Abstract
GABAA receptors are located on the majority of neurons in the central and peripheral nervous system, where they mediate important actions of the neurotransmitter gamma-aminobutyric acid. Early in development the trophic properties of GABA allow a healthy development of the nervous system. Most neurons have a high intracellular Cl-concentration early in life due to the late functional expression of the Cl-pump KCC2, therefore GABA has excitatory effects at this stage. Upon higher expression and activation of KCC2 GABA takes on its inhibitory effects while glutamate functions as the major excitatory neurotransmitter. Like all multisubunit membrane proteins the GABAA receptor is assembled in the ER and travels through the Golgi and remaining secretory pathway to the cell surface, where it mediates GABA actions either directly at the synapses or at extrasynaptic sites responding to ambient GABA to provide a basal tonic inhibitory state. In order to adapt to changing needs and information states, the GABAergic system is highly dynamic. That includes subtype specific trafficking to different locations in the cell, regulation of mobility by interaction with scaffold molecules, posttranslational modifications, that either directly affect channel function or the interaction with other proteins and finally the dynamic exchange between surface and intracellular receptor pools, that either prepare receptors for recycling to the surface or degradation. Here we give an overview of the current understanding of GABAA receptor functional and molecular dynamics that play a major part in maintaining the balance between excitation and inhibition and in changes in network activity.
Keywords: GABAA receptor, receptor trafficking, receptor clustering, inhibition
Introduction
Healthy brain function depends on a precise regulation of neuronal activity. While signalling is initiated by excitatory neurotransmitters, mostly glutamate, the inhibitory neurotransmitters (glycine and GABA) prevent the system from overreacting by damping postsynaptic depolarization that leads to a reduced likelihood of the initiation of action potentials. GABA is the predominant inhibitory neurotransmitter in the brain and acts either via Cl- channels (GABAA receptors) or via the slower metabotropic receptors (GABAB receptors). The striking heterogeneity of GABAA receptors and partially non-overlapping distribution and distinct pharmacology of the subtypes suggests, that these individual subtypes play an important individual role in orchestrating neuronal inhibition and therefore maintain a healthy balance between excitation and inhibition (E/I balance). Varying expression of GABAA receptor genes and subtype specific trafficking are mechanisms to define the identity of cell types, that are only beginning to be understood in detail.
The GABAA Receptor
The GABAA receptor is a member of the cys loop ligand-gated ion-channel superfamily that also comprises acetylcholine receptors, 5-hydroxytryptamine type 3 (5-HT3) receptors and glycine receptors. This structural design is an old theme in evolution. Only recently the X-ray structure of a prokaryotic pentameric ligand-gated ion channel has been published (Hilf and Dutzler, 2008). The high homology to the acetylcholine receptor implies, that the GABAA receptor has a structure, where five subunits are arranged around a central pore that in the case of the GABAA receptor is permeable to Cl− and HCO3− ions (Unwin, 1995). The receptor was originally purified from cow brain by affinity chromatography in the 1980s, but modern cloning techniques revealed a huge heterogeneity of subunits, that have been subdivided into families according to sequence homology (Sigel et al., 1983). Today we know six alpha, three beta, three gamma, one delta, one epsilon, one theta and one pi subunits. Three rho subunits constitute a family of pharmacologically distinct GABAergic Cl− channels that have been termed GABAC receptors. The membrane topology of each subunit is the same as in other members of cys loop ligand-gated ion-channels with a large extracellular N-terminus, that contains the information for subunit assembly, four hydrophobic helices, that cross the membrane and a relatively large intracellular loop between transmembrane regions 3 and 4. The intracellular domain mediates the interaction with cytoplasmic proteins and is the target for posttranslational modifications like phosphorylation, palmitoylation and ubiquitination. Not all mathematically possible subunit combinations occur in vivo, as there exist restrictions in the assembly process and the surface delivery (Rudolph and Möhler, 2004). The most prominent combination consists of alpha, beta and gamma subunits or alpha, beta and delta subunits. A minor population seems to contain only alpha and beta subunits in the adult wildtype organism, but this type of receptor gains importance in animals, where the gamma or delta subunit has been genetically deleted (Bencsits et al., 1999; Günther et al., 1995; Mortensen and Smart, 2006; Tretter et al., 2001). The epsilon subunit seems to be capable of substituting either (but not both) of the alpha subunits, one of the beta subunits and possibly the gamma2 subunit in a 2alpha2beta1gamma pentamer (Bollan et al., 2008). Rho subunits form functional homooligomers that occur in vivo. It has also been shown that beta3 and delta subunits can form homooligomeric receptors, that reach the cell surface in heterologous cells, but these channels are not gated by GABA and whether or not such homomeric channels occur in vivo in the nervous system is still a matter of speculation.
A large amount of effort has been made to elucidate the subunit stoichiometry and arrangement of subunits in the pentamer. Due to the heterogeneity of GABAA receptor subunits with similar molecular weights, this has proved to present a major challenge. Biochemical and electrophysiological approaches using expression of recombinant receptors in heterologous cells led to various results (Baumann et al., 2001; Chang et al., 1996; Farrar et al., 1999; Knight et al., 2000; Tretter et al., 1997). The current accepted conclusion is that GABAA receptor channels are combined from two alpha, two beta and one gamma or delta subunits. This hypothesis is further supported by the fact, that native receptors can contain two different alpha or beta subunits, but whether this stoichiometry is true for all receptors in the brain remains unclear (Li and Blas, 1997). A number of studies have also addressed the arrangement of subunits within the receptor. This work suggests that subunits specifically interact with each other predominantly at the interfaces of their extracellular amino termini and therefore assembly is not arbitrary, but follows the rules of protein–protein interactions (Bollan et al., 2003; Klausberger et al., 2000, 2001a,b). In receptors with two alpha, two beta and one gamma subunit, an arrangement of alpha-beta-alpha-beta-gamma clockwise when viewed form the synaptic cleft has been deduced from subunit interaction analysis and electrophysiological studies on tandem constructs (Baumann et al., 2002).
The subunit composition determines the functional as well as the pharmacological properties of the individual receptor type. Varying functional receptor parameters are: the concentration of ligand, that is necessary to obtain activation (EC50), the rate of activation following the exposure to the ligand, the rate and extent of desensitization in the presence of the ligand, the deactivation of the current following agonist removal, the mean open and closed times, burst durations and the open probability, when the receptor is fully occupied with ligand (Farrant and Nusser, 2005).
The GABAA receptor is especially interesting in pharmacological terms, as there are binding sites for a multitude of physiologically, clinically or chemically relevant substances that modify the function of the receptor in a positive or negative way. Binding of substances in vivo leads to a neuronal inhibition that is either enhanced (sedative, anxiolytic, narcotic, muscle relaxant effect) or reduced (spasmic, convulsive, epileptogenic effect but also enhanced cognitive functions depending on substance and dose). The most prominent substances that bind to the GABAA receptor on distinct sites are GABA itself, benzodiazepines, gaboxadol, loreclezole, furosemide, barbiturates, neurosteroids, anesthetics and alcohol (Korpi et al., 2002). The individual subtypes exhibit specific response patterns to the drugs, a fact that carries the major hope of researchers and pharmaceutical companies to develop drugs, that specifically target individual receptor populations to treat psychiatric disorders efficiently, but at the same time avoid unwanted side effects (Korpi and Sinkkonen, 2006).
GABAergic Effects in Development
Early in development the expression of the NKCC1 transporter, which is driven by sodium and potassium gradients, leads to the accumulation of high chloride concentrations inside the young neuron. The first functional synapses to be formed are GABAergic, as glutamatergic inputs require a more mature postsynaptic target. The high intracellular chloride concentration induces an efflux of chloride ions through the activated GABAA receptor, thereby depolarizing the cell. At this stage GABA exerts also trophic effects by positively stimulating neuronal migration, cell division and neuritic growth. The activation of GABAA receptors also generates calcium currents by activating voltage-dependent calcium channels. The resulting depolarization is sufficient to remove the voltage dependent magnesium block from NMDA receptors, which contributes to the development of a more mature neuronal circuit. Additionally to its pure excitatory effects GABA can also have a shunting effect on glutamatergic activity by clamping the membrane potential to the chloride equilibrium potential, which raises the spike threshold and reduces network activity. The pure inhibitory effects of the GABAA receptors later in development (between postnatal day 3 and 12) are initiated by the downregulation of NKCC1 and through a Calcium dependent transcriptional regulation the expression of a new transporter is enhanced: the K-Cl cotransporter KCC2. KCC2 pumps chloride ions out of the cell, thereby converting GABAergic transmission from excitatory to inhibitory. GABA itself mediates this transition by miniature postsynaptic currents independent of action potentials (Ben-Ari, 2002). Blaesse et al. (2006) studied neurons of the lateral superior olive in the brainstem and found, that the low amounts of KCC2, that can be found in the plasma membrane of immature neurons are transport-inactive monomers and that oligomerization later in development is essential for their chloride transport activity.
In contrast to the equilibrium potentials of sodium and potassium ions, the equilibrium potential for chloride ions lies very close to the resting membrane potential, so that only small changes in the activity of chloride transporters can reduce inhibition or even invert the direction of the chloride ion flux. This is frequently the case in changes of the state of neuronal activity or under pathological conditions like epilepsy, oxidative stress or chronic pain (Cohen et al., 2002; Coull et al., 2003; Rivera et al., 2002; Woodin et al., 2003).
Surface expression and fast changes in the activity of KCC2 like during oxidative stress are mediated by regulating the phosphorylation state of KCC2 (Lee et al., 2007; Wake et al., 2007). Nerve injury frequently results in a decrease of KCC2 messenger RNA (Nabekura et al., 2002). Additionally KCC2 seems to have an impact on neuronal morphology that is independent of the transporter activity. Cortical neurons from KCC2 knock out mice exhibit an abnormal morphology of dendritic spines that can be rescued by overexpression of a transport inactive mutant of KCC2. Therefore KCC2 plays a vital role in the maturation of excitatory and inhibitory synapses during neuronal maturation (Li et al., 2007).
The GABAergic Synapse
The ultrastructure of inhibitory synapses mostly appears symmetric, which means, that the pre- and postsynaptic structure appears equally dense as opposed to glutamatergic synapses, where the postsynapse is extremely dark (PSD, postsynaptic density). GABAergic terminals mostly synapse onto dendritic shafts and the nerve cell soma while glutamatergic synapses target preferentially dendritic spines. The sequence of events that lead to the formation of inhibitory synapses is still unknown. Cell adhesion molecules are prime candidates to establish a connection between the presynaptic axonal bouton and the postsynaptic site (Craig et al., 2006; Shapiro et al., 2007). The neurexin/neuroligin system has received considerable attention in this regard (Craig and Kang, 2007). The presynaptic transmembrane protein beta-neurexin binds to the postsynaptic neuroligins (NL1-4), therefore bridging the synaptic cleft. This interaction can trigger synaptogenesis in non-neuronal cells as shown by Scheiffele et al. (2000). Mixed cultures of neurons and transfected fibroblast cells revealed that expression of beta-neurexin induces clusters of the postsynaptic marker gephyrin and vice versa, the expression of neuroligins recruits GAD-65 positive vesicles in the presynaptic terminals (Graf et al., 2004). Similarly, alpha-neurexins induce clustering of gephyrin, GABAA receptor subunit gamma2 and neuroligin-2, but not of PSD-95 or neuroligin-1/3/4 (Kang et al., 2008). Nevertheless these proteins do not seem to be the original pioneers of synaptogenesis in vivo, as the brains and cultured neurons from NL1-4 knock out mice exhibit an unaltered density of synaptic contacts. Nevertheless the animals die shortly after birth due to respiratory failure, which is a consequence of reduced GABAergic, glycinergic and glutamatergic synaptic transmission in the brainstem that controls respiration (Chih et al., 2006; Levinson and El-Husseini, 2005; Varoqueaux et al., 2006; Washbourne et al., 2004). Neuroligins enhance synapse formation in vitro, but obviously are not required for the generation of synapses in vivo. Overexpression or deletion of neuroligins enhances or respectively decreases synaptic responses (neuroligin-1 for excitatory synapses, neuroligin-2 for inhibitory synapses), but the effect is activity dependent. Therefore neuroligins do not establish synapses per se, but support their maturation by recruiting synaptic proteins and influence synaptic activity (Chubykin et al., 2007). The scaffold protein of excitatory synapses, PSD 95, binds to all neuroligins, although neuroligin-2 is preferentially found at inhibitory synapses. The expression level of PSD 95 seems to influence the relative amounts of excitatory and inhibitory synapses and therefore critically affects the E/I balance (for details see Keith and El-Husseini in this issue) (Liu, 2004).
