Abstract
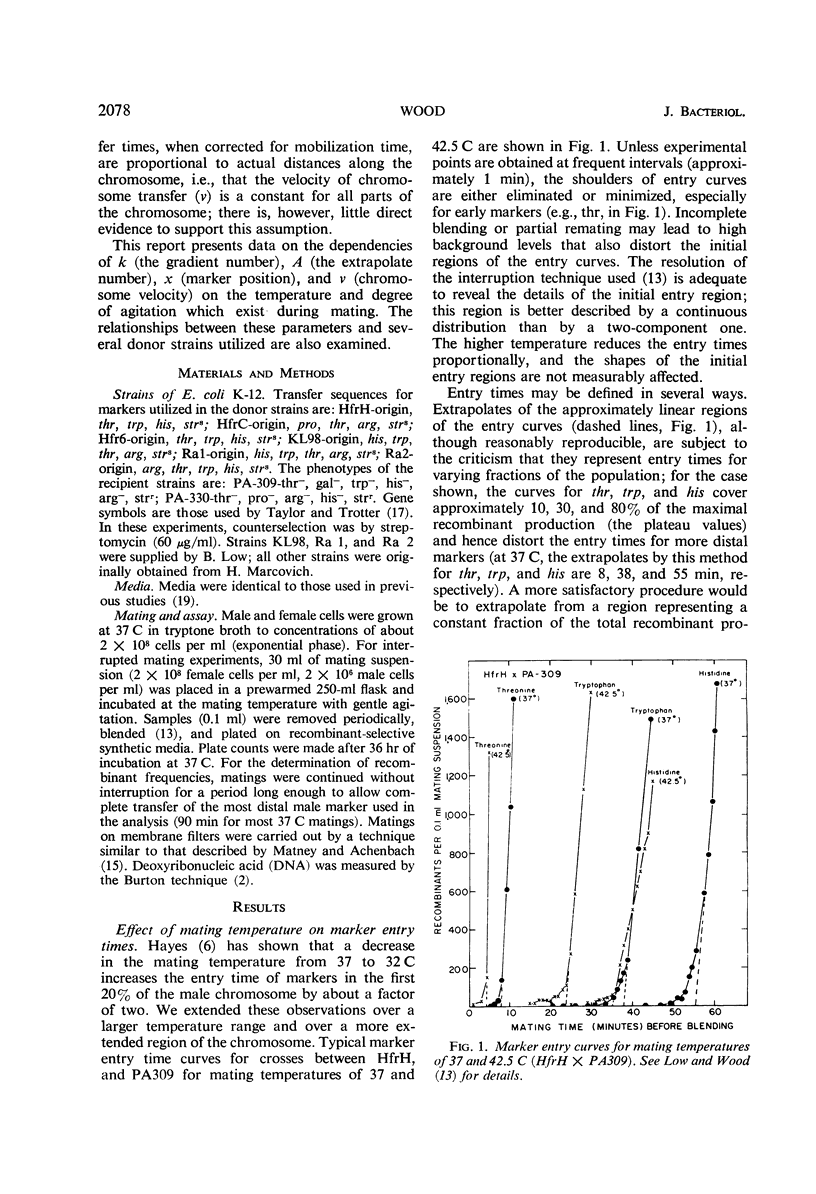
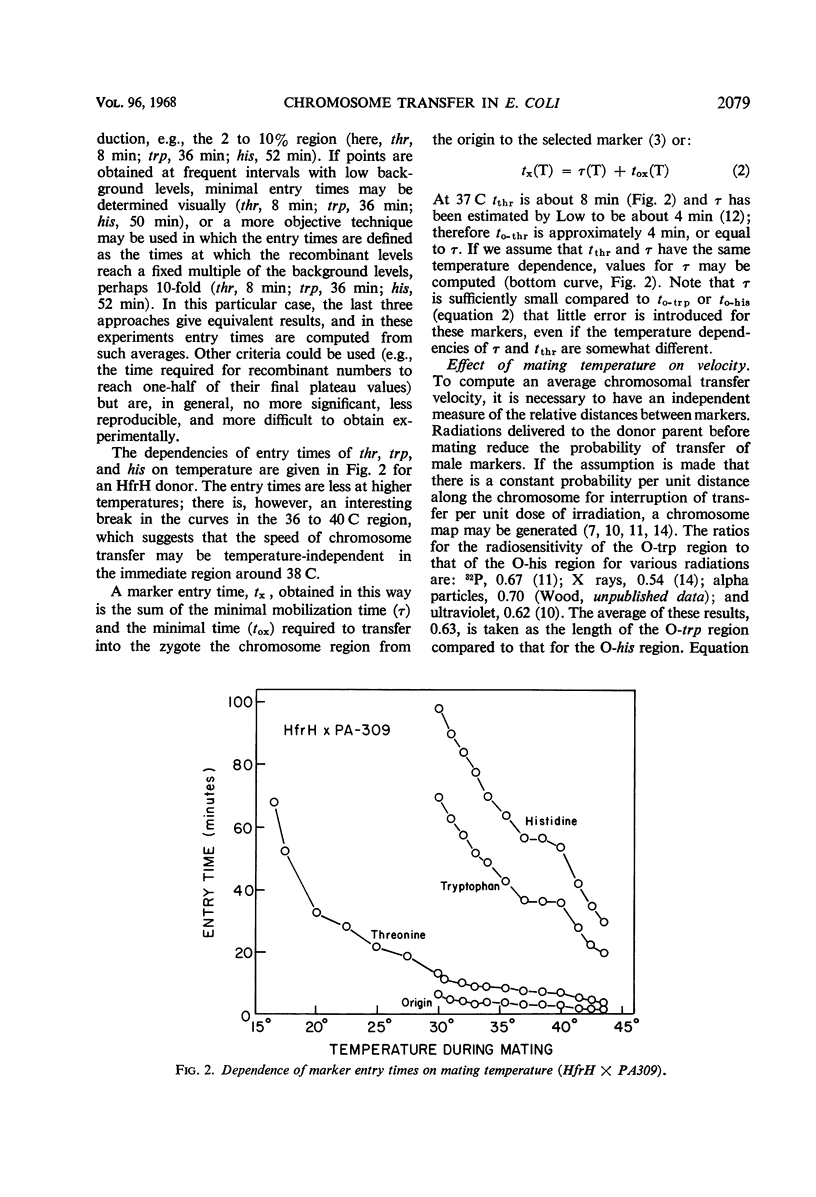
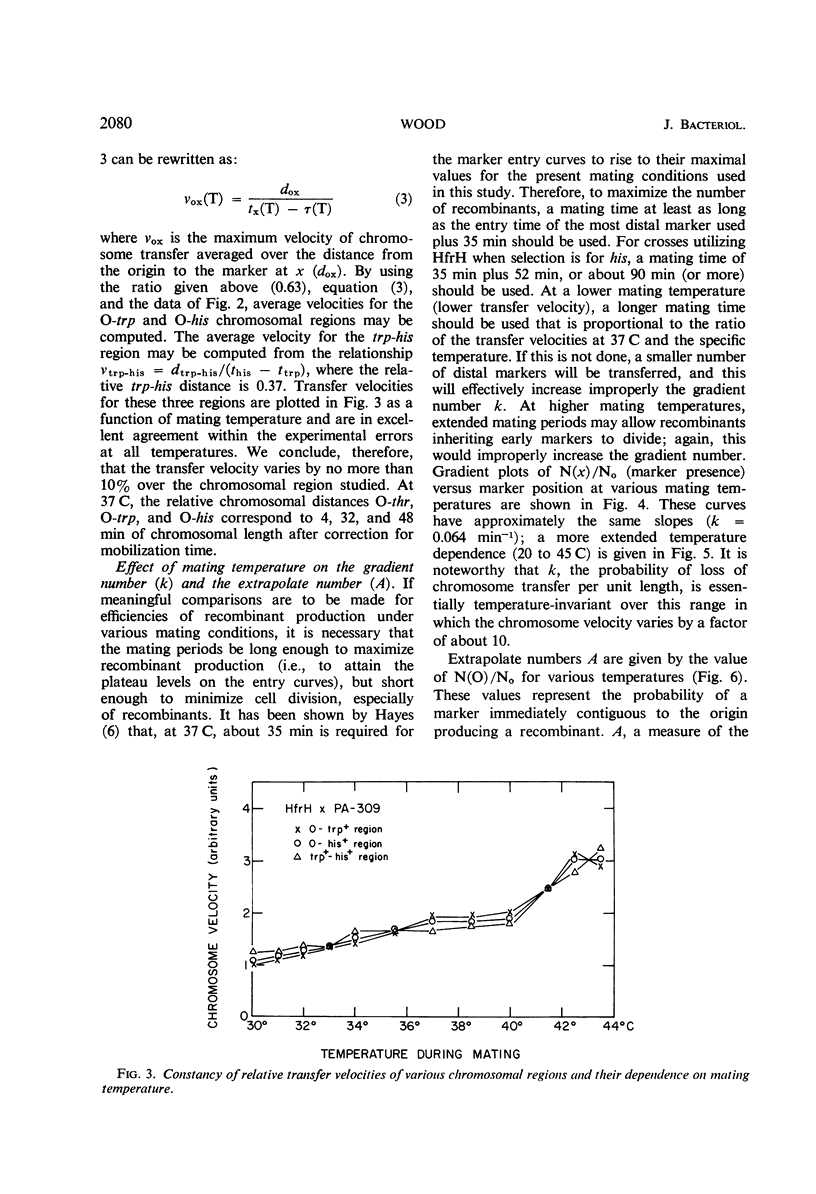
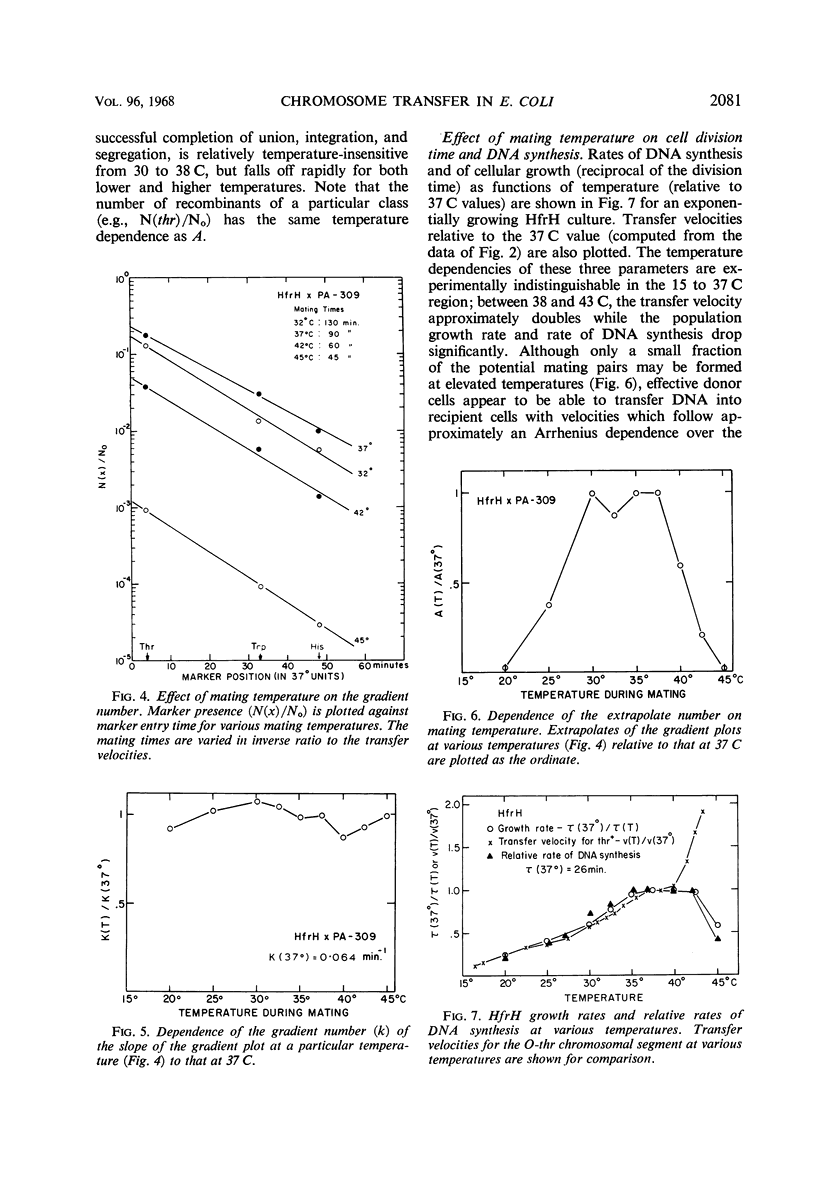
Recombinant production in Escherichia coli K-12 can be described by three parameters: (i) the distance x of a selected male marker from the donor origin; (ii) the gradient constant k (the probability of interruption of the donor chromosome per unit distance during transfer into a recipient cell); and (iii) the extrapolate number A (the probability that a donor cell will produce a recombinant inheriting the donor marker contiguous with the origin). It is usually assumed that chromosomal distances can be measured by marker entry times, i.e., that the velocity of chromosome transfer v is constant along the chromosome. The dependencies of k, A, x, and v on temperature, agitation during mating, and donor strain were studied. The transfer velocity of the HfrH chromosomal region from the origin to his increases 15-fold between 16 and 43 C, and the chromosomal regions studied have the same temperature dependence that was found for the separate transfer velocities of the O-trp and trp-his