Abstract
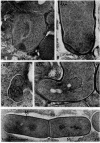
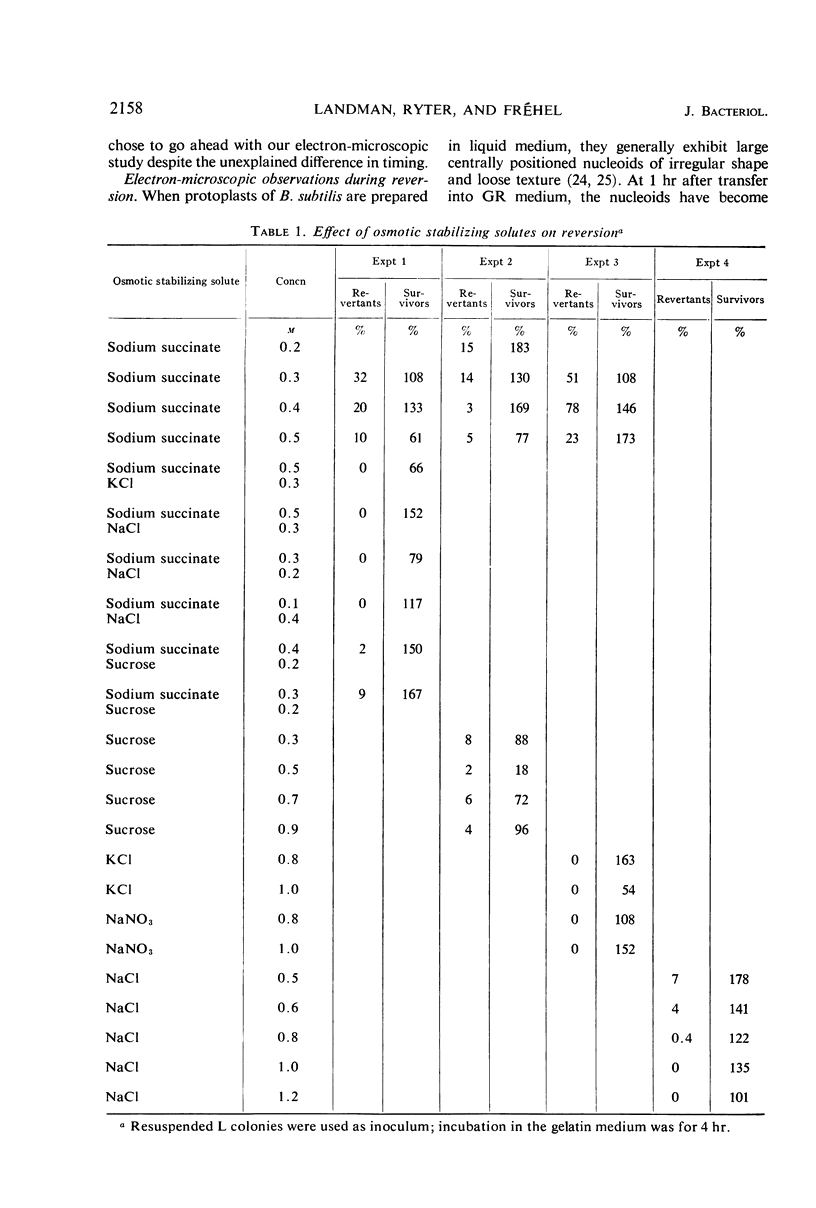
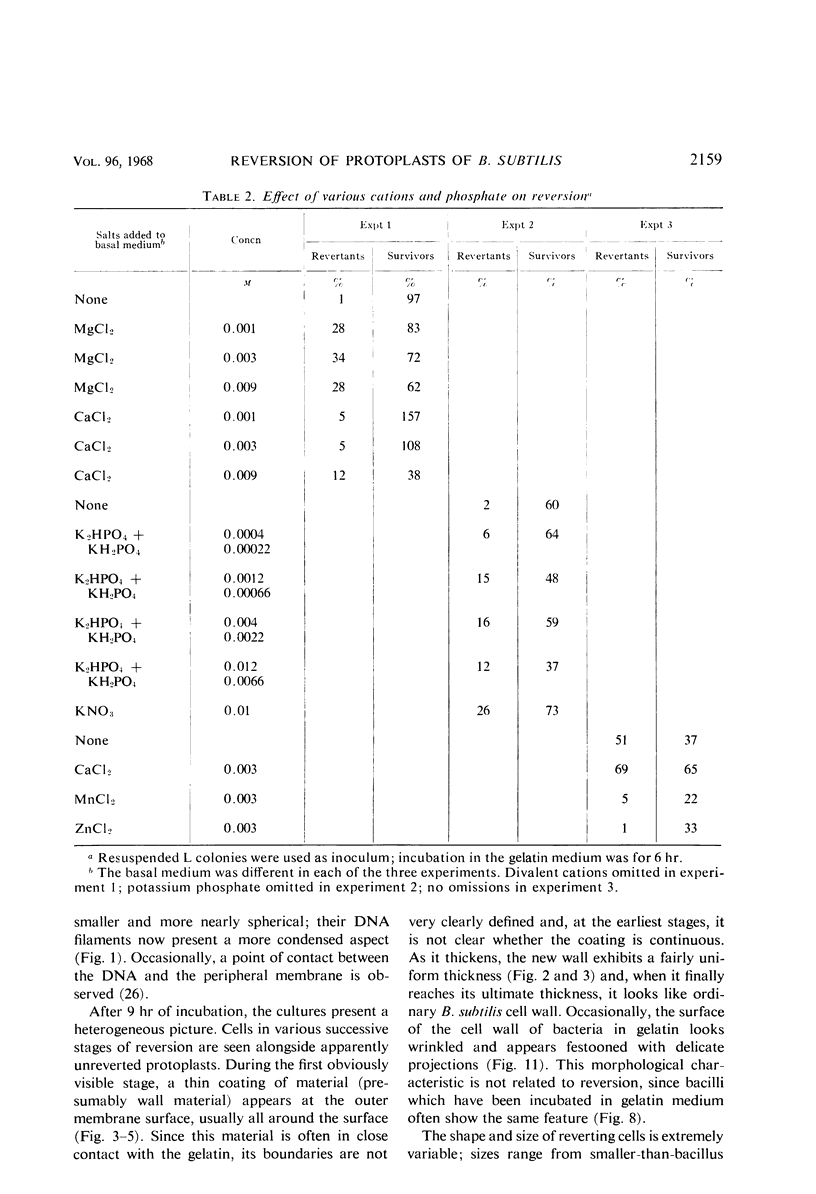
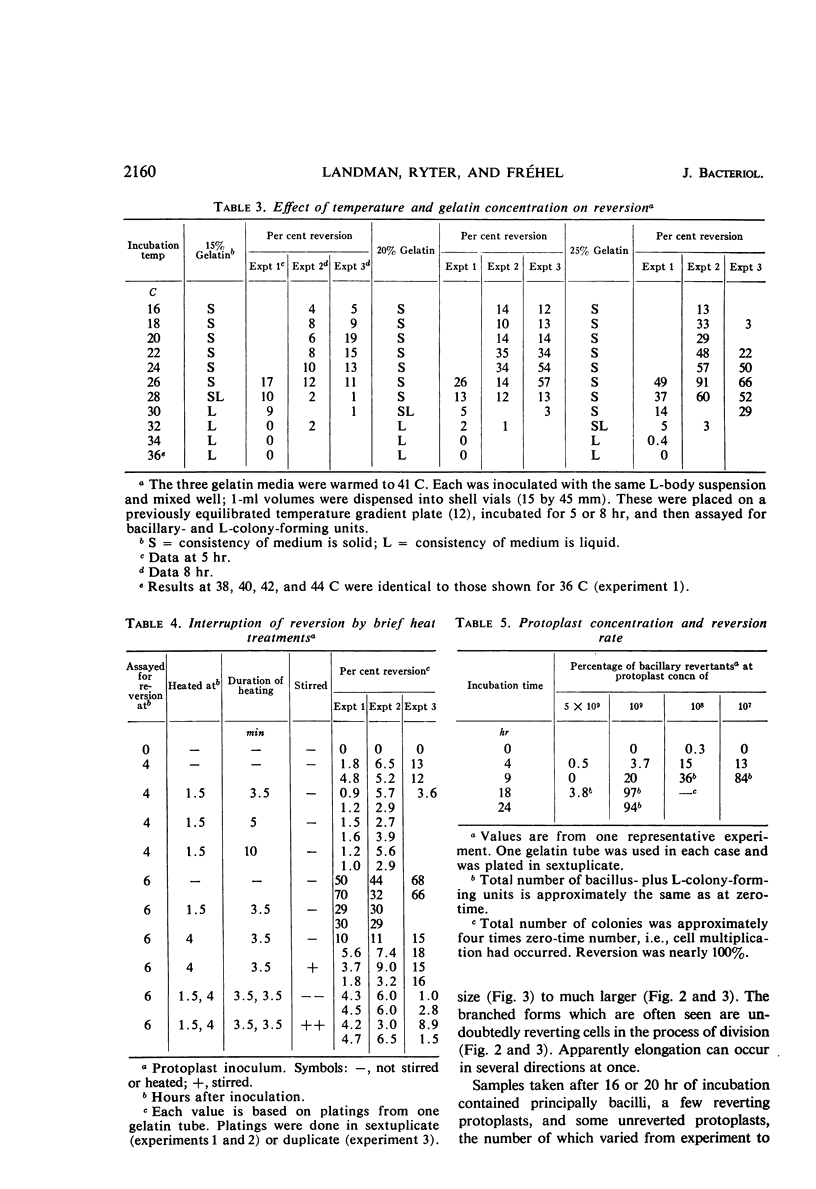
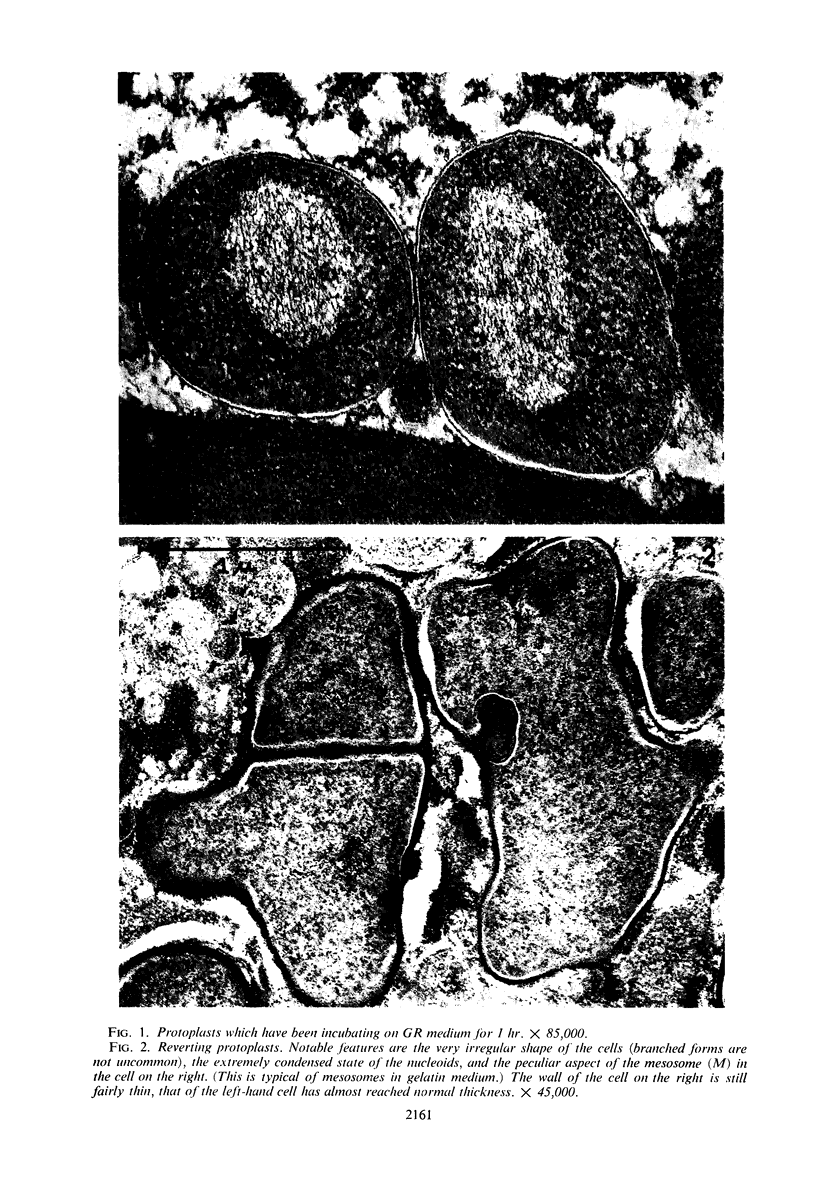
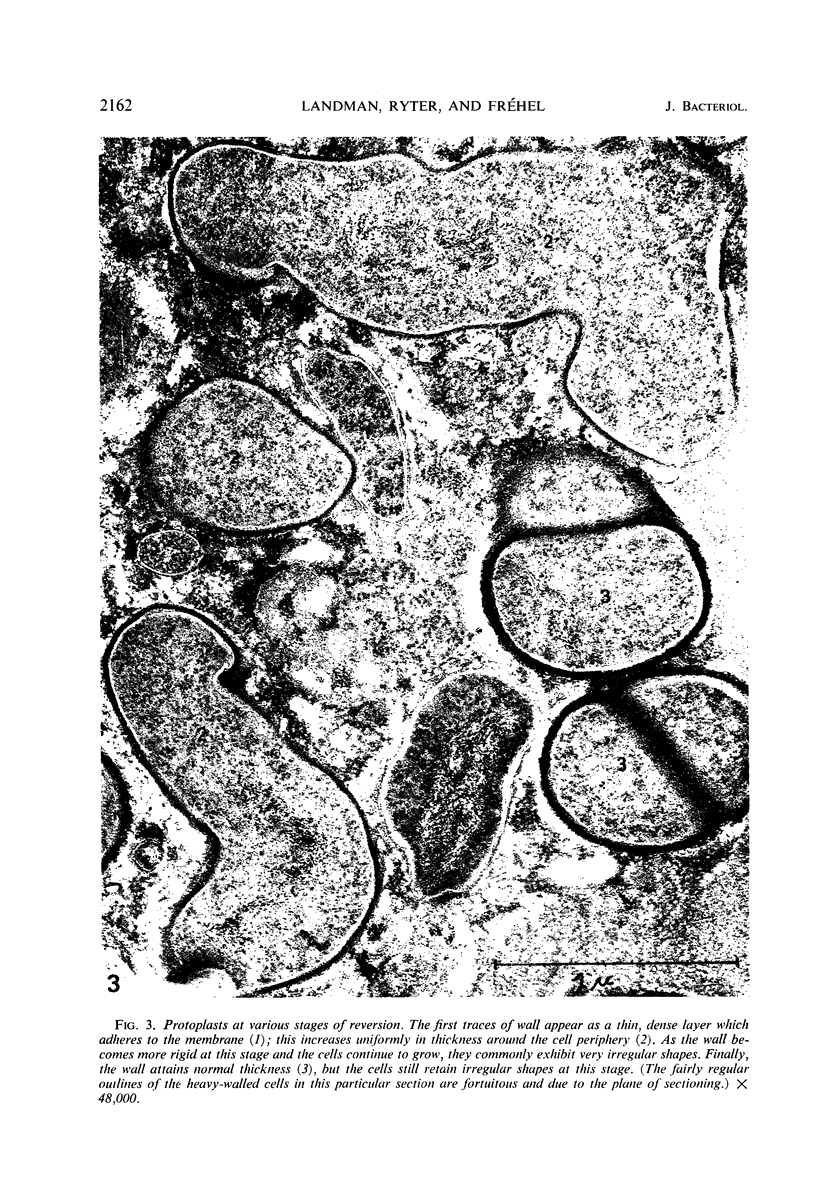
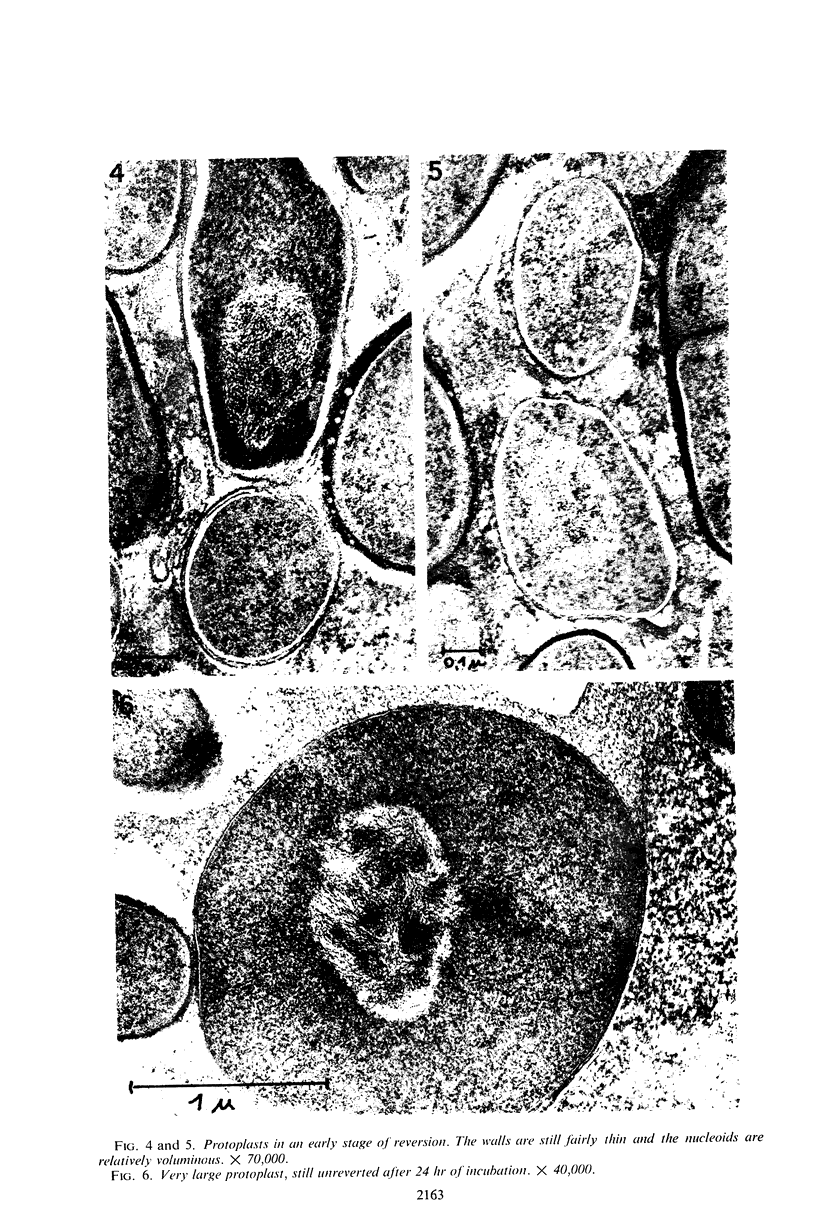
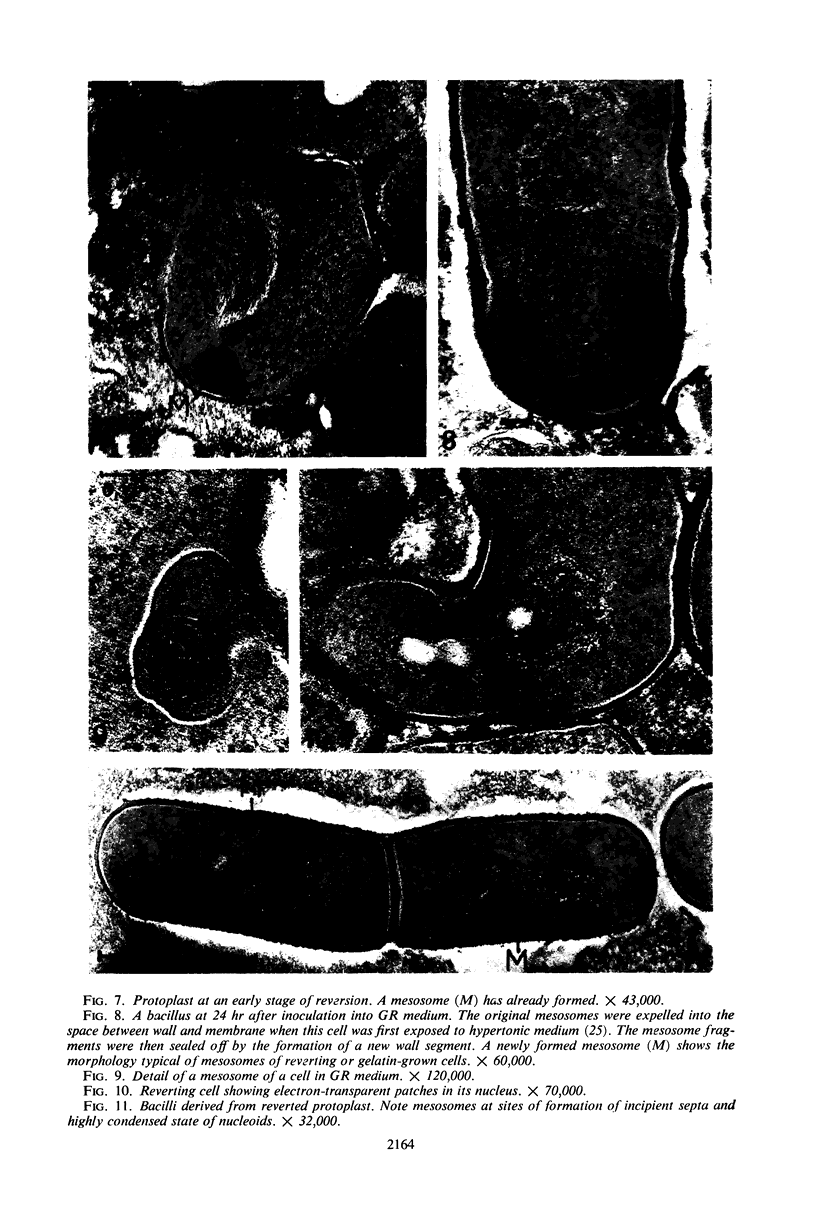
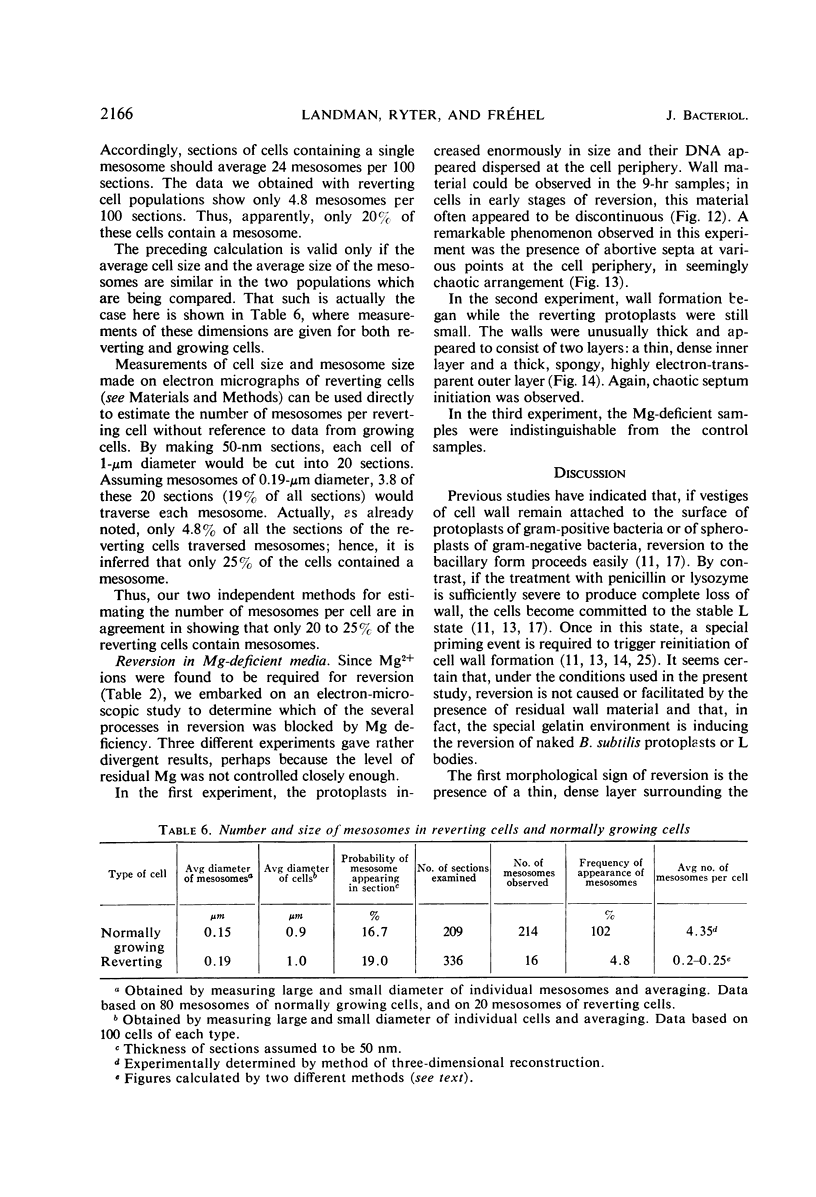
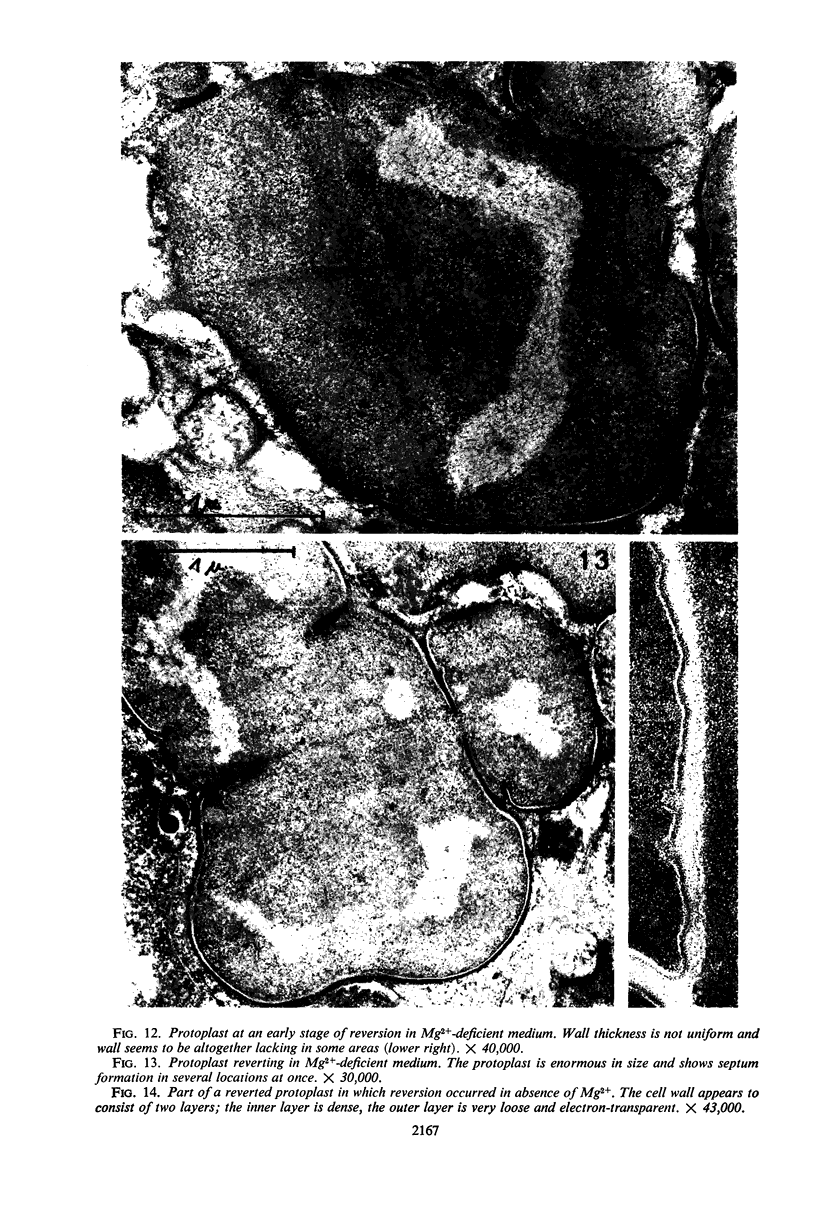
Protoplasts of Bacillus subtilis plated on SD medium form L colonies in quantitative yield and propagate in the L form indefinitely. L bodies or protoplasts placed in 25% gelatin medium form bacillary colonies. Details of the reversion of naked bodies to the walled form are reported. In 25% gelatin medium, reversion begins earlier (about 50% reversion in 4 hr) than the multiplication of bacilli. Thus, virtually all the observed bacillary forms are themselves revertants and not