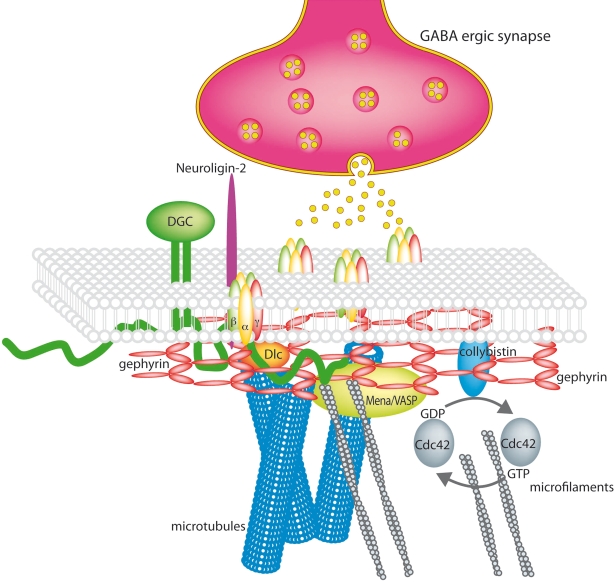
A key organizer molecule of GABAergic synapses is the 93kD protein gephyrin. It was originally copurified with the glycine receptor from spinal cord, but was also prominently found at GABAergic synapses in the absence of the glycine receptor (Kneussel et al., 1999). In contrast to the glycine receptor, it did not copurify with any GABAA receptor under standard biochemical conditions, therefore an intermediate linker protein was postulated. One hot candidate would have been the 14kD protein GABARAP, that interacts with the GABAA receptor gamma2 subunit as well as with gephyrin, but confocal microscopy as well as electronmicroscopic studies revealed, that only minute amounts of GABARAP are found in the synapses, while the majority is located in intracellular compartments implying a role of GABARAP in trafficking rather than in synaptic clustering.
Recently it has been demonstrated that gephyrin is capable of a detergent sensitive direct interaction with GABAA receptor alpha subunits (Tretter et al., 2008). The synaptic formation of gephyrin and GABAA receptor clusters are mutually dependent on each other: neuronal cultures from gephyrin knock out mice show an extensive loss of alpha2 and gamma2 containing GABAA receptor clusters and vice versa in GABAA receptor gamma2 subunit knock out mice the synaptic gephyrin clusters are absent and the remaining alpha-beta containing receptors are diffusely distributed on the cell surface (Craig et al., 1996; Essrich et al., 1998; Günther et al., 1995). However the exact role of the gamma2 subunit in synaptic targeting is still unknown. Alldred et al. (2005) showed, that in gamma2 deficient neurons only transfection with exogenous gamma2, but not alpha2 rescues synaptic clustering of GABAA receptors, the recruitment of gephyrin and normal amplitude and frequency of mIPSCs. They identified the fourth transmembrane region of the gamma2 subunit as the essential domain to mediate the effects. A way of communication between presynaptic inputs and postsynaptic organization has been shown by the fact, that reducing gephyrin expression for instance with small hairpin RNAs results in a reduction of opposite GABAergic presynaptic boutons (Yu et al., 2007).
Several gephyrin interacting proteins have been identified meanwhile: the guanine nucleotide exchange factor (GEF) collybistin, that catalyzes GTP-GDP exchange on Rho family GTPase Cdc42, that plays an important role in controlling cell shape, by affecting the actin cytoskeleton (Kins et al., 2000; Reid et al., 1999). It might support the trafficking of proteins to the subsynaptic actin cytoskeleton (Kneussel and Betz, 2000). One of the two known splice variants, collybistin II, recruits otherwise intracellular gephyrin to the membrane of heterologous cells, where interaction with coexpressed receptors can take place (Harvey et al., 2004; Kins et al., 2000). Collybistin knock out mice exhibit a region specific loss of postsynaptic gephyrin and GABAA receptor clusters in the hippocampus and the basolateral amygdala. As a result synaptic plasticity is changed and results in an increase of LTP (longterm potentiation) and a decrease of LTD (longterm depression), two recordable functional parameters, that are believed to underlie learning and memory. Behavioural tests revealed, that these mice have increased levels of anxiety and an impaired capability of spatial learning (Papadopoulos et al., 2007).
An indirect interaction of gephyrin with the actin cytoskeleton is mediated by the actin binding proteins profilin I and IIA and by the microfilament adaptors of the Mena/VASP family that also interact with each other and regulate microfilament dynamics (Lambrechts et al., 2000). Local transport of synaptic proteins might be mediated by the gephyrin interactors Dlc-1 and Dlc-2, that are homologous to dynein light chain and provide a link to microtubules and microfilaments (Fuhrmann et al., 2002). These proteins are not especially enriched in inhibitory synapses and electron microscopy even identified a fraction of gephyrin-positive synapses negative for Dlc. Dlc immunoreactivity is found throughout the cytosol, frequently associated with the cytoskeleton. This implies a function of Dlc in active transport and the electron microscopy data represent a snapshot of cargo linked to the cytoskeleton by the adaptor Dlc. Dynein itself is involved in several migration processes as the retrograde transport of vesicles in axons. Overexpression of a gephyrin mutant with a deleted binding site to Dlc does not result in a reduced targeting of gephyrin to the synapses implying a potential role in the retrograde transport of the protein. Dlc1/2 also occur in excitatory synapses of dendritic spines, where they bind to GKAP/PSD-95. Although final proof is missing, these data suggest an important role of Dlc proteins in regulating synaptic strength through transport mechanisms of scaffold proteins in inhibitory and excitatory postsynaptic specializations.
Some GABAergic synapses containing the alpha1 or alpha2 subunit are associated with the dystrophin-glycoprotein complex (DGC) that links the extracellular matrix (ECM) with the cytoskeleton. The DGC has a prominent role at the neuromuscular junction for the maturation and the maintenance of the synapse, but its precise role at inhibitory synapses is still not fully understood. The transmembrane protein beta-dystroglycan binds alpha-dystroglycan at the extracellular side of the membrane and dystrophin and utrophin intracellularly. Binding partners of the DGC are the ECM proteins agrin and laminin, but also the presynaptic protein neurexin. Dystroglycan appears at GABAergic synapses after the presynaptic GAD and the postsynaptic GABAA receptors and gephyrin, but independent of the presence of gephyrin or dystrophin. The absence of dystrophin in mdx mice results in a reduction of GABAA receptor cluster number and size (Kneussel and Betz, 2000). Interestingly, upon elimination of dystroglycan, the whole DGC disappears from GABAergic synapses without having an obvious effect on receptor clusters. When receptor clusters are disrupted, for instance in gamma2 or gephyrin mutant mice, the DGC still localises opposite GABAergic terminals, but is absent from glutamatergic synapses. The DGC obviously does not induce synaptogenesis, but helps with the maturation and stabilization of a subset of GABAergic synapses (Brünig et al., 2002; Lévi et al., 2002) (Figure 1).
Figure 1.
The GABAergic synapse (modified and reproduced with permission from Lüscher and Keller 2004). Synaptic GABAA receptors are stabilized by a submembranous lattice of gephyrin by direct interaction. Cytoskeleton associated proteins are Dlc1/2 and Mena/VASP. Collybistin, a guanine nucleotide exchange factor is membrane associated and interacts with gephyrin. The dystrophin-glycoprotein complex (DGC) stabilises the synapse and neuroligins bridge the synaptic cleft by interaction with presynaptic neurexins.
Synaptic and Extrasynaptic GABAA Receptors and Different Forms of Inhibition
The postsynaptic site contains clusters of 10 to several 100 receptors directly opposite to the presynaptic terminal, the remaining receptors are located perisynaptically (<30 μm distant from the centre synapse) or at extrasynaptic sites. The activation of synaptic receptors is transient carrying over the information from the action potentials of the presynaptic neuron, that cause high concentrations of GABA (1.5–3 mM) to be suddenly released into the cleft. The signal is terminated by the removal of the transmitter from the synaptic cleft by presynaptic GABA transporters or passive diffusion away from the synapse and deactivation of postsynaptic receptors (Jensen et al., 2003). The subunit composition of the postsynaptic GABAA receptors varies between types of synapses and seems to be influenced by pre- and postsynaptic cues. Receptors with strong synaptic localization frequently contain the alpha1, alpha2 or alpha3 subunit. Several efforts have been undertaken to quantify the individual subtypes in welldefined interneuron/pyramidal cell or interneuron/interneuron synapses by electron microscopy and proved the importance of the identity of both cell types. This makes sense in neuronal networks, as it provides every synapse with its own identity and explains the enormous potential for regulation and information processing (Klausberger et al., 2002; Nyíri et al., 2001). Bitufted interneurons, that synapse onto dendrites of neocortical pyramidal cells use postsynaptic alpha5 containing receptors as their IPSCs can be reduced by the alpha5-selective inverse agonist IAalpha5, but are insensitive to the alpha1 preferring zolpidem (Ali and Thomson, 2007). This shows, that the subcellular targeting of GABAA receptor subtypes must be a complex process far from being understood yet, as alpha5 has long been regarded as a subunit mostly occurring in extrasynaptic receptors.
Extrasynaptic receptors are exposed to persistent amounts of ambient GABA (microM), partly derived from synaptic spill over or other non-neuronal sources. This rather low concentration of GABA especially activates receptor subtypes with high affinity but low efficacy for the agonist (which means an inefficiency of coupling GABA binding to channel gating) and provides the neuron with a so called “tonic” inhibition (Farrant and Nusser, 2005). This source of inhibition is not negligible compared to the “phasic” inhibition of synaptic GABA receptors. It is responsible for generating 75% of the total inhibitory charge received by hippocampal neurons (Mody and Pearce, 2004). It is mostly carried by receptors containing the delta subunit in neurons of the neocortex, thalamus, striatum, the dentate gyrus granule cells and cerebellar granule cells where it is mostly combined with the alpha4 and alpha6 subunit (Wisden et al., 2002). Other candidates are sole alpha/beta combinations without gamma found in hippocampal neurons, receptors containing the epsilon subunit that even reveal GABA independent openings or the alpha5 subunit in the CA1/CA3 pyramidal cells of the hippocampus mostly combined with the gamma2 subunit. Some alpha5 also occurs in synapses (Serwanski et al., 2006). Deletion of the alpha5 subunit results in the upregulation of otherwise in this celltype undetectable receptors containing the delta subunit that provide some small rescuing tonic inhibition. Complete abolishment of tonic inhibition in CA3 pyramidal cells from alpha5/delta subunit double knockouts results in the occurrence of spontaneous gamma oscillations (Glykys et al., 2008). In cerebellar granule cells the tonic inhibition is solely carried by extrasynaptic receptors containing the delta subunit. Deletion of this subunit is compensated through homeostatic plasticity by a change in magnitude of the voltage-independent K+ channel TASK 1 (Brickley et al., 2001). Both subunits have attracted considerable interest per se: alpha5 seems to be involved in hippocampus-dependent spatial learning as tested in the water maze and trace fear conditioning. A recent study implied a role of alpha5 containing receptors in mediating the sedative properties of benzodiazepines (Collinson et al., 2002; Crestani et al., 2002; Savić et al., 2008). Receptors containing the delta subunit are especially sensitive targets of endogenous and exogenous neuroactive steroids, that enhance the efficacy of GABA at the receptors and therefore increase tonic inhibition (Belelli and Lambert, 2005). Other interesting compounds like gaboxadol are by themselves more efficacious than GABA on these receptors. Gaboxadol has potential for therapeutic use as a novel sleep aid or the treatment of premenstrual dysphoric disorder (Glykys and Mody, 2007).
GABAA Receptor Interacting Proteins
GABAA receptor intracellular loops are the sites of interaction with intracellular proteins that either modify the functional properties of the receptors or mediate their maturation, intracellular transport and stabilization at the synapse.
Kinases and phosphatases
Kinases known to phosphorylate GABAA receptors include the cAMP-dependent protein kinase (PKA), Protein kinase C (PKC), Ca2+/calmodulin dependent kinase II (CaMK-II), Protein kinase B (Akt) and tyrosine kinases of the Src family.
PKA can either potentiate or depress GABAA receptor function (Angelotti et al., 1993; Kapur and Macdonald, 1996; McDonald et al., 1998; Moss et al., 1992; Nusser et al., 1999; Poisbeau et al., 1999; Porter et al., 1990). PKA is recruited to GABAA receptors containing the beta1 or beta3 subunit via the adapter protein AKAP79/150 (Brandon et al., 2003). It phosphorylates beta3 on serine 408 and 409, thereby enhancing receptor function. Beta1 is solely phosphorylated on serine 409, which inhibits receptor function. Beta2 is not affected by PKA in HEK293 cells (McDonald et al., 1998). Activation of PKA can also trigger the phenomenon of rebound potentiation (RP) of GABAA receptor function, a long-term upregulation of inhibitory synaptic transmission in cerebellar Purkinje cells following climbing fiber activation (Kano et al., 1992, 1996). Recent studies started to bring light into the cascade of events in RP and identified more involved players like GABAB receptors, protein phosphatase 1 (PP1) and metabotropic glutamate receptor type 1 (mGluR1) (Sugiyama et al., 2008, see below).
Protein kinase C (PKC) is recruited to the GABAA receptor via the anchoring protein RACK-1 and similar to PKA has different effects on receptor function depending on the subunit composition (Brandon et al., 2002; Connolly et al., 1999; Herring et al., 2005; Jovanovic et al., 2004; Krishek et al., 1994; Leidenheimer et al., 1992; Lin et al., 1996; Poisbeau et al., 1999). PKA and PKC mediate the effects of serotonin on the GABAergic system via 5HT4 and 5HT2 receptors respectively in the prefrontal cortex (Yan, 2002). PKA is also the mediator of the influence of the dopamine system on GABAA receptors in the nucleus accumbens (Chen et al., 2006). The dopamine D3 receptor in the nucleus accumbens (NAC) is believed to play an important role in reinforcement and reward. A component of this effect might be, that D3 receptor agonists reduce GABAergic mIPSCs in NAC slices by inhibiting adenylyl cyclase and therefore the activity of PKA. PKA negatively regulates the endocytosis of the GABAA receptor via phosphorylation of an atypical binding motif for the clathrin AP-2 adaptor complex (see below).