regions. These data and radiation studies on chromosome transfer indicate that, at a given temperature, chromosomal transfer velocity varies by less than 10% as the distance of any given region from the origin increases. The gradient constant k is temperature-independent between 20 and 45 C if mating times at different temperatures are inversely proportional to the chromosome velocities; also, k is insensitive to agitation during mating and is not decreased by mating on membrane filters. However, the extrapolate number A is highly temperature-dependent, having its maximum value between 30 and 38 C. These results suggest that the spontaneous interruption of transfer which produces the gradient of transfer is a property of the chromosome itself and not of the fragility of the connection between mating cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON T. F. Recombination and segregation in Escherichia coli. Cold Spring Harb Symp Quant Biol. 1958;23:47–58. doi: 10.1101/sqb.1958.023.01.007. [DOI] [PubMed] [Google Scholar]
- CLARK A. J., ADELBERG E. A. Bacterial conjugation. Annu Rev Microbiol. 1962;16:289–319. doi: 10.1146/annurev.mi.16.100162.001445. [DOI] [PubMed] [Google Scholar]
- Curtiss R., 3rd, Stallions D. R. Energy requirements for specific pair formation during conjugation in Escherichia coli K-12. J Bacteriol. 1967 Aug;94(2):490–492. doi: 10.1128/jb.94.2.490-492.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYES W. The kinetics of the mating process in Escherichia coli. J Gen Microbiol. 1957 Feb;16(1):97–119. doi: 10.1099/00221287-16-1-97. [DOI] [PubMed] [Google Scholar]
- Joset F., Wood T. H. Modification of conjugation in Escherichia coli K-12 by ultraviolet irradiation. Genetics. 1966 Feb;53(2):343–356. doi: 10.1093/genetics/53.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krisch R. Effects on genetic recombination of Escherichia coli K 12 produced by P-32 decay in the Hfr male. Genet Res. 1965 Nov;6(3):454–468. doi: 10.1017/s001667230000433x. [DOI] [PubMed] [Google Scholar]
- LOW B., WOOD T. H. A QUICK AND EFFICIENT METHOD FOR INTERRUPTION OF BACTERIAL CONJUGATION. Genet Res. 1965 Jul;6:300–303. doi: 10.1017/s001667230000416x. [DOI] [PubMed] [Google Scholar]
- Low B. Low recombination frequency for markers very near the origin in conjugation in E. coli. Genet Res. 1965 Nov;6(3):469–473. doi: 10.1017/s0016672300004341. [DOI] [PubMed] [Google Scholar]
- MATNEY T. S., ACHENBACH N. E. New uses for membrane filters III. Bacterial mating procedure. J Bacteriol. 1962 Oct;84:874–875. doi: 10.1128/jb.84.4.874-875.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittard J., Walker E. M. Conjugation in Escherichia coli: recombination events in terminal regions of transferred deoxyribonucleic acid. J Bacteriol. 1967 Nov;94(5):1656–1663. doi: 10.1128/jb.94.5.1656-1663.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Revised linkage map of Escherichia coli. Bacteriol Rev. 1967 Dec;31(4):332–353. doi: 10.1128/br.31.4.332-353.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOD T. H., MARCOVICH H. EFFECTS ON GENETIC RECOMBINATION OF ESCHERICHIA COLI K-12 PRODUCED BY X-RAY AND ALPHA-PARTICLE IRRADIATION OF THE FEMALE. Genetics. 1964 May;49:779–786. doi: 10.1093/genetics/49.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood T. H. Genetic recombination in Escherichia coli: clone heterogeneity and the kinetics of segregation. Science. 1967 Jul 21;157(3786):319–321. doi: 10.1126/science.157.3786.319. [DOI] [PubMed] [Google Scholar]


