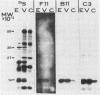
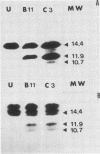
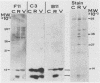
Abstract
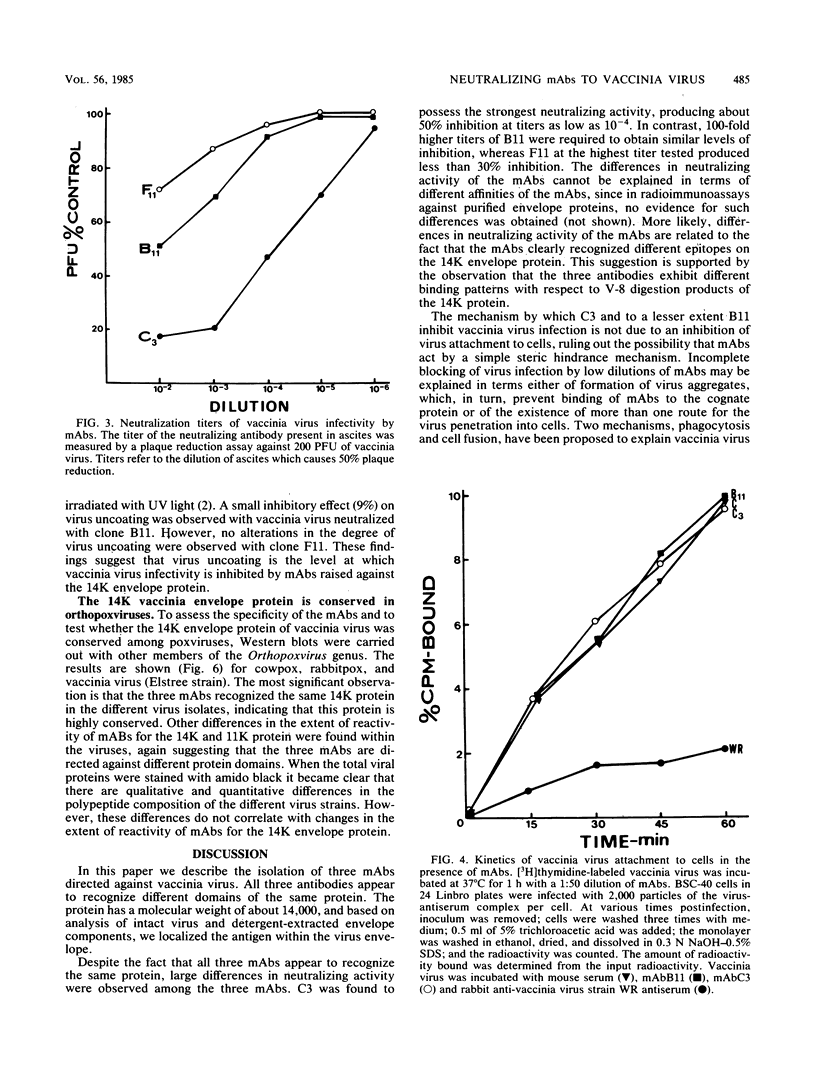
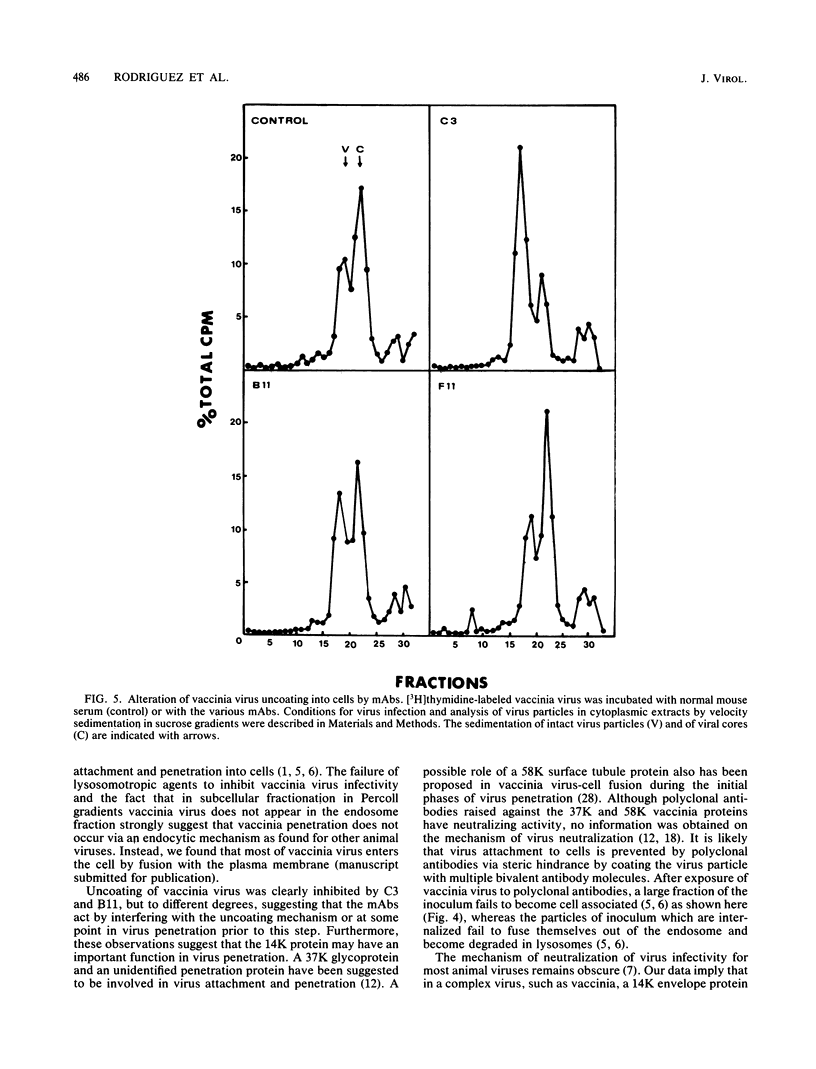
Cells producing neutralizing monoclonal antibodies (mAbs) to UV-inactivated vaccinia virus strain WR were derived by fusion of hyperimmunized mouse spleen cells with mouse myeloma cells. Three mAbs that reacted strongly with purified virus envelopes as determined by enzyme-linked immunosorbent assay were studied. The three mAbs recognized a 14,000-molecular-weight (14K) envelope protein of vaccinia virus and were shown to be immunoglobulin G2b (mAbC3 and mAbB11) and immunoglobulin M (mAbF11). By using ascites, one of the antibodies, mAbC3, neutralized (50%) virus infectivity with a titer of about 10(-4), whereas the others exhibited lower neutralization titers of 10(-2) to 10(-3). The binding of the mAbs to vaccinia virus did not alter virus attachment to cells. However, virus uncoating was extensively blocked by mAbC3, whereas mAbB11 and mAbF11 had little or no effect. The three mAbs recognized a similar 14K protein in cowpox, rabbitpox, and vaccinia Elstree strains, indicating a high degree of protein conservation among orthopoxviruses. Based on the binding of mAbs to V-8 protease cleavage products of the 14K protein, the extent of protein recognition for other poxviruses, and differences in the degree of virus neutralization and of virus uncoating into cells, we suggest that the three mAbs recognize different domains of vaccinia 14K viral envelope protein. Furthermore, our findings indicate that the 14K protein may play a role in virus penetration.