No adapter protein is known for CaMK-II, although the enzyme can also be pulled down with the GABAA receptor (McAinsh, Tretter, Moss unpublished observations). Its effects on the GABAA receptor differ in the neuronal environment compared to HEK293 cells implying the contribution of some essential neuronal factor (Houston and Smart, 2006). In vitro phosphorylation sites of the GABAA receptor by CaMK-II are beta1-serine 384 and 408, 409, beta2-serine 410, beta3-serine 383 and 408, 409, gamma2S/L-serine 348 and threonine 350, gamma2L-serine 343 (Machu et al., 1993; McDonald and Moss, 1994, 1997). CaMK-II also plays an important role in rebound potentiation, but the full mechanism and sequence of events has not been yet elucidated.
Additionally, GABAA receptors are substrates for a number of phosphatases. Ca/calmodulin-dependent phosphatase 2B (Calcineurin) binds directly to the intracellular loop of the GABAA receptor gamma2 subunit, thereby dephosphorylating the receptor and inducing long-term depression (LTD) of synaptic efficacy at inhibitory synapses of the CA1 region of the hippocampus, a mechanism, that is believed to contribute to learning and memory (Wang et al., 2003). Protein phosphatase 1alpha (PP1A) regulates the phosphorylation of GABAA receptor beta subunits through PKA. Terunuma et al. (2004) proposed the following model: activated G-protein coupled receptors increase intracellular cAMP levels that enhance PKA activity. PKA phosphorylates GABAA receptors but also the GABAA receptor associated protein PRIP-1 (Phospholipase C-related inactive protein type I) that under basal conditions binds PP1A and keeps it in an inactive form. The phosphorylated PRIP-1 releases PP1A that results in dephosphorylation of the GABAA receptor beta subunit and a dynamic regulation of synaptic efficiency. Apart from the direct regulation of receptor function by the phosphorylation state, the PRIP protein family (PRIP-1 and PRIP-2) is also involved in GABAA receptor endocytosis, which is another factor to regulate synaptic inhibition (Kanematsu, 2007, see below). Similarly, brain-derived neurotrophic factor (BDNF) produces short-term and long-term effects via modulation of the phosphorylation state of GABAA receptors. BDNF signalling via TrkB receptors results in the phosphorylation of serines 408/409 in the beta3 subunit by PKC, thereby transiently increasing mIPSC amplitudes. Phosphorylation of serines 408/409 then increases binding affinity and recruitment of PP2A that dephosphorylates the receptor and subsequently reduces the efficacy of synaptic inhibition in the longterm (Jovanovic et al., 2004).
Interacting proteins involved in GABAA receptor trafficking
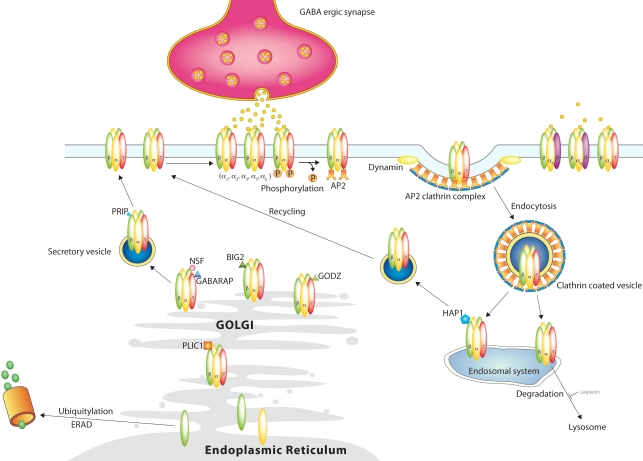
A number of proteins have been demonstrated to interact directly with GABAA receptor intracellular domains and to be important for regulating the membrane transport and localization of GABAA receptors (Figure 2).
Figure 2.
GABAA receptor trafficking. GABAA receptors are synthesized and assembled in the Endoplasmic Reticulum and matured in the Golgi and reach the surface outside of synapses through the secretory path. Synaptic receptors reach their destination through lateral movement in the plasma membrane, where they mediate phasic inhibition. Extrasynaptic receptors are activated by lower concentrations of GABA in the extracellular space and mediate tonic inhibition. Phosphorylation not only affects receptor function, but also regulates their removal from the surface through clathrin mediated endocytosis. From the endosomal system the receptors are either recycled to the surface or degraded in the lysosomes. This degradation can be blocked by leupeptin. Another degradation system works through the proteasome after ubiquitylation.
GABARAP (GABA receptor associated protein)
Upon its discovery in a yeast two-hybrid screen with the intracellular loop of the gamma2 subunit, GABARAP was regarded as the prime candidate for synaptic GABAA receptor clustering. More recent studies have suggested that in fact GABARAP may be an intracellular trafficking factor for the receptor and/or to be an important regulator of channel kinetics (Everitt et al., 2004; Luu et al., 2006). GABARAP is mainly localized in intracellular compartments, such as endoplasmatic reticulum, Golgi structures and intracellular vesicles near the synapses, but only a small fraction is directly colocalized with synaptic GABAA receptors (Kittler and Moss, 2001; Kneussel and Betz, 2000; Leil et al., 2004). Overexpression of GABARAP in heterologous cells or cultured hippocampal neurons enhances the cell surface expression of gamma2 containing GABAA receptors. This effect depends on polymerized microtubules (Chen et al., 2005). GABARAP has also recently been demonstrated to be important for plasticity of GABAA receptor function during rebound potentiation in the cerebellum (Kawaguchi and Hirano, 2007). The GABARAP knock out mouse appears healthy and exhibits normal synaptic GABAA receptor clustering (O'Sullivan et al., 2005). This implies that its function can be replaced by other cellular proteins, for instance other members of the same protein family which exhibit high homology to GABARAP including GATE-16 (Golgi-associated ATPase enhancer of 16kDa), that supports intra-Golgi transport. Another family member is GEC-1, that like GABARAP binds the gamma2 subunit, tubulin and NSF and plays an important role in surface expression of kappa-opioid receptors (Chen et al., 2006). The GABARAP family is structurally and functionally related to ubiquitin, but the final acceptor molecule of the E1-3 enzyme catalyzed reaction is not a protein, but the membrane lipid phosphatidylethanolamine, that might facilitate insertion into membranes (Chen and Olsen, 2007). GABARAP and GATE-16 are mammalian homologues of the yeast protein Aut7 associated with autophagosomes. Autophagy is responsible for the majority of intracellular protein degradation in particular during starvation and apoptosis. It also plays a role in the remodelling of mammalian cells and pathogenesis, where small aggregates of abnormal proteins are degraded by autophagy. Cytoplasmic constituents including organelles are enwrapped by a membrane sac called isolation membrane. The resulting autophagosomes fuse with endosomes or lysosomes, so that lysosomal hydrolases can degrade the cytoplasm derived contents. The process was originally studied in yeast, where more than 16 genes were found to be required for autophagosome formation. Most of them have homologues in higher eukaryotes including mammals. Both, GABARAP and GATE-16 can reside on autophagosomal membranes. An ubiquitin like conjugation system is required in the elongation of the isolation system. GABARAP and GATE-16 become processed by Aut2/Apg4 homologues to expose a C-terminal glycine and are catalyzed by Apg7 (an E1 like enzyme) and Aut1/Apg3 (an E2 like enzyme). They also interact with other proteins in the autophagic pathway, like ULK1 (Mizushima et al., 2002).
A protein interacting with GABARAP, GRIP (Glutamate receptor interacting protein), has attracted some interest, as it is a PDZ (postsynaptic density protein, Discs large, Zonula occludens-1) protein, localized to glutamatergic synapses (Kittler et al., 2004). It has been detected in GABAergic synapses in cultured hippocampal neurons and at GABAergic synapses of the intact brain (Yu et al., 2008). GRIP1 and GABARAP colocalize in intracellular compartments (Golgi), where they might be part of the trafficking system of GABAA receptors. In agreement with this, a recent study by Marsden and coworkers proved, that both GABARAP and GRIP1 play a role in the NMDA induced plasticity of GABAergic synapses. The (chemical) activation of NMDA receptors not only removes AMPA receptors from the membrane leading to LTD, but also increases the synaptic GABAA receptor surface expression in hippocampal neurons. By using specific inhibitors and RNAi they showed the involvement of CaMKII, GABARAP, GRIP and NSF, though the full sequence of events remains to be determined (Marsden et al., 2007).
Plic-1
Plic-1, which was also identified as a GABAA receptor binding partner using the two-hybrid system, interacts with the intracellular loop of the GABAA receptor alpha1, alpha2, alpha3, alpha6 and beta1-3, but not gamma2 or delta subunits. Its name stems from its interaction with IAP (integrin-associated protein, CD47) and vimentin-containing intermediate filaments (Protein linking IAP and cytoskeleton). Plic-1 has an N-terminal ubiquitin like domain, and an ubiquitin-associated domain in the C-terminus which mediates the interaction with the GABAA receptor subunits. Electron microscopy revealed its presence in clathrin-coated pits, the border of the Golgi apparatus, the ER and directly at GABAergic synapses. Its main function at synapses seems to be assistance with receptor insertion into the membrane without affecting channel function. Furthermore it stabilises intracellular receptors, probably by inhibiting polyubiquitination and proteosomal degradation (Bedford et al., 2001).
Huntingtin-associated protein 1 (HAP1)
HAP1 was originally found associated with huntingtin, the protein product of the Huntington's disease gene. Yeast two-hybrid screens revealed its interaction with the GABAA receptor beta1-3 subunits (Kittler et al., 2004). It is a cytosolic protein with coiled-coil domains and several N-myristoylation sites that probably facilitate its association with membranes of the cell surface or intracellular vesicles. It exists as two C-terminal splice variants, HAP1a and HAP1b (75/85kDa). It directly interacts with the p150glued subunit of the dynein/dynactin microtubule-based motor complex, suggesting a role for HAP1 in intracellular trafficking events (Engelender et al., 1997; Gauthier et al., 2004; Li et al., 1998). HAP1 is located in dendrites, axons and perinuclear structures in the soma. Its overexpression in cultured cortical neurons enhances the stability of internalized GABAA receptors, facilitates their recycling to the plasma membrane and therefore increases the number of cell surface receptors. This correlates with a significant increase in the mean amplitude of mIPSCs, without changing their frequency or kinetic parameters. In vivo, HAP1 dependent trafficking of GABAA receptors has been demonstrated to be important for regulating the GABAergic system in the hypothalamus, that influences eating behaviour (Dragatsis et al., 2004; Sheng et al., 2006). The precise mechanism of HAP1 action is still unknown, as essentially both of the two most likely scenarios are possible: enhancement of recycling or inhibition of degradation.
GABAA receptor interacting factor-1 (GRIF-1)
GRIF-1 was identified as a protein interacting with the beta2 subunit (Beck et al., 2002). It contains alpha-helical structures and a coiled-coil domain, which might be involved in protein-interactions. It is a homologue of HAP1 and the Drosophila protein Milton, that is essential for the kinesin-mediated transport of mitochondria to nerve terminals (Stowers et al., 2002). The role of GRIF-1 in regulating GABAAR function remains unclear, however it can interact directly with kinesin motor proteins (Brickley et al., 2005). This makes a participation in trafficking and transport of receptors a realistic suggestion. Interaction between GABAA receptors and GRIF-1 has not been demonstrated in vivo and no functional studies have yet been carried out to directly address what role this protein may have in regulating GABAA receptor function.
Brefeldin A-inhibited GDP/GTP exchange factor 2 (BIG2)
BIG2 is a 200kDa GDP/GTP exchange factor on the small G-protein ADP-ribosylation factors (ARF) that are involved in the trafficking of proteins through the Trans-Golgi Network (TGN) into the exocytotic pathway. BIG2 binds to the intracellular loop of all beta subunits (Charych et al., 2004). Co-expression of BIG2 and the beta3 subunit results in the loss of the beta3-containing GABAA receptors from ER and Golgi. It is currently proposed that BIG2 might play a role in the transport of newly assembled GABAA receptors by clathrin/AP-1 coated vesicles to the synaptic plasma membrane and might also play a role in receptor recycling (Shen et al., 2006; Shin et al., 2004).
Golgi-specific DHHC zinc finger protein (GODZ)
The GABAA receptor gamma2 subunit intracellular loop is subject to palmitoylation on five cysteins, that influences GABAA receptor clustering and cell surface stability (Keller et al., 2004; Rathenberg et al., 2004). In a yeast two-hybrid screen GODZ was found as a gamma 2 interacting protein that recognizes the cysteine rich domain of gamma 1-3 next to the GABARAP binding site. GODZ contains a DHHC-CRD domain and is predicted to be a protein with four transmembrane regions and an intracellular N- and C-terminus and to have palmitoyltransferase activity. GODZ is mainly located in the Golgi apparatus, and when overexpressed in HEK cells, the gamma2 subunit gets mostly trapped intracellularly together with GODZ. Upon coexpression with alpha2beta3gamma2, GODZ partly leaves the Golgi to guide the receptor to the surface. It most probably is the neuronal enzyme, that in vivo palmitolates the GABAA receptor gamma2 subunit. In agreement with this, disrupting GODZ function or expression levels using dominant negative or RNA interference approaches results in a significant reduction in the number of synaptic GABAA receptors and the amplitude of mIPSCs (Fang et al., 2006).