the offspring of a few growing clones. The optimal temperature for reversion is 26 C in 25% gelatin. When cells reverting at 26 C are warmed to 40 C for 3 min, reversion is delayed markedly, whereas viability is unaffected. For electron microscopy, a dense protoplast inoculum was placed on a gelatin surface, incubated, and then fixed in situ. There was no multiplication, but crowding delayed reversion markedly. Successive events of reversion are as follows. The loose nucleoid of the protoplasts condenses in response to the gelatin medium and condenses further and further as reversion proceeds. A thin coat of wall develops around the bodies of various sizes and shapes and then increases uniformly in thickness until a wall of normal aspect is formed. Rod-shaped cells grow out from these bodies—sometimes in several directions at once. A few mesosomes begin to appear only after a thin coat of wall has been formed. These are dense, atypical structures compartmented by membranes. They are located at the cell periphery and do not seem to be in contact with the nucleoids. Quantitative estimates showed that only 20 to 25% of revertant cells or cells grown on gelatin contain even a single mesosome. The others have no mesosome at all. Mesosomes thus do not appear to play a significant role in reversion, and normal mesosome functions must presumably be performed elsewhere in the cell in gelatin-grown bacilli. The role of cell wall, its synthesis, and its chemical nature in successive steps in reversion are discussed.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAM D. ELECTRON MICROSCOPE OBSERVATIONS ON INTACT CELLS, PROTOPLASTS, AND THE CYTOPLASMIC MEMBRANE OF BACILLUS STEAROTHERMOPHILUS. J Bacteriol. 1965 Mar;89:855–873. doi: 10.1128/jb.89.3.855-873.