Full text
PDF

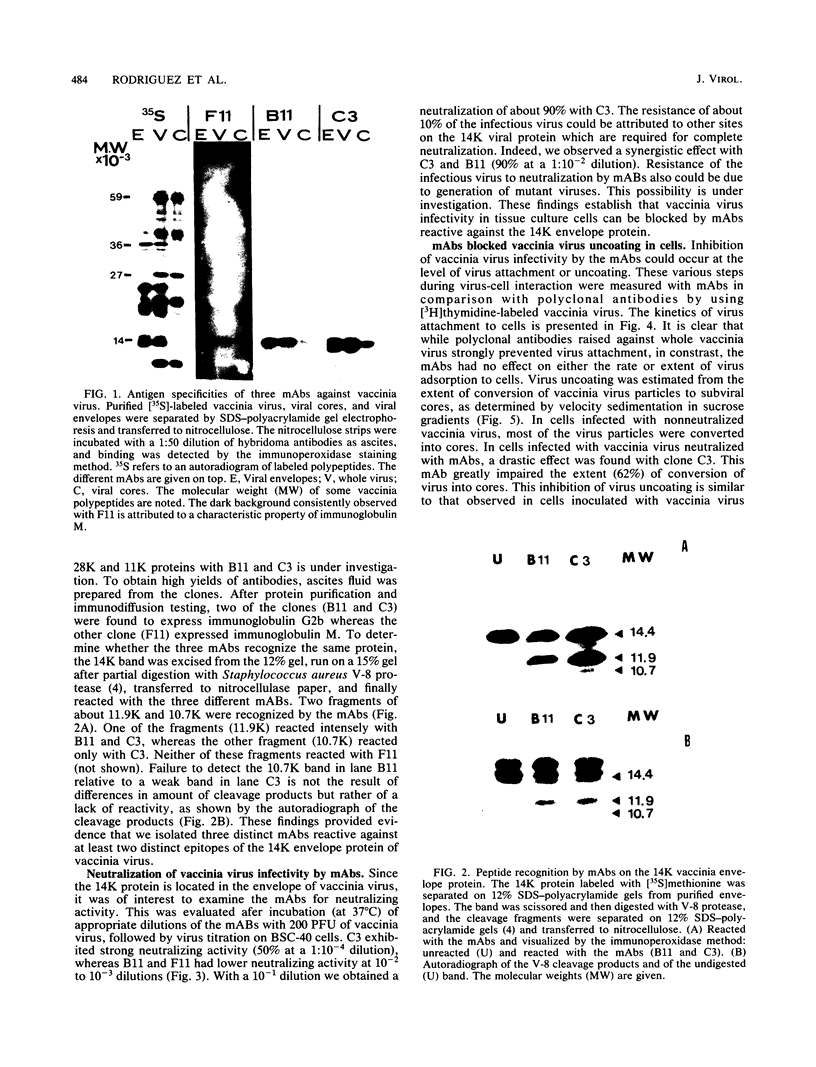


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J. A., Metz D. H., Young M. R. The mode of entry of vaccinia virus into L cells. J Gen Virol. 1973 Dec;21(3):533–537. doi: 10.1099/0022-1317-21-3-533. [DOI] [PubMed] [Google Scholar]
- Bablanian R., Coppola G., Scribani S., Esteban M. Inhibition of protein synthesis by vaccinia virus. III. The effect of ultraviolet-irradiated virus on the inhibition of protein synthesis. Virology. 1981 Jul 15;112(1):1–12. doi: 10.1016/0042-6822(81)90606-1. [DOI] [PubMed] [Google Scholar]
- Behbehani A. M. The smallpox story: life and death of an old disease. Microbiol Rev. 1983 Dec;47(4):455–509. doi: 10.1128/mr.47.4.455-509.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Dales S. Pentration of animal viruses into cells. Prog Med Virol. 1965;7:1–43. [PubMed] [Google Scholar]
- Dimmock N. J. Mechanisms of neutralization of animal viruses. J Gen Virol. 1984 Jun;65(Pt 6):1015–1022. doi: 10.1099/0022-1317-65-6-1015. [DOI] [PubMed] [Google Scholar]
- Esposito J. J., Obijeski J. F., Nakano J. H. Orthopoxvirus DNA: strain differentiation by electrophoresis of restriction endonuclease fragmented virion DNA. Virology. 1978 Aug;89(1):53–66. doi: 10.1016/0042-6822(78)90039-9. [DOI] [PubMed] [Google Scholar]
- Esteban M. Defective vaccinia virus particles in interferon-treated infected cells. Virology. 1984 Feb;133(1):220–227. doi: 10.1016/0042-6822(84)90443-4. [DOI] [PubMed] [Google Scholar]
- Golini F., Kates J. R. Transcriptional and translational analysis of a strongly expressed early region of the vaccinia virus genome. J Virol. 1984 Feb;49(2):459–470. doi: 10.1128/jvi.49.2.459-470.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holowczak J. A. Uncoating of poxviruses. I. Detection and characterization of subviral particles in the uncoating process. Virology. 1972 Oct;50(1):216–232. doi: 10.1016/0042-6822(72)90362-5. [DOI] [PubMed] [Google Scholar]
- Ichihashi Y., Oie M. Adsorption and penetration of the trypsinized vaccinia virion. Virology. 1980 Feb;101(1):50–60. doi: 10.1016/0042-6822(80)90482-1. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K. The purification fo four strains of poxvirus. Virology. 1962 Sep;18:9–18. doi: 10.1016/0042-6822(62)90172-1. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Mackett M., Smith G. L., Moss B. General method for production and selection of infectious vaccinia virus recombinants expressing foreign genes. J Virol. 1984 Mar;49(3):857–864. doi: 10.1128/jvi.49.3.857-864.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackett M., Smith G. L., Moss B. Vaccinia virus: a selectable eukaryotic cloning and expression vector. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7415–7419. doi: 10.1073/pnas.79.23.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackett M., Yilma T., Rose J. K., Moss B. Vaccinia virus recombinants: expression of VSV genes and protective immunization of mice and cattle. Science. 1985 Jan 25;227(4685):433–435. doi: 10.1126/science.2981435. [DOI] [PubMed] [Google Scholar]