Radixin
Radixin is a member of the ERM (ezrin/radixin/moesin) family of proteins that are structurally similar with a N-terminal FERM domain and a C-terminal F-actin binding site that links them to the cytoskeleton. Radixin interacts with several transmembrane proteins, one of them being the alpha5 subunit of the GABAA receptor. Only minute amounts of alpha 5 receptors occur synaptically, the majority is located in clusters at extrasynaptic sites (Serwanski et al., 2006). Radixin needs to be activated by phosphorylation to open an intramolecular binding between the N- and C-terminus before it can link the alpha5 subunit to actin. Radixin clusters alpha5 receptors independent of the gephyrin system, although the advantage of cluster formation at extrasynaptic sites is not clear yet. After dominant negative disruption of the interaction with radixin, unclustered alpha5 receptors remain fully functional in mediating tonic inhibition (Loebrich et al., 2006).
gC1q-R
The multifunctional protein gC1q-R is known to be involved in oxidative phosphorylation in mitochondria. Additionally it occurs in several other cellular locations in non-neuronal cells. Schaerer et al. (2001) identified this 34kD protein as a strong interactor with GABAA receptor beta subunits. As its extramitochondrial abundance in GABAergic neurons is low, its role with regard to this receptor is unknown. The binding site on beta2 contains seven positively charged aminoacids and serine 410, a known phosphorylation site, that modifies receptor function.
GABAA Receptor Mobility in the Postsynaptic Membrane
The physical properties of the lipid bilayer allow a relative high mobility of embedded proteins as long as they do not encounter obstacles like other proteins (picket fence) or are trapped by intracellular interactors. This is the reason, why receptors are slowed down in the synapse and get accumulated opposite of synaptic terminals. Single particle tracking (SPT) of organic dye or quantum dot labelling of receptors have successfully been used to observe the exchange of glutamate and glycine receptors between the synapse and the extrasynaptic space (Choquet and Triller, 2003; Dahan et al., 2003; Groc et al., 2007; Meier et al., 2001). Published studies on the GABAA receptor used other techniques to show, that synaptic receptors are mainly recruited from extrasynaptic areas and not directly inserted into the synapse from intracellular sources. Thomas et al. (2005) introduced the V257C mutation into the alpha1 subunit of the GABAA receptor, that is functionally silent under normal conditions, but allows irreversible inactivation in the pore through covalent coupling of MTSES, once the channel has been opened by GABA. Whole-cell GABA activated currents revealed a relatively slow movement of unblocked new receptors from intracellular pools into the synaptic or extrasynaptic compartments. Although constitutive receptor endocytosis constantly removes receptors from the surface, most of these receptors are recycled back and not replaced with “new” unblocked receptors. On the contrary, the exchange between the synapse and the extrasynaptic space seems to be very rapid. The authors inactivated action potentials by TTX to avoid the activation of peri-and extrasynaptic receptors through GABA spillover and recorded mIPSCs and evoked IPSCs before, during and after an 8 min application of MTSES. The massive depression of mIPSC amplitudes during MTSES treatment fully recovered in the following 10–15 min. They proved, that these new unblocked receptors do not stem from newly born or recycled receptor pools, as the drugs brefeldin-A (blocking the export from the endoplasmatic reticulum), botulinum toxin B or N-ethylmaleimide (interfering with exocytosis) did not change the observed effect. This is a major difference to (non-NMDA) glutamate receptors: mEPSCs are substantially affected by the presence of N-ethylmaleimide confirming a direct route of intracellular receptors to excitatory synapses. They estimated a turnover rate of 1 out of 14 receptors per minute and synapse.
Bogdanov and coworkers come to a similar conclusion with their imaging approach (Bogdanov et al., 2006). They transfected phluorin-tagged beta3 subunits with an alpha-bungarotoxin binding site at its N-terminus into hippocampal neurons to observe their fate on the cell surface. They showed, that insertion of GABAA receptors into the membrane occurs at extrasynaptic sites but their residence time there is relatively short due to continuous endocytosis mediated by the clathrin adaptor AP-2 or translocation to synaptic sites, where receptors get stabilized. The study of Jacob et al. (2005) shows, that this stabilization results in lower FRAP (fluorescence recovery after photobleaching) rates indicating lower rates of lateral mobility for synaptic receptors compared with their extrasynaptic counterparts. To date the only known candidate as a scaffold protein at GABAergic synapses is gephyrin that is thought to stabilize synaptic GABAA receptor clusters (Essrich et al., 1998; Kneussel et al., 1999; Lévi et al., 2004). Jacob et al. (2005) used RNA interference (RNAi) to reduce gephyrin expression and found, that this does not affect total cell surface receptor expression, but reduces receptor cluster numbers. The remaining clusters exhibited enhanced mobility. The role of gephyrin has been extensively investigated at glycinergic synapses, as its strong direct interaction with the glycine receptor has long been established. The regulated lateral diffusion has been shown to play a role during synaptogenesis and is an important mechanism for the dynamics of synaptic strength.
Mechanisms Involved in the Maintenance of the E/I Balance: Cell Surface Stability, Endocytosis and Recycling, and Plasticity
Changes in the levels of excitability are rapidly answered by homeostatic changes in voltage-dependent conductances (intrinsic homeostatic plasticity) or adjustments of synaptic strength (synaptic homeostatic plasticity, synaptic scaling) in order to maintain the stability of the neuronal circuits (Mody, 2005; Turrigiano and Nelson, 2000). There might be a multitude of cellular mechanisms that take part in the regulation of postsynaptic receptor numbers and their functionality. Synaptic efficacy and the overall excitation state of neurons depends on the amount, the type, the functional state and the location of neurotansmitter receptors on the cell surface (Collingridge et al., 2004). Change in the functional properties of the receptors through posttranslational modifications or interaction with endogenous ligands or the receptor numbers in the plasma membrane is known as plasticity. Historically, the first spectacular discoveries have been LTP and LTD of glutamatergic synapses. Today it has become clear, that essentially almost every ion channel and synapse and as a result, every cell or circuit undergoes plastic change, which allows neuronal output to adapt to specific needs (McBain, 2008).
Constitutive endocytosis of GABAA receptors
GABAA receptors on the surface of cortical neurons at steady state undergo significant constitutive endocytosis of 17–25% depending on maturity of neurons (Kittler et al., 2004). An important factor of GABAA receptor dynamics is the dynamin-dependent constitutive endocytosis via the clathrin pathway. Like many other cell surface receptors (ionotropic glutamate receptors, beta-adrenergic receptors, opioid receptors) GABAA receptors are recruited to clathrin-coated pits that are mostly located at peri- and extrasynaptic sites. An adaptor protein (AP-2 complex) binds the receptor and facilitates the interaction with clathrin. The AP-2 protein complex consists of four subunits, the alpha, beta, mu and sigma subunit. Cargo is bound by the beta and mu subunit, that mostly recognize a dileucine motif or the canonical YXXHyd motif (where X stands for any aminoacid and hyd for a bulky hydrophobic amino acid) (Clague, 1998; LeBorgne and Hoflack, 1998). The GABAA receptor contains several AP-2 binding motifs. One of them is a dileucine motif on the beta2 subunit (L343, L344) (Herring et al., 2003). This motif is conserved in beta1 and beta3, but it is not clear, whether it is active in this context. A typical tyrosine based motif has been found in the gamma2 subunit (Y367 in the context YECL). This motif exhibits one of the strongest known interactions with the mu2 subunit, that is regulated by the phosphorylation state of the tyrosine (Kittler et al., 2008). This site is supported in an additive way by an atypical binding site in the beta3 subunit (S408/409), that is also regulated by phosphorylation (Kittler et al., 2005). Additionally, the GTPase dynamin facilitates vesicle formation. The disruption of the dynamin-amphiphysin interaction with a sequence specific peptide results in higher mIPSC peak amplitudes (Kittler et al., 2000). The involvement of dynamin has been shown in HEK cells with a dominant negative form of the protein (K44A) (Herring et al., 2003).
A significant portion of internalized receptors (30%) is rapidly recycled back to the cell surface. Degradation of internalized receptors does not occur instantaneously, but over longer periods of time. In 6 h 29% of originally internalized receptors are degraded in lysosomes. This process can be specifically followed up by its sensitivity to leupeptin (Kittler et al., 2005).
Regulated endocytosis of GABAA receptors
Kinases not only modulate channel function by phosphorylating the GABAA receptor directly, but also mediate the effect of extracellular stimuli like growth factors or the crosstalk with other neurotransmitter systems on the trafficking of the receptor. A well known example is the activation of PKC, that induces dynamin-dependent endocytosis of GABAA receptors containing a gamma subunit (Connolly et al., 1999; Herring et al., 2005). Binding of the AP-2 complex to a dileucine motif within the beta 2 subunit is involved in the process. One suspected candidate as a trigger is brain-derived neurotrophic factor (BDNF), that plays an important role in development, but also at mature synapses (McAllister, 1999). Several studies describe a decrease or increase of IPSCs depending on the brain region. A BDNF induced reduction of spontaneous or evoked IPSCs was observed in the rat hippocampal CA1 region by Tanaka et al. (1997) and Frerking et al. (1998) and in another study of the hippocampus by Brünig et al. (2001). Similar observations were made in cerebellar granule cells by Cheng et al. or in the paraventricular nucleus of the hypothalamus (Cheng and Yeh, 2003; Hewitt and Bains, 2006). The connection between BDNF, PKC and IPSCs becomes obvious in BDNF knock out mice, where the rescue of IPSC amplitudes through exogenous BDNF can be blocked by the postsynaptic application of a PKC inhibitory peptide (Henneberger et al., 2002). In other regions, like the rat visual cortex, BDNF potentiates mIPSC amplitudes as a result of an increased receptor cell surface expression (Mizoguchi et al., 2003). Jovanovic et al. (2004) observed a biphasic effect of BDNF on GABAA receptor trafficking, namely an initial rapid increase, followed by a decrease in mIPSC amplitudes. Both effects are blocked by the PKC inhibitor calphostin C. An example for neurotransmitter receptor crosstalk is the activation of the dopamine D3 receptor in the GABAergic medium spiny neurons of the nucleus accumbens. Activation of the D3 receptor resulted in a reduction of GABAA receptor currents, that was dependent on PKA activity, dynamin and an atypical AP-2 binding motif on the beta3 subunit with a critical PKA site, that needs to be dephosphorylated to mediate the effect (Chen et al., 2006; Kittler et al., 2005).
The glia derived proinflammatory cytokine tumor necrosis factor alpha (TNF-alpha) also promotes GABAA receptor endocytosis, but at the same time AMPA receptor exocytosis, thereby influencing the E/I balance, which has been observed in the hippocampus (Leidenheimer, 2008; Stellwagen et al., 2005).
Activity dependent regulation of GABAergic inhibition
Blocking presynaptic action potentials and therefore synaptic activity by TTX or the activity of ionotropic glutamate receptors in neuronal cultures for 24 h reduces the amplitudes of mIPSCs as a result of a loss of GABAA receptors containing the alpha2, beta3 and gamma2 subunit from the synapse (Kilman et al., 2002; Saliba et al., 2007). The opposite phenomenon has been observed upon enhancing neuronal activity. TTX treatment did not change the rate of endocytosis or receptor half-life at the cell surface, but increased the polyubiquitination of the beta3 subunit significantly, therefore pointing to a role of the proteasome. Pulse chase analysis showed, that newly translated subunits or assembled receptors in the ER exhibited a reduced stability, as polyubiquitination directly targeted them to the proteasome for degradation, therefore reducing the amount of functional receptor, which is delivered to the synapse through the secretory pathway. A similar mechanism has been shown for the NMDA receptor and PSD proteins, but not for AMPA receptors, where ubiquitination influences endocytosis (Burbea et al., 2002; Colledge et al., 2003; Kato et al., 2005; Patrick et al., 2003). Blocking ubiquitination of the beta3 subunit by mutating 12 lysine residues to arginines resulted in significantly higher cell surface levels of the mutant as a result of increased receptor insertion into the membrane (Saliba et al., 2007). Although other subunits might also be the target of polyubiquitination, the beta3 subunit is critical for the exit of assembled receptors from the ER, and therefore rate limiting (Lüscher and Keller, 2004).
Live imaging of phluorin tagged beta3 subunits bearing a bungarotoxin binding site (BBS) and the use of fluorescent bungarotoxin showed, that the mutant beta3 subunit exhibits much higher insertion rates into the plasma membrane than the wildtype beta3 subunit (Bogdanov et al., 2006). This effect remained the same, whether TTX was present or not. ER associated degradation (ERAD) via the proteosome is therefore a means to regulate neuronal inhibition by GABAA receptors in response to changing neuronal activity. The ubiquitin-like protein Plic-1 that has been identified as a GABAA receptor interacting protein, might play some regulatory role by reducing proteosomal degradation and assisting membrane insertion of receptors (Bedford et al., 2001).