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anraku N., Landman O. E. Control of the synthesis of macromolecules during amino acid and thymine starvation in Bacillus subtilis. J Bacteriol. 1968 May;95(5):1813–1827. doi: 10.1128/jb.95.5.1813-1827.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLE R. M., HAHN J. J. Cell wall replication in Streptococcus pyogenes. Science. 1962 Mar 2;135(3505):722–724. doi: 10.1126/science.135.3505.722. [DOI] [PubMed] [Google Scholar]
- FITZ-JAMES P. FATE OF THE MESOSOMES OF BACILLUS MEGATERIUM DURING PROTOPLASTING. J Bacteriol. 1964 Jun;87:1483–1491. doi: 10.1128/jb.87.6.1483-1491.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrandes B., Chaix P., Ryter A. Localisation des cytochromes de Bacillus subtilis dans les structures mésosomiques. C R Acad Sci Hebd Seances Acad Sci D. 1966 Nov 21;263(21):1632–1635. [PubMed] [Google Scholar]
- Ghosh B. K., Murray R. G. Fine structure of Listeria monocytogenes in relation to protoplast formation. J Bacteriol. 1967 Jan;93(1):411–426. doi: 10.1128/jb.93.1.411-426.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANCOCK R., PARK J. T. Cell-wall synthesis by Staphylococcus aureus in the presence of chloramphenicol. Nature. 1958 Apr 12;181(4615):1050–1052. doi: 10.1038/1811050a0. [DOI] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANDMAN O. E., BAUSUM H. T., MATNEY T. S. Temperaturegradient plates for growth of microorganisms. J Bacteriol. 1962 Mar;83:463–469. doi: 10.1128/jb.83.3.463-469.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANDMAN O. E., GINOZA H. S. Genetic nature of stable L forms of Salmonella paratyphi. J Bacteriol. 1961 Jun;81:875–886. doi: 10.1128/jb.81.6.875-886.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANDMAN O. E., HALLE S. ENZYMICALLY AND PHYSICALLY INDUCED INHERITANCE CHANGES IN BACILLUS SUBTILIS. J Mol Biol. 1963 Dec;7:721–738. doi: 10.1016/s0022-2836(63)80119-9. [DOI] [PubMed] [Google Scholar]