- Moss B., Smith G. L., Gerin J. L., Purcell R. H. Live recombinant vaccinia virus protects chimpanzees against hepatitis B. Nature. 1984 Sep 6;311(5981):67–69. doi: 10.1038/311067a0. [DOI] [PubMed] [Google Scholar]
- Panicali D., Davis S. W., Weinberg R. L., Paoletti E. Construction of live vaccines by using genetically engineered poxviruses: biological activity of recombinant vaccinia virus expressing influenza virus hemagglutinin. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5364–5368. doi: 10.1073/pnas.80.17.5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panicali D., Paoletti E. Construction of poxviruses as cloning vectors: insertion of the thymidine kinase gene from herpes simplex virus into the DNA of infectious vaccinia virus. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4927–4931. doi: 10.1073/pnas.79.16.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondle C. J., Dumbell K. R. A poxvirus antigen associated with pathogenicity for rabbits. J Hyg (Lond) 1982 Dec;89(3):383–388. doi: 10.1017/s0022172400070959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarov I., Joklik W. K. Characterization of intermediates in the uncoating of vaccinia virus DNA. Virology. 1972 Nov;50(2):593–602. doi: 10.1016/0042-6822(72)90410-2. [DOI] [PubMed] [Google Scholar]
- Smith G. L., Mackett M., Moss B. Infectious vaccinia virus recombinants that express hepatitis B virus surface antigen. Nature. 1983 Apr 7;302(5908):490–495. doi: 10.1038/302490a0. [DOI] [PubMed] [Google Scholar]
- Smith G. L., Murphy B. R., Moss B. Construction and characterization of an infectious vaccinia virus recombinant that expresses the influenza hemagglutinin gene and induces resistance to influenza virus infection in hamsters. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7155–7159. doi: 10.1073/pnas.80.23.7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern W., Dales S. Biogenesis of vaccinia: isolation and characterization of a surface component that elicits antibody suppressing infectivity and cell-cell fusion. Virology. 1976 Nov;75(1):232–241. doi: 10.1016/0042-6822(76)90022-2. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner A., Baxby D. Structural polypeptides of Orthopoxvirus: their distribution in various members and location within the virion. J Gen Virol. 1979 Dec;45(3):537–545. doi: 10.1099/0022-1317-45-3-537. [DOI] [PubMed] [Google Scholar]
- Walls H. H., Ziegler D. W., Nakano J. H. Characterization of antibodies to orthopoxviruses in human sera by radioimmunoassay. Bull World Health Organ. 1981;59(2):253–262. [PMC free article] [PubMed] [Google Scholar]
- Wiktor T. J., Macfarlan R. I., Reagan K. J., Dietzschold B., Curtis P. J., Wunner W. H., Kieny M. P., Lathe R., Lecocq J. P., Mackett M. Protection from rabies by a vaccinia virus recombinant containing the rabies virus glycoprotein gene. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7194–7198. doi: 10.1073/pnas.81.22.7194. [DOI] [PMC free article] [PubMed] [Google Scholar]