Plasticity of GABAergic synapses influencing the balance between excitation and inhibition
The different forms of plasticity at excitatory synapses certainly have been dominating research since their first discovery by Bliss and Lomo in the 1970s as they have been considered to lie at the base of learning and memory. Plasticity at inhibitory synapses was discovered later and the different forms have only emerged gradually. The Purkinje cells of the cerebellar cortex are at the centre of interest in this regard. Repetitive depolarization of Purkinje cells results in the induction of three forms of inhibitory synaptic plasticity: Rebound potentiation (Kano et al., 1992), DSI (Llano et al., 1991) and DPI (Duguid and Smart, 2004). While DSI and DPI rely on retrograde messengers, rebound potentiation is a purely postsynaptic phenomenon, where the main regulatory players in the postsynaptic membrane seem to be GABAB and mGluRI receptors. Postsynaptic depolarization of a Purkinje neuron leads to an increase of the intracellular Ca2+ concentration, that together with calmodulin binds to and activates Ca2+/Calmodulin-dependent protein kinase II (CaMKII), thereby potentiating GABAA receptor function. Simultaneous activation of GABAB receptors suppresses the induction of rebound potentiation through the activation of protein phosphatase I (PP-1). The group of Hirano suggests, that PP-1 reduces GABAA receptor function by controlling the autophosphorylation of CaMKII (Sugiyama et al., 2008). The central molecule of the whole process seems to be DARPP-32. mGluRI activates adenylyl cyclase acting via a Gs protein and GABAB receptors inhibit adenylyl cyclase through Gi/Go proteins. The resulting cAMP levels influence the activity of PKA that in its activated state catalyzes phosphorylation of DARPP-32, a strong inhibitor of PP-1. The increase of Ca2+/Calmodulin additionally activates calcineurin that shifts the equilibrium towards the unphosphorylated form of DARPP-32, which has no effect on PP-1. The role of mGluRI in this context is not beyond doubt, as Duguid et al. (2007) report, that any pharmacological blockage of mGluRI activation did not affect the induction of rebound potentiation in their hands. Another recent study does not entirely support the hypothesis on the role of PP-1, as they have some evidence that CaMK-II modulation of GABAA receptors critically requires Ca2+/CaM binding to CaMK-II but not autophosphorylation (C. Houston and TG Smart, personal communication). PP-1 can also directly dephosphorylate the GABAA receptor.
Rebound potentiation seems to be a far more complex process, as several other factors, like a structural change of GABARAP and the involvement of tyrosine kinases is currently discussed (Kawaguchi and Hirano, 2006, 2007)
The increase of intracellular calcium during Purkinje cell activation also leads to a postsynaptic release of endocannabinoids only when the stimulation is modest. They act as retrograde messengers on presynaptic CB1 receptors to transiently suppress the release of GABA (DSI). Repetitive climbing fibre stimulation or strong somatic depolarization results in a rapid increase of Ca2+ and the simultaneous release of postsynaptic glutamate and endocannabinoids into the extrasynaptic space. Glutamate activates postsynaptic mGluRI receptors in an autocrine manner and through Gq/11 proteins enhances the release of endocannabinoids. On the other hand, glutamate also activates presynaptic NMDA receptors, which induce an increase in presynaptic GABA release. Glutamate therefore enhances synaptic depression (DSI) before inducing synaptic potentiation (DPI) (Duguid et al., 2007).
Summary
The delicate balance between excitation and inhibition in the mature nervous system is mainly carried by the excitatory neurotransmitter glutamate, that acts on AMPA, NMDA, kainate and metabotropic glutamate receptors and the mostly inhibitory neurotransmitter GABA, that acts on GABAA, GABAB and GABAc receptors. The highly heterogenous GABAA receptors are trafficked to specific subcellular locations by mechanisms that only start to be understood. Novel GABAA receptor interacting proteins have been shown to be involved in trafficking, stabilization and anchoring of the receptors. Their functional properties and stability is further regulated by post-translational modifications like phosphorylation, palmitoylation and ubiquitination. Subtype heterogeneity and receptor dynamics (through trafficking, regulation of stability and plasticity) allow the fine tuning of inhibition in neural networks, that when disturbed, results in pathological states like epilepsy, anxiety and depression.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by a Marie Curie fellowship of the European Community (VT) and NIH/NINDS grants NS 046478, 048045, 051195, 056359, P01NS054900 (SJM). We thank Drs Josef T. Kittler and Catriona Houston for comments on the manuscript.
References
- Ali A. B., Thomson A. M. (2007). Synaptic alpha5 subunit-containing GABAA receptors mediate IPSPs elicited by dendrite-preferring cells in rat neocortex. Cereb. Cortex [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Alldred M., Mulder-Rosi J., Lingenfelter S., Chen G., Lüscher B. (2005). Distinct gamma2 subunit domains mediate clustering and synaptic function of postsynaptic GABAA receptors and gephyrin. J. Neurosci. 25, 594–603 10.1523/JNEUROSCI.4011-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelotti T., Uhler M., Macdonald R. (1993). Enhancement of recombinant gamma-aminobutyric acid type A receptor currents by chronic activation of cAMP-dependent protein kinase. Mol. Pharmacol. 44, 1202–1210 [PubMed] [Google Scholar]
- Baumann S., Baur R., Sigel E. (2001). Subunit arrangement of gamma-aminobutyric acid type A receptors. J. Biol. Chem. 276, 36275–36280 10.1074/jbc.M105240200 [DOI] [PubMed] [Google Scholar]
- Baumann S., Baur R., Sigel E. (2002). Forced subunit assembly in alpha1beta2gamma2 GABAA receptors. Insight into the absolute arrangement. J. Biol. Chem. 277, 46020–46025 10.1074/jbc.M207663200 [DOI] [PubMed] [Google Scholar]
- Beck M., Brickley K., Wilkinson H., Sharma S., Smith M., Chazot P., Pollard S., Stephenson F. (2002). Identification, molecular cloning, and characterization of a novel GABAA receptor-associated protein, GRIF-1. J. Biol. Chem. 277, 30079–30090 10.1074/jbc.M200438200 [DOI] [PubMed] [Google Scholar]
- Bedford F., Kittler J., Muller E., Thomas P., Uren J., Merlo D., Wisden W., Triller A., Smart T., Moss S. (2001). GABA(A) receptor cell surface number and subunit stability are regulated by the ubiquitin-like protein Plic-1. Nat. Neurosci. 4, 908–916 10.1038/nn0901-908 [DOI] [PubMed] [Google Scholar]
- Belelli D., Lambert J. (2005). Neurosteroids: endogenous regulators of the GABA(A) receptor. Nat. Rev. Neurosci. 6, 565–575 10.1038/nrn1703 [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. (2002). Excitatory actions of gaba during development: the nature of the nurture. Nat. Rev. Neurosci. 3, 728–739 10.1038/nrn920 [DOI] [PubMed] [Google Scholar]
- Bencsits E., Ebert V., Tretter V., Sieghart W. (1999). A significant part of native gamma-aminobutyric AcidA receptors containing alpha4 subunits do not contain gamma or delta subunits. J. Biol. Chem. 274, 19613–19616 10.1074/jbc.274.28.19613 [DOI] [PubMed] [Google Scholar]
- Blaesse P., Guillemin I., Schindler J., Schweizer M., Delpire E., Khiroug L., Friauf E., Nothwang H. (2006). Oligomerization of KCC2 correlates with development of inhibitory neurotransmission. J. Neurosci. 26, 10407–10419 10.1523/JNEUROSCI.3257-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanov Y., Michels G., Armstrong-Gold C., Haydon P., Lindstrom J., Pangalos M., Moss S. (2006). Synaptic GABAA receptors are directly recruited from their extrasynaptic counterparts. EMBO J. 25, 4381–4389 10.1038/sj.emboj.7601309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollan K., King D., Robertson L., Brown K., Taylor P., Moss S. J., Connolly C. (2003). GABA(A) receptor composition is determined by distinct assembly signals within alpha and beta subunits. J. Biol. Chem. 278, 4747–4755 10.1074/jbc.M210229200 [DOI] [PubMed] [Google Scholar]
- Bollan K. A., Baur R., Hales T. G., Sigel E., Connolly C. N. (2008). The promiscuous role of the epsilon subunit in GABAA receptor biogenesis. Mol. Cell. Neurosci. 37, 610–621 [DOI] [PubMed] [Google Scholar]
- Brandon N., Jovanovic J., Colledge M., Kittler J., Brandon J., Scott J., Moss S. (2003). A-kinase anchoring protein 79/150 facilitates the phosphorylation of GABA(A) receptors by cAMP-dependent protein kinase via selective interaction with receptor beta subunits. Mol. Cell. Neurosci. 22, 87–97 10.1016/S1044-7431(02)00017-9 [DOI] [PubMed] [Google Scholar]
- Brandon N., Jovanovic J., Smart T., Moss S. (2002). Receptor for activated C kinase-1 facilitates protein kinase C-dependent phosphorylation and functional modulation of GABA(A) receptors with the activation of G-protein-coupled receptors. J. Neurosci. 22, 6353–6361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley K., Smith M., Beck M., Stephenson F. (2005). GRIF-1 and OIP106, members of a novel gene family of coiled-coil domain proteins: association in vivo and in vitro with kinesin. J. Biol. Chem. 280, 14723–14732 10.1074/jbc.M409095200 [DOI] [PubMed] [Google Scholar]
- Brickley S., Revilla V., Cull-Candy S., Wisden W., Farrant M. (2001). Adaptive regulation of neuronal excitability by a voltage-independent potassium conductance. Nature 409, 88–92 10.1038/35051086 [DOI] [PubMed] [Google Scholar]
- Brünig I., Penschuck S., Berninger B., Benson J., Fritschy J. (2001). BDNF reduces miniature inhibitory postsynaptic currents by rapid downregulation of GABA(A) receptor surface expression. Eur. J. Neurosci. 13, 1320–1328 10.1046/j.0953-816x.2001.01506.x [DOI] [PubMed] [Google Scholar]
- Brünig I., Suter A., Knuesel I., Lüscher B., Fritschy J. (2002). GABAergic terminals are required for postsynaptic clustering of dystrophin but not of GABA(A) receptors and gephyrin. J. Neurosci. 22, 4805–4813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbea M., Dreier L., Dittman J., Grunwald M., Kaplan J. (2002). Ubiquitin and AP180 regulate the abundance of GLR-1 glutamate receptors at postsynaptic elements in C. elegans. Neuron 35, 107–120 10.1016/S0896-6273(02)00749-3 [DOI] [PubMed] [Google Scholar]
- Chang Y., Wang R., Barot S., Weiss D. (1996). Stoichiometry of a recombinant GABAA receptor. J. Neurosci. 16, 5415–5424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charych E., Yu W., Miralles C., Serwanski D., Li X., Rubio M., Blas A. D. (2004). The brefeldin A-inhibited GDP/GTP exchange factor 2, a protein involved in vesicular trafficking, interacts with the beta subunits of the GABA receptors. J. Neurochem. 90, 173–189 10.1111/j.1471-4159.2004.02481.x [DOI] [PubMed] [Google Scholar]
- Chen G., Kittler J., Moss S., Yan Z. (2006). Dopamine D3 receptors regulate GABAA receptor function through a phospho-dependent endocytosis mechanism in nucleus accumbens. J. Neurosci. 26, 2513–2521 10.1523/JNEUROSCI.4712-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Chang C., Leil T., Olcese R., Olsen R. (2005). GABAA receptor-associated protein regulates GABAA receptor cell-surface number in Xenopus laevis oocytes. Mol. Pharmacol. 68, 152–159 [DOI] [PubMed] [Google Scholar]
- Chen Z., Olsen R. (2007). GABAA receptor associated proteins: a key factor regulating GABAA receptor function. J. Neurochem. 100, 279–294 10.1111/j.1471-4159.2006.04206.x [DOI] [PubMed] [Google Scholar]
- Cheng Q., Yeh H. (2003). Brain-derived neurotrophic factor attenuates mouse cerebellar granule cell GABA(A) receptor-mediated responses via postsynaptic mechanisms. J. Physiol. 548, 711–721 10.1113/jphysiol.2002.037846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chih B., Gollan L., Scheiffele P. (2006). Alternative splicing controls selective trans-synaptic interactions of the neuroligin-neurexin complex. Neuron 51, 171–178 10.1016/j.neuron.2006.06.005 [DOI] [PubMed] [Google Scholar]