- Leene W., van Iterson W. Tetranitro--blue tetrazolium reduction in Bacillus subtilis. J Cell Biol. 1965 Oct;27(1):237–241. doi: 10.1083/jcb.27.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nermut M. V., Murray R. G. Ultrastructure of the cell wall of Bacillus polymyxa. J Bacteriol. 1967 Jun;93(6):1949–1965. doi: 10.1128/jb.93.6.1949-1965.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RYTER A., JACOB F. ETUDE AU MICROSCOPE 'ELECTRONIQUE DE LA LIAISON ENTRE NOYAU ET M'ESOSOME CHEZ BACILLUS SUBTILIS. Ann Inst Pasteur (Paris) 1964 Sep;107:384–400. [PubMed] [Google Scholar]
- RYTER A., LANDMAN O. E. ELECTRON MICROSCOPE STUDY OF THE RELATIONSHIP BETWEEN MESOSOME LOSS AND THE STABLE L STATE (OR PROTOPLAST STATE) IN BACILLUS SUBTILIS. J Bacteriol. 1964 Aug;88:457–467. doi: 10.1128/jb.88.2.457-467.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryter A., Frehel C., Ferrandes B. Comportement des mésosomes lors de l'attaque de Bacillus subtilis par le lysozyme en milieu hyper- ou hypotonique. C R Acad Sci Hebd Seances Acad Sci D. 1967 Oct 23;265(17):1259–1262. [PubMed] [Google Scholar]
- Ryter A., Jacob F. Etude morphologique de la liaison du noyau à la membrane chez E. coli et chez les protoplastes de B. subtilis. Ann Inst Pasteur (Paris) 1966 Jun;110(6):801–812. [PubMed] [Google Scholar]
- Sedar A. W., Burde R. M. The demonstration of the succinic dehydrogenase system in Bacillus subtilis using tetranitro--blue tetrazolium combined with techniques of electron microscopy. J Cell Biol. 1965 Oct;27(1):53–66. doi: 10.1083/jcb.27.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D., Pooley H. M., Thompson J. S. Autolytic enzyme system of Streptococcus faecalis. 3. Localization of the autolysin at the sites of cell wall synthesis. J Bacteriol. 1967 Nov;94(5):1525–1530. doi: 10.1128/jb.94.5.1525-1530.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAITUZIS Z., DOETSCH R. N. FLAGELLA OF SALMONELLA TYPHIMURIUM SPHEROPLASTS. J Bacteriol. 1965 Jun;89:1586–1593. doi: 10.1128/jb.89.6.1586-1593.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN ITERSON, LEENE W. A CYTOCHEMICAL LOCALIZATION OF REDUCTIVE SITES IN A GRAM-POSITIVE BACTERIUM. TELLURITE REDUCTION IN BACILLUS SUBTILIS. J Cell Biol. 1964 Mar;20:361–375. doi: 10.1083/jcb.20.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Kamp JA O. P., van Iterson W., van Deenen L. L. Studies of the phospholipids and morphology of protoplasts of Bacillus megaterium. Biochim Biophys Acta. 1967;135(5):862–884. doi: 10.1016/0005-2736(67)90056-9. [DOI] [PubMed] [Google Scholar]