Abstract
The first total synthesis of gliocladin C, a fungal-derived marine alkaloid containing a rare trioxopiperazine fragment, is reported. This asymmetric synthesis establishes the absolute configuration of this structurally novel natural product.
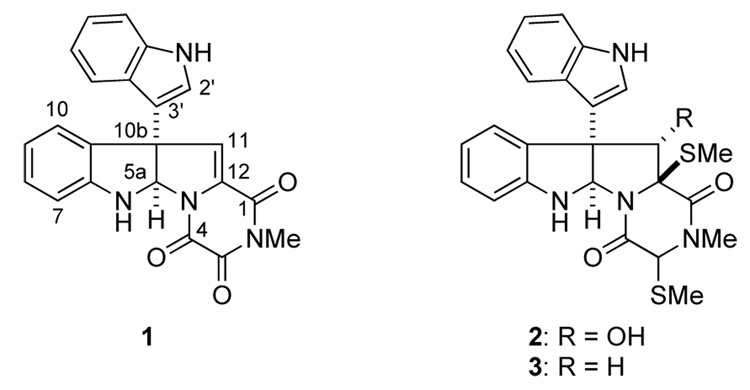
Fungi found in marine organisms have proven to be a rich source of architecturally novel and biologically active natural products.1 In 2004, Usami and co-workers reported the isolation of the indole alkaloid gliocladin C (1) from a strain of Gliocladium roseum, originally obtained from the sea hare Aplysia kurodai (Figure 1).2 Coisolated with gliocladin C were the sulfur-containing analogs gliocladins A (2) and B (3), the former being related closely in structure to epidithiodiketopiperazine congeners leptosin D,3 gliocladine A4 and T988A.5 Gliocladins A–C exhibited cytotoxic activity against P388 lymphocytic leukemia in cell culture, with gliocladin C (1) being most potent (2.4 µg/mL).2
Figure 1.
Gliocladins A (2), B (3) and C (1).
The proposed gross structure and relative configuration of gliocladin C (1) was based on mass spectrometric and spectroscopic data, with the absolute configuration being undefined.2 The most novel structural feature of gliocladin C is the trioxopiperazine ring, which is an extremely rare feature of natural products, never before seen in conjunction with a pyrrolidinoindoline fragment.6,7 We report in this disclosure the first total synthesis of gliocladin C (1) and proof that its absolute configuration is as depicted in Figure 1.
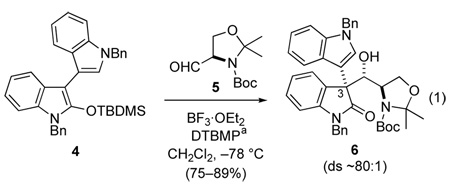
Oxindoles having a β-aminoethyl substituent at C3 are time-tested precursors of pyrrolidinoindolines.8 We recently reported9 that elaborate, enantiopure structures of this type containing an aryl or heteroaryl substituent at the quaternary C3 stereocenter could be quickly assembled by the Mukaiyama aldol reaction10 of 2-siloxyindoles and the serine-derived aldehyde 5 (equation 1).11 (+)-Oxindole 6, which can be prepared in this fashion on a large scale in five steps from isatin, was the starting point for our construction of (+)-gliocladin C (1).
aDTBMP = 2,6-di-tert-butyl-4-methylpyridine