- Choquet D., Triller A. (2003). The role of receptor diffusion in the organization of the postsynaptic membrane. Nat. Rev. Neurosci. 4, 251–265 10.1038/nrn1077 [DOI] [PubMed] [Google Scholar]
- Chubykin A., Atasoy D., Etherton M., Brose N., Kavalali E., Gibson J., Südhof T. (2007). Activity-dependent validation of excitatory versus inhibitory synapses by neuroligin-1 versus neuroligin-2. Neuron 54, 919–931 10.1016/j.neuron.2007.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clague M. (1998). Molecular aspects of the endocytic pathway. Biochem. J. 336, 271–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen I., Navarro V., Clemenceau S., Baulac M., Miles R. (2002). On the origin of interictal activity in human temporal lobe epilepsy in vitro. Science 298, 1418–1421 10.1126/science.1076510 [DOI] [PubMed] [Google Scholar]
- Colledge M., Snyder E., Crozier R., Soderling J., Jin Y., Langeberg L., Lu H., Bear M., Scott J. (2003). Ubiquitination regulates PSD-95 degradation and AMPA receptor surface expression. Neuron 40, 595–607 10.1016/S0896-6273(03)00687-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge G., Isaac J., Wang Y. (2004). Receptor trafficking and synaptic plasticity. Nat. Rev. Neurosci. 5, 952–962 10.1038/nrn1556 [DOI] [PubMed] [Google Scholar]
- Collinson N., Kuenzi F., Jarolimek W., Maubach K., Cothliff R., Sur C., Smith A., Otu F., Howell O., Atack J., McKernan R., Seabrook G., Dawson G., Whiting P., Rosahl T. (2002). Enhanced learning and memory and altered GABAergic synaptic transmission in mice lacking the alpha 5 subunit of the GABAA receptor. J. Neurosci. 22, 5572–5580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly C., Kittler J., Thomas P., Uren J., Brandon N., Smart T., Moss S. (1999). Cell surface stability of gamma-aminobutyric acid type A receptors. Dependence on protein kinase C activity and subunit composition. J. Biol. Chem. 274, 36565–36572 10.1074/jbc.274.51.36565 [DOI] [PubMed] [Google Scholar]
- Coull J., Boudreau D., Bachand K., Prescott S., Nault F., Sík A., Koninck P. D., Koninck Y. D. (2003). Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature 424, 938–942 10.1038/nature01868 [DOI] [PubMed] [Google Scholar]
- Craig A., Banker G., Chang W., McGrath M., Serpinskaya A. (1996). Clustering of gephyrin at GABAergic but not glutamatergic synapses in cultured rat hippocampal neurons. J. Neurosci. 16, 3166–3177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig A., Graf E., Linhoff M. (2006). How to build a central synapse: clues from cell culture. Trends Neurosci. 29, 8–20 10.1016/j.tins.2005.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig A., Kang Y. (2007). Neurexin-neuroligin signaling in synapse development. Curr. Opin. Neurobiol. 17, 43–52 10.1016/j.conb.2007.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crestani F., Keist R., Fritschy J., Benke D., Vogt K., Prut L., Blüthmann H., Möhler H., Rudolph U. (2002). Trace fear conditioning involves hippocampal alpha5 GABA(A) receptors. Proc. Natl. Acad. Sci. USA 99, 8980–8985 10.1073/pnas.142288699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahan M., Lévi S., Luccardini C., Rostaing P., Riveau B., Triller A. (2003). Diffusion dynamics of glycine receptors revealed by single-quantum dot tracking. Science 302, 442–445 10.1126/science.1088525 [DOI] [PubMed] [Google Scholar]
- Dragatsis I., Zeitlin S., Dietrich P. (2004). Huntingtin-associated protein 1 (Hap1) mutant mice bypassing the early postnatal lethality are neuroanatomically normal and fertile but display growth retardation. Hum. Mol. Genet. 13, 3115–3125 10.1093/hmg/ddh328 [DOI] [PubMed] [Google Scholar]
- Duguid I. C., Pankratov Y., Moss G. W. J., Smart T. G. (2007). Somatodendritic release of glutamate regulates synaptic inhibition in cerebellar Purkinje cells via autocrine mGluRI activation. J. Neurosci. 27, 12464–12474 10.1523/JNEUROSCI.0178-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duguid I. C., Smart T. G. (2004). Retrograde activation of presynaptic NMDA receptors enhances GABA release at cerebellar interneuron-Purkinje cell synapses. Nat. Neurosci. 7, 525–533 10.1038/nn1227 [DOI] [PubMed] [Google Scholar]
- Engelender S., Sharp A., Colomer V., Tokito M., Lanahan A., Worley P., Holzbaur E., Ross C. (1997). Huntingtin-associated protein 1 (HAP1) interacts with the p150Glued subunit of dynactin. Hum. Mol. Genet. 6, 2205–2212 10.1093/hmg/6.13.2205 [DOI] [PubMed] [Google Scholar]
- Essrich C., Lorez M., Benson J., Fritschy J., Lüscher B. (1998). Postsynaptic clustering of major GABAA receptor subtypes requires the gamma 2 subunit and gephyrin. Nat. Neurosci. 1, 563–571 10.1038/2798 [DOI] [PubMed] [Google Scholar]
- Everitt A., Luu T., Cromer B., Tierney M., Birnir B., Olsen R., Gage P. (2004). Conductance of recombinant GABA(A) channels is increased in cells co-expressing GABA(A) receptor-associated protein. J. Biol. Chem. 279, 21701–21706 10.1074/jbc.M312806200 [DOI] [PubMed] [Google Scholar]
- Fang C., Deng L., Keller C., Fukata M., Fukata Y., Chen G., Lüscher B. (2006). GODZ-mediated palmitoylation of GABA(A) receptors is required for normal assembly and function of GABAergic inhibitory synapses. J. Neurosci. 26, 12758–12768 10.1523/JNEUROSCI.4214-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant M., Nusser Z. (2005). Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat. Rev. Neurosci. 6, 215–229 10.1038/nrn1625 [DOI] [PubMed] [Google Scholar]
- Farrar S., Whiting P., Bonnert T., McKernan R. (1999). Stoichiometry of a ligand-gated ion channel determined by fluorescence energy transfer. J. Biol. Chem. 274, 10100–10104 10.1074/jbc.274.15.10100 [DOI] [PubMed] [Google Scholar]
- Frerking M., Malenka R., Nicoll R. (1998). Brain-derived neurotrophic factor (BDNF) modulates inhibitory, but not excitatory, transmission in the CA1 region of the hippocampus. J. Neurophysiol. 80, 3383–3386 [DOI] [PubMed] [Google Scholar]
- Fuhrmann J., Kins S., Rostaing P., Far O. E., Kirsch J., Sheng M., Triller A., Betz H., Kneussel M. (2002). Gephyrin interacts with Dynein light chains 1 and 2, components of motor protein complexes. J. Neurosci. 22, 5393–5402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier L., Charrin B., Borrell-Pagès M., Dompierre J., Rangone H., Cordelières F., Mey J. D., MacDonald M., Lessmann V., Humbert S., Saudou F. (2004). Huntingtin controls neurotrophic support and survival of neurons by enhancing BDNF vesicular transport along microtubules. Cell 118, 127–138 10.1016/j.cell.2004.06.018 [DOI] [PubMed] [Google Scholar]
- Glykys J., Mann E., Mody I. (2008). Which GABA(A) receptor subunits are necessary for tonic inhibition in the hippocampus? J. Neurosci. 28, 1421–1426 10.1523/JNEUROSCI.4751-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glykys J., Mody I. (2007). Activation of GABAA receptors: views from outside the synaptic cleft. Neuron 56, 763–770 10.1016/j.neuron.2007.11.002 [DOI] [PubMed] [Google Scholar]
- Graf E., Zhang X., Jin S., Linhoff M., Craig A. (2004). Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell 119, 1013–1026 10.1016/j.cell.2004.11.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groc L., Lafourcade M., Heine M., Renner M., Racine V., Sibarita J., Lounis B., Choquet D., Cognet L. (2007). Surface trafficking of neurotransmitter receptor: comparison between single-molecule/quantum dot strategies. J. Neurosci. 27, 12433–12437 10.1523/JNEUROSCI.3349-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günther U., Benson J., Benke D., Fritschy J. M., Reyes G., Knoflach F., Crestani F., Aguzzi A., Arigoni M., Lang Y. (1995). Benzodiazepine-insensitive mice generated by targeted disruption of the gamma 2 subunit gene of gamma-aminobutyric acid type A receptors. Proc. Natl. Acad. Sci. USA 92, 7749–7753 10.1073/pnas.92.17.7749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey K., Duguid I., Alldred M., Beatty S., Ward H., Keep N., Lingenfelter S., Pearce B., Lundgren J., Owen M., Smart T., Lüscher B., Rees M., Harvey R. (2004). The GDP-GTP exchange factor collybistin: an essential determinant of neuronal gephyrin clustering. J. Neurosci. 24, 5816–5826 10.1523/JNEUROSCI.1184-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneberger C., Jüttner R., Rothe T., Grantyn R. (2002). Postsynaptic action of BDNF on GABAergic synaptic transmission in the superficial layers of the mouse superior colliculus. J. Neurophysiol. 88, 595–603 [DOI] [PubMed] [Google Scholar]
- Herring D., Huang R., Singh M., Dillon G., Leidenheimer N. (2005). PKC modulation of GABAA receptor endocytosis and function is inhibited by mutation of a dileucine motif within the receptor beta 2 subunit. Neuropharmacology 48, 181–194 10.1016/j.neuropharm.2004.09.015 [DOI] [PubMed] [Google Scholar]
- Herring D., Huang R., Singh M., Robinson L., Dillon G., Leidenheimer N. (2003). Constitutive GABAA receptor endocytosis is dynamin-mediated and dependent on a dileucine AP2 adaptin-binding motif within the beta 2 subunit of the receptor. J. Biol. Chem. 278, 24046–24052 10.1074/jbc.M301420200 [DOI] [PubMed] [Google Scholar]
- Hewitt S., Bains J. (2006). Brain-derived neurotrophic factor silences GABA synapses onto hypothalamic neuroendocrine cells through a postsynaptic dynamin-mediated mechanism. J. Neurophysiol. 95, 2193–2198 10.1152/jn.01135.2005 [DOI] [PubMed] [Google Scholar]
- Hilf R. J., Dutzler R. (2008). X-ray structure of a prokaryotic pentameric ligand-gated ion channel. Nature 452, 375–379 10.1038/nature06717 [DOI] [PubMed] [Google Scholar]
- Houston C., Smart T. (2006). CaMK-II modulation of GABA(A) receptors expressed in HEK293, NG108-15 and rat cerebellar granule neurons. Eur. J. Neurosci. 24, 2504–2514 10.1111/j.1460-9568.2006.05145.x [DOI] [PubMed] [Google Scholar]
- Jacob T., Bogdanov Y., Magnus C., Saliba R., Kittler J., Haydon P., Moss S. (2005). Gephyrin regulates the cell surface dynamics of synaptic GABAA receptors. J. Neurosci. 25, 10469–10478 10.1523/JNEUROSCI.2267-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen K., Chiu C., Sokolova I., Lester H., Mody I. (2003). GABA transporter-1 (GAT1)-deficient mice: differential tonic activation of GABAA versus GABAB receptors in the hippocampus. J. Neurophysiol. 90, 2690–2701 10.1152/jn.00240.2003 [DOI] [PubMed] [Google Scholar]
- Jovanovic J., Thomas P., Kittler J., Smart T., Moss S. (2004). Brain-derived neurotrophic factor modulates fast synaptic inhibition by regulating GABA(A) receptor phosphorylation, activity, and cell-surface stability. J. Neurosci. 24, 522–530 10.1523/JNEUROSCI.3606-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanematsu T., Fuji M., Mizokami A., Kittler J. T., Nabekura J., Moss S. J., Hirata M. (2007). Phospholipase C-related inactive protein is implicated in the constitutive internalization of GABAA receptors mediated by clathrin and AP2 adaptor complex. J. Neurochem. 10, 898–905 10.1111/j.1471-4159.2006.04399.x [DOI] [PubMed] [Google Scholar]
- Kang Y., Zhang X., Dobie F., Wu H., Craig A. M. (2008). Induction of GABAergic postsynaptic differentiation by alpha-neurexins. J. Biol. Chem. 283, 2323–2334 10.1074/jbc.M703957200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano M., Kano M., Fukunaga K., Konnerth A. (1996). Ca(2+)-induced rebound potentiation of gamma-aminobutyric acid-mediated currents requires activation of Ca2+/calmodulin-dependent kinase II. Proc. Natl. Acad. Sci. USA 93, 13351–13356 10.1073/pnas.93.23.13351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano M., Rexhausen U., Dreessen J., Konnerth A. (1992). Synaptic excitation produces a long-lasting rebound potentiation of inhibitory synaptic signals in cerebellar Purkinje cells. Nature 356, 601–604 10.1038/356601a0 [DOI] [PubMed] [Google Scholar]
- Kapur J., Macdonald R. (1996). Cyclic AMP-dependent protein kinase enhances hippocampal dentate granule cell GABAA receptor currents. J. Neurophysiol. 76, 2626–2634 [DOI] [PubMed] [Google Scholar]
