4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.