- Kato A., Rouach N., Nicoll R., Bredt D. (2005). Activity-dependent NMDA receptor degradation mediated by retrotranslocation and ubiquitination. Proc. Natl. Acad. Sci. USA 102, 5600–5605 10.1073/pnas.0501769102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi S., Hirano T. (2006). Integrin alpha3beta1 suppresses long-term potentiation at inhibitory synapses on the cerebellar Purkinje neuron. Mol. Cell. Neurosci. 31, 416–426 10.1016/j.mcn.2005.10.012 [DOI] [PubMed] [Google Scholar]
- Kawaguchi S., Hirano T. (2007). Sustained structural change of GABA(A) receptor-associated protein underlies long-term potentiation at inhibitory synapses on a cerebellar Purkinje neuron. J. Neurosci. 27, 6788–6799 10.1523/JNEUROSCI.1981-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller C., Yuan X., Panzanelli P., Martin M., Alldred M., Sassoè-Pognetto M., Lüscher B. (2004). The gamma2 subunit of GABA(A) receptors is a substrate for palmitoylation by GODZ. J. Neurosci. 24, 5881–5891 10.1523/JNEUROSCI.1037-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilman V., van Rossum M. C., Turrigiano G. G. (2002). Activity deprivation reduces miniature IPSC amplitude by decreasing the number of postsynaptic GABA(A) receptors clustered at neocortical synapses. J. Neurosci. 22, 1328–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kins S., Betz H., Kirsch J. (2000). Collybistin, a newly identified brain-specific GEF, induces submembrane clustering of gephyrin. Nat. Neurosci. 3, 22–29 10.1038/71096 [DOI] [PubMed] [Google Scholar]
- Kittler J., Arancibia-Carcamo I., Moss S. (2004). Association of GRIP1 with a GABA(A) receptor associated protein suggests a role for GRIP1 at inhibitory synapses. Biochem. Pharmacol. 68, 1649–1654 10.1016/j.bcp.2004.07.028 [DOI] [PubMed] [Google Scholar]
- Kittler J., Chen G., Honing S., Bogdanov Y., McAinsh K., Arancibia-Carcamo I., Jovanovic J., Pangalos M., Haucke V., Yan Z., Moss S. (2005). Phospho-dependent binding of the clathrin AP2 adaptor complex to GABAA receptors regulates the efficacy of inhibitory synaptic transmission. Proc. Natl. Acad. Sci. USA 102, 14871–14876 10.1073/pnas.0506653102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler J., Chen G., Kukhtina V., Vahedi-Faridi A., Gu Z., Tretter V., Smith K., McAinsh K., Arancibia-Carcamo I., Saenger W., Haucke V., Yan Z., Moss S. (2008). Regulation of synaptic inhibition by phospho-dependent binding of the AP2 complex to a YECL motif in the GABAA receptor gamma2 subunit. Proc. Natl. Acad. Sci. USA 105, 3616–3621 10.1073/pnas.0707920105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler J., Delmas P., Jovanovic J., Brown D., Smart T., Moss S. (2000). Constitutive endocytosis of GABAA receptors by an association with the adaptin AP2 complex modulates inhibitory synaptic currents in hippocampal neurons. J. Neurosci. 20, 7972–7977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler J., Moss S. (2001). Neurotransmitter receptor trafficking and the regulation of synaptic strength. Traffic 2, 437–448 10.1034/j.1600-0854.2001.20702.x [DOI] [PubMed] [Google Scholar]
- Klausberger T., Ehya N., Fuchs K., Fuchs T., Ebert V., SartoS I., Sieghart W. (2001a). Detection and binding properties of GABA(A) receptor assembly intermediates. J. Biol. Chem. 276, 16024–16032 10.1074/jbc.M009508200 [DOI] [PubMed] [Google Scholar]
- Klausberger T., Fuchs K., Mayer B., Ehya N., Sieghart W. (2000). GABA(A) receptor assembly. Identification and structure of gamma(2) sequences forming the intersubunit contacts with alpha(1) and beta(3) subunits. J. Biol. Chem. 275, 8921–8928 10.1074/jbc.275.12.8921 [DOI] [PubMed] [Google Scholar]
- Klausberger T., Roberts J., Somogyi P. (2002). Cell type- and input-specific differences in the number and subtypes of synaptic GABA(A) receptors in the hippocampus. J. Neurosci. 22, 2513–2521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T., Sarto I., Ehya N., Fuchs K., Furtmuller R., Mayer B., Huck S., Sieghart W. (2001b). Alternate use of distinct intersubunit contacts controls GABAA receptor assembly and stoichiometry. J. Neurosci. 21, 9124–9133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneussel M., Betz H. (2000). Clustering of inhibitory neurotransmitter receptors at developing postsynaptic sites: the membrane activation model. Trends Neurosci. 23, 429–435 10.1016/S0166-2236(00)01627-1 [DOI] [PubMed] [Google Scholar]
- Kneussel M., Brandstätter J., Laube B., Stahl S., Müller U., Betz H. (1999). Loss of postsynaptic GABA(A) receptor clustering in gephyrin-deficient mice. J. Neurosci. 19, 9289–9297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight A., Stephenson F., Tallman J., Ramabahdran T. (2000). Monospecific antibodies as probes for the stoichiometry of recombinant GABA(A) receptors. Recept. Channels. 7, 213–226 [PubMed] [Google Scholar]
- Korpi E., Gründer G., Lüddens H. (2002). Drug interactions at GABA(A) receptors. Prog. Neurobiol. 67, 113–159 10.1016/S0301-0082(02)00013-8 [DOI] [PubMed] [Google Scholar]
- Korpi E., Sinkkonen S. (2006). GABA(A) receptor subtypes as targets for neuropsychiatric drug development. Pharmacol. Ther. 109, 12–32 10.1016/j.pharmthera.2005.05.009 [DOI] [PubMed] [Google Scholar]
- Krishek B., Xie X., Blackstone C., Huganir R., Moss S., Smart T. (1994). Regulation of GABAA receptor function by protein kinase C phosphorylation. Neuron 12, 1081–1095 10.1016/0896-6273(94)90316-6 [DOI] [PubMed] [Google Scholar]
- Lambrechts A., Braun A., Jonckheere V., Aszodi A., Lanier L., Robbens J., Colen I. V., Vandekerckhove J., Fässler R., Ampe C. (2000). Profilin II is alternatively spliced, resulting in profilin isoforms that are differentially expressed and have distinct biochemical properties. Mol. Cell. Biol. 20, 8209–8219 10.1128/MCB.20.21.8209-8219.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llano I., Leresche N., Marty A. (1991). Calcium entry icreases the sensitivity of cerebellar Purkinje cells to applied GABA and decreases inhibitory synaptic currents. Neuron 6, 565–574 10.1016/0896-6273(91)90059-9 [DOI] [PubMed] [Google Scholar]
- LeBorgne R., Hoflack B. (1998). Mechanisms of protein sorting and coat assembly: insights from the clathrin-coated vesicle pathway. Curr. Opin. Cell Biol. 10, 499–503 10.1016/S0955-0674(98)80065-3 [DOI] [PubMed] [Google Scholar]
- Lee H., Walker J., Williams J., Goodier R., Payne J., Moss S. (2007). Direct protein kinase C-dependent phosphorylation regulates the cell surface stability and activity of the potassium chloride cotransporter KCC2. J. Biol. Chem. 282, 29777–29784 10.1074/jbc.M705053200 [DOI] [PubMed] [Google Scholar]
- Leidenheimer N. (2008). Regulation of excitation by GABA(A) receptor internalization. Results Probl. Cell Differ. 44, 1–28 [DOI] [PubMed] [Google Scholar]
- Leidenheimer N., McQuilkin S., Hahner L., Whiting P., Harris R. (1992). Activation of protein kinase C selectively inhibits the gamma-aminobutyric acidA receptor: role of desensitization. Mol. Pharmacol. 41, 1116–1123 [PubMed] [Google Scholar]
- Leil T., Chen Z., Chang C., Olsen R. (2004). GABAA receptor-associated protein traffics GABAA receptors to the plasma membrane in neurons. J. Neurosci. 24, 11429–11438 10.1523/JNEUROSCI.3355-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévi S., Grady R., Henry M., Campbell K., Sanes J., Craig A. (2002). Dystroglycan is selectively associated with inhibitory GABAergic synapses but is dispensable for their differentiation. J. Neurosci. 22, 4274–4285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévi S., Logan S., Tovar K., Craig A. (2004). Gephyrin is critical for glycine receptor clustering but not for the formation of functional GABAergic synapses in hippocampal neurons. J. Neurosci. 24, 207–217 10.1523/JNEUROSCI.1661-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson J., El-Husseini A. (2005). Building excitatory and inhibitory synapses: balancing neuroligin partnerships. Neuron 48, 171–174 10.1016/j.neuron.2005.09.017 [DOI] [PubMed] [Google Scholar]
- Li H., Khirug S., Cai C., Ludwig A., Blaesse P., Kolikova J., Afzalov R., Coleman S., Lauri S., Airaksinen M., Keinänen K., Khiroug L., Saarma M., Kaila K., Rivera C. (2007). KCC2 interacts with the dendritic cytoskeleton to promote spine development. Neuron 56, 1019–1033 10.1016/j.neuron.2007.10.039 [DOI] [PubMed] [Google Scholar]
- Li M., Blas A. D. (1997). Coexistence of two beta subunit isoforms in the same gamma-aminobutyric acid type A receptor. J. Biol. Chem. 272, 123–126 10.1074/jbc.272.26.16564 [DOI] [PubMed] [Google Scholar]
- Li S., Gutekunst C., Hersch S., Li X. (1998). Interaction of huntingtin-associated protein with dynactin P150Glued. J. Neurosci. 18, 1261–1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y., Angelotti T., Dudek E., Browning M., Macdonald R. (1996). Enhancement of recombinant alpha 1 beta 1 gamma 2L gamma-aminobutyric acidA receptor whole-cell currents by protein kinase C is mediated through phosphorylation of both beta 1 and gamma 2L subunits. Mol. Pharmacol. 50, 185–195 [PubMed] [Google Scholar]
- Liu G. (2004). Local structural balance and functional interaction of excitatory and inhibitory synapses in hippocampal dendrites. Nat. Neurosci. 7, 373–379 10.1038/nn1206 [DOI] [PubMed] [Google Scholar]
- Loebrich S., Bähring R., Katsuno T., Tsukita S., Kneussel M. (2006). Activated radixin is essential for GABAA receptor alpha5 subunit anchoring at the actin cytoskeleton. EMBO J. 25, 987–999 10.1038/sj.emboj.7600995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher B., Keller C. (2004). Regulation of GABAA receptor trafficking, channel activity, and functional plasticity of inhibitory synapses. Pharmacol. Ther. 102, 195–221 10.1016/j.pharmthera.2004.04.003 [DOI] [PubMed] [Google Scholar]
- Luu T., Gage P., Tierney M. (2006). GABA increases both the conductance and mean open time of recombinant GABAA channels co-expressed with GABARAP. J. Biol. Chem. 281, 35699–35708 10.1074/jbc.M605590200 [DOI] [PubMed] [Google Scholar]
- Machu T., Firestone J., Browning M. (1993). Ca2+/calmodulin-dependent protein kinase II and protein kinase C phosphorylate a synthetic peptide corresponding to a sequence that is specific for the gamma 2L subunit of the GABAA receptor. J. Neurochem. 61, 375–377 10.1111/j.1471-4159.1993.tb03582.x [DOI] [PubMed] [Google Scholar]
- Marsden K., Beattie J., Friedenthal J., Carroll R. (2007). NMDA receptor activation potentiates inhibitory transmission through GABA receptor-associated protein-dependent exocytosis of GABA(A) receptors. J. Neurosci. 27, 14326–14337 10.1523/JNEUROSCI.4433-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister A. (1999). Subplate neurons: a missing link among neurotrophins, activity, and ocular dominance plasticity? Proc. Natl. Acad. Sci. USA 96, 13600–13602 10.1073/pnas.96.24.13600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBain C. (2008). New directions in synaptic and network plasticity – a move away from NMDA receptor mediated plasticity. J. Physiol. 586, 1473–1474 10.1113/jphysiol.2008.151183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald B., Amato A., Connolly C., Benke D., Moss S., Smart T. (1998). Adjacent phosphorylation sites on GABAA receptor beta subunits determine regulation by cAMP-dependent protein kinase. Nat. Neurosci. 1, 23–28 10.1038/223 [DOI] [PubMed] [Google Scholar]
- McDonald B., Moss S. (1994). Differential phosphorylation of intracellular domains of gamma-aminobutyric acid type A receptor subunits by calcium/calmodulin type 2-dependent protein kinase and cGMP-dependent protein kinase. J. Biol. Chem. 269, 18111–18117 [PubMed] [Google Scholar]
- McDonald B., Moss S. (1997). Conserved phosphorylation of the intracellular domains of GABA(A) receptor beta2 and beta3 subunits by cAMP-dependent protein kinase, cGMP-dependent protein kinase protein kinase C and Ca2+/calmodulin type II-dependent protein kinase. Neuropharmacology 36, 1377–1385 [DOI] [PubMed] [Google Scholar]
- Meier J., Vannier C., Sergé A., Triller A., Choquet D. (2001). Fast and reversible trapping of surface glycine receptors by gephyrin. Nat. Neurosci. 4, 253–260 10.1038/85099 [DOI] [PubMed] [Google Scholar]
- Mizushima N., Ohsumi Y., Yoshimori T. (2002) Autophagosome formation in mammalian cells. Cell Struct. Funct. 27, 421–429 10.1247/csf.27.421 [DOI] [PubMed] [Google Scholar]
- Mizoguchi Y., Kanematsu T., Hirata M., Nabekura J. (2003). A rapid increase in the total number of cell surface functional GABAA receptors induced by brain-derived neurotrophic factor in rat visual cortex. J. Biol. Chem. 278, 44097–44102 10.1074/jbc.M305872200 [DOI] [PubMed] [Google Scholar]
- Mody I. (2005). Aspects of the homeostaic plasticity of GABAA receptor-mediated inhibition. J. Physiol. 562, 37–46 10.1113/jphysiol.2004.077362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mody I., Pearce R. (2004). Diversity of inhibitory neurotransmission through GABA(A) receptors. Trends Neurosci. 27, 569–575 10.1016/j.tins.2004.07.002 [DOI] [PubMed] [Google Scholar]
- Mortensen M., Smart T. (2006). Extrasynaptic alphabeta subunit GABAA receptors on rat hippocampal pyramidal neurons. J. Physiol. 577, 841–856 10.1113/jphysiol.2006.117952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss S., Smart T., Blackstone C., Huganir R. (1992). Functional modulation of GABAA receptors by cAMP-dependent protein phosphorylation. Science 257, 661–665 10.1126/science.1323140 [DOI] [PubMed] [Google Scholar]
- Nabekura J., Ueno T., Okabe A., Furuta A., Iwaki T., Shimizu-Okabe C., Fukuda A., Akaike N. (2002). Reduction of KCC2 expression and GABAA receptor-mediated excitation after in vivo axonal injury. J. Neurosci. 22, 4412–4417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z., Sieghart W., Mody I. (1999). Differential regulation of synaptic GABAA receptors by cAMP-dependent protein kinase in mouse cerebellar and olfactory bulb neurones. J. Physiol. 521, 421–435 10.1111/j.1469-7793.1999.00421.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyíri G., Freund T., Somogyi P. (2001). Input-dependent synaptic targeting of alpha(2)-subunit-containing GABA(A) receptors in synapses of hippocampal pyramidal cells of the rat. Eur. J. Neurosci. 13, 428–442 10.1046/j.1460-9568.2001.01407.x [DOI] [PubMed] [Google Scholar]
- O'Sullivan G., Kneussel M., Elazar Z., Betz H. (2005). GABARAP is not essential for GABA receptor targeting to the synapse. Eur. J. Neurosci. 22, 2644–2648 [DOI] [PubMed] [Google Scholar]
- Papadopoulos T., Korte M., Eulenburg V., Kubota H., Retiounskaia M., Harvey R., Harvey K., O'Sullivan G., Laube B., Hülsmann S., Geiger J., Betz H. (2007). Impaired GABAergic transmission and altered hippocampal synaptic plasticity in collybistin-deficient mice. EMBO J. 26, 3888–3899 10.1038/sj.emboj.7601819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick G., Bingol B., Weld H., Schuman E. (2003). Ubiquitin-mediated proteasome activity is required for agonist-induced endocytosis of GluRs. Curr. Biol. 13, 2073–2081 10.1016/j.cub.2003.10.028 [DOI] [PubMed] [Google Scholar]
- Poisbeau P., Cheney M., Browning M., Mody I. (1999). Modulation of synaptic GABAA receptor function by PKA and PKC in adult hippocampal neurons. J. Neurosci. 19, 674–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter N., Twyman R., Uhler M., Macdonald R. (1990). Cyclic AMP-dependent protein kinase decreases GABAA receptor current in mouse spinal neurons. Neuron 5, 789–796 10.1016/0896-6273(90)90338-G [DOI] [PubMed] [Google Scholar]
- Rathenberg J., Kittler J., Moss S. (2004). Palmitoylation regulates the clustering and cell surface stability of GABAA receptors. Mol. Cell. Neurosci. 26, 251–257 10.1016/j.mcn.2004.01.012 [DOI] [PubMed] [Google Scholar]
- Reid T., Bathoorn A., Ahmadian M., Collard J. (1999). Identification and characterization of hPEM-2, a guanine nucleotide exchange factor specific for Cdc42. J. Biol. Chem. 274, 33587–33593 10.1074/jbc.274.47.33587 [DOI] [PubMed] [Google Scholar]
- Rivera C., Li H., Thomas-Crusells J., Lahtinen H., Viitanen T., Nanobashvili A., Kokaia Z., Airaksinen M., Voipio J., Kaila K., Saarma M. (2002). BDNF-induced TrkB activation down-regulates the K+–Cl− cotransporter KCC2 and impairs neuronal Cl− extrusion. J. Cell Biol. 159, 747–752 10.1083/jcb.200209011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph U., Möhler H. (2004). Analysis of GABAA receptor function and dissection of the pharmacology of benzodiazepines and general anesthetics through mouse genetics. Annu. Rev. Pharmacol. Toxicol. 44, 475–498 10.1146/annurev.pharmtox.44.101802.121429 [DOI] [PubMed] [Google Scholar]
- Saliba R., Michels G., Jacob T., Pangalos M., Moss S. (2007). Activity-dependent ubiquitination of GABA(A) receptors regulates their accumulation at synaptic sites. J. Neurosci. 27, 13341–13351 10.1523/JNEUROSCI.3277-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savić M., Huang S., Furtmüller R., Clayton T., Huck S., Obradović D., Ugresić N., Sieghart W., Bokonjić D., Cook J. (2008). Are GABAA receptors containing alpha5 subunits contributing to the sedative properties of benzodiazepine site agonists? Neuropsychopharmacology 33, 332–339 10.1038/sj.npp.1301403 [DOI] [PubMed] [Google Scholar]
- Schaerer M., Kannenberg K., Hunziker P., Baumann S., Sigel E. (2001). Interaction between GABA(A) receptor beta subunits and the multifunctional protein gC1q-R. J. Biol. Chem. 276, 26597–26604 10.1074/jbc.M102534200 [DOI] [PubMed] [Google Scholar]
- Scheiffele P., Fan J., Choih J., Fetter R., Serafini T. (2000). Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell 101, 657–669 10.1016/S0092-8674(00)80877-6 [DOI] [PubMed] [Google Scholar]
- Serwanski D., Miralles C., Christie S., Mehta A., Li X., Blas A. D. (2006). Synaptic and nonsynaptic localization of GABAA receptors containing the alpha5 subunit in the rat brain. J. Comp. Neurol. 499, 458–470 10.1002/cne.21115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro L., Love J., Colman D. (2007). Adhesion molecules in the nervous system: structural insights into function and diversity. Annu. Rev. Neurosci. 30, 451–474 10.1146/annurev.neuro.29.051605.113034 [DOI] [PubMed] [Google Scholar]
- Shen X., Xu K., Fan Q., Pacheco-Rodriguez G., Moss J., Vaughan M. (2006). Association of brefeldin A-inhibited guanine nucleotide-exchange protein 2 (BIG2) with recycling endosomes during transferrin uptake. Proc. Natl. Acad. Sci. USA 103, 2635–2640 10.1073/pnas.0510599103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng G., Chang G., Lin J., Yu Z., Fang Z., Rong J., Lipton S., Li S., Tong G., Leibowitz S., Li X. (2006). Hypothalamic huntingtin-associated protein 1 as a mediator of feeding behavior. Nat. Med. 12, 526–533 10.1038/nm1382 [DOI] [PubMed] [Google Scholar]
- Shin H., Morinaga N., Noda M., Nakayama K. (2004). BIG2, a guanine nucleotide exchange factor for ADP-ribosylation factors: its localization to recycling endosomes and implication in the endosome integrity. Mol. Biol. Cell 15, 5283–5294 10.1091/mbc.E04-05-0388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigel E., Stephenson F. A., Mamalaki C., Barnard E. A. (1983). The gamma-aminobutyric acid/benzodiazepine receptor complex of bovine cerebral cortex. J. Biol. Chem. 258, 6965–6971 [PubMed] [Google Scholar]
- Stellwagen D., Beattie E., Seo J., Malenka R. (2005). Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor-alpha. J. Neurosci. 25, 3219–3228 10.1523/JNEUROSCI.4486-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowers R., Megeath L., Górska-Andrzejak J., Meinertzhagen I., Schwarz T. (2002). Axonal transport of mitochondria to synapses depends on milton, a novel Drosophila protein. Neuron 36, 1063–1077 10.1016/S0896-6273(02)01094-2 [DOI] [PubMed] [Google Scholar]
- Sugiyama Y., Kawaguchi S., Hirano T. (2008). mGluR1-mediated facilitation of long-term potentiation at inhibitory synapses on a cerebellar Purkinje neuron. Eur. J. Neurosci. 27, 884–896 10.1111/j.1460-9568.2008.06063.x [DOI] [PubMed] [Google Scholar]
- Tanaka T., Saito H., Matsuki N. (1997). Inhibition of GABAA synaptic responses by brain-derived neurotrophic factor (BDNF) in rat hippocampus. J. Neurosci. 17, 2959–2966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terunuma M., Jang I., Ha S., Kittler J., Kanematsu T., Jovanovic J., Nakayama K., Akaike N., Ryu S., Moss S., Hirata M. (2004). GABAA receptor phospho-dependent modulation is regulated by phospholipase C-related inactive protein type 1, a novel protein phosphatase 1 anchoring protein. J. Neurosci. 24, 7074–7084 10.1523/JNEUROSCI.1323-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P., Mortensen M., Hosie A., Smart T. (2005). Dynamic mobility of functional GABAA receptors at inhibitory synapses. Nat. Neurosci. 8, 889–897 [DOI] [PubMed] [Google Scholar]
- Tretter V., Ehya N., Fuchs K., Sieghart W. (1997). Stoichiometry and assembly of a recombinant GABAA receptor subtype. J. Neurosci. 17, 2728–2737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretter V., Hauer B., Nusser Z., Mihalek R., Höger H., Homanics G., Somogyi P., Sieghart W. (2001). Targeted disruption of the GABA(A) receptor delta subunit gene leads to an up-regulation of gamma 2 subunit-containing receptors in cerebellar granule cells. J. Biol. Chem. 276, 10532–10538 10.1074/jbc.M011054200 [DOI] [PubMed] [Google Scholar]
- Tretter V., Jacob T., Mukherjee J., Fritschy J., Pangalos M., Moss S. (2008). The clustering of GABA(A) receptor subtypes at inhibitory synapses is facilitated via the direct binding of receptor alpha 2 subunits to gephyrin. J. Neurosci. 28, 1356–1365 10.1523/JNEUROSCI.5050-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano G., Nelson S. (2000). Hebb and homeostasis in neuronal plasticity. Curr. Opin. Neurobiol. 10, 358–364 10.1016/S0959-4388(00)00091-X [DOI] [PubMed] [Google Scholar]
- Unwin N. (1995). Acetylcholine receptor channel imaged in the open state. Nature 373, 37–43 10.1038/373037a0 [DOI] [PubMed] [Google Scholar]
- Varoqueaux F., Aramuni G., Rawson R., Mohrmann R., Missler M., Gottmann K., Zhang W., Südhof T., Brose N. (2006). Neuroligins determine synapse maturation and function. Neuron 51, 741–754 10.1016/j.neuron.2006.09.003 [DOI] [PubMed] [Google Scholar]
- Wake H., Watanabe M., Moorhouse A., Kanematsu T., Horibe S., Matsukawa N., Asai K., Ojika K., Hirata M., Nabekura J. (2007). Early changes in KCC2 phosphorylation in response to neuronal stress result in functional downregulation. J. Neurosci. 27, 1642–1650 10.1523/JNEUROSCI.3104-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Liu S., Haditsch U., Tu W., Cochrane K., Ahmadian G., Tran L., Paw J., Wang Y., Mansuy I., Salter M., Lu Y. (2003). Interaction of calcineurin and type-A GABA receptor gamma 2 subunits produces long-term depression at CA1 inhibitory synapses. J. Neurosci. 23, 826–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washbourne P., Dityatev A., Scheiffele P., Biederer T., Weiner J., Christopherson K., El-Husseini A. (2004). Cell adhesion molecules in synapse formation. J. Neurosci. 24, 9244–9249 10.1523/JNEUROSCI.3339-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisden W., Cope D., Klausberger T., Hauer B., Sinkkonen S., Tretter V., Lujan R., Jones A., Korpi E., Mody I., Sieghart W., Somogyi P. (2002). Ectopic expression of the GABA(A) receptor alpha6 subunit in hippocampal pyramidal neurons produces extrasynaptic receptors and an increased tonic inhibition. Neuropharmacology 43, 530–549 10.1016/S0028-3908(02)00151-X [DOI] [PubMed] [Google Scholar]
- Woodin M., Ganguly K., Poo M. (2003). Coincident pre- and postsynaptic activity modifies GABAergic synapses by postsynaptic changes in Cl− transporter activity. Neuron 39, 807–820 10.1016/S0896-6273(03)00507-5 [DOI] [PubMed] [Google Scholar]
- Yan Z. (2002). Regulation of GABAergic inhibition by serotonin signaling in prefrontal cortex: molecular mechanisms and functional implications. Mol. Neurobiol. 26, 203–216 10.1385/MN:26:2-3:203 [DOI] [PubMed] [Google Scholar]
- Yu W., Charych E., Serwanski D., Li R., Ali R., Bahr B., Blas A. D. (2008). Gephyrin interacts with the glutamate receptor interacting protein 1 isoforms at GABAergic synapses. J. Neurochem. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W., Jiang M., Miralles CP., Li R., Chen G., de Blas AL. (2007). Gephyrin clustering is required for the stability of GABAergic synapses. Mol. Cell. Neurosci. 36, 484–500 10.1016/j.mcn.2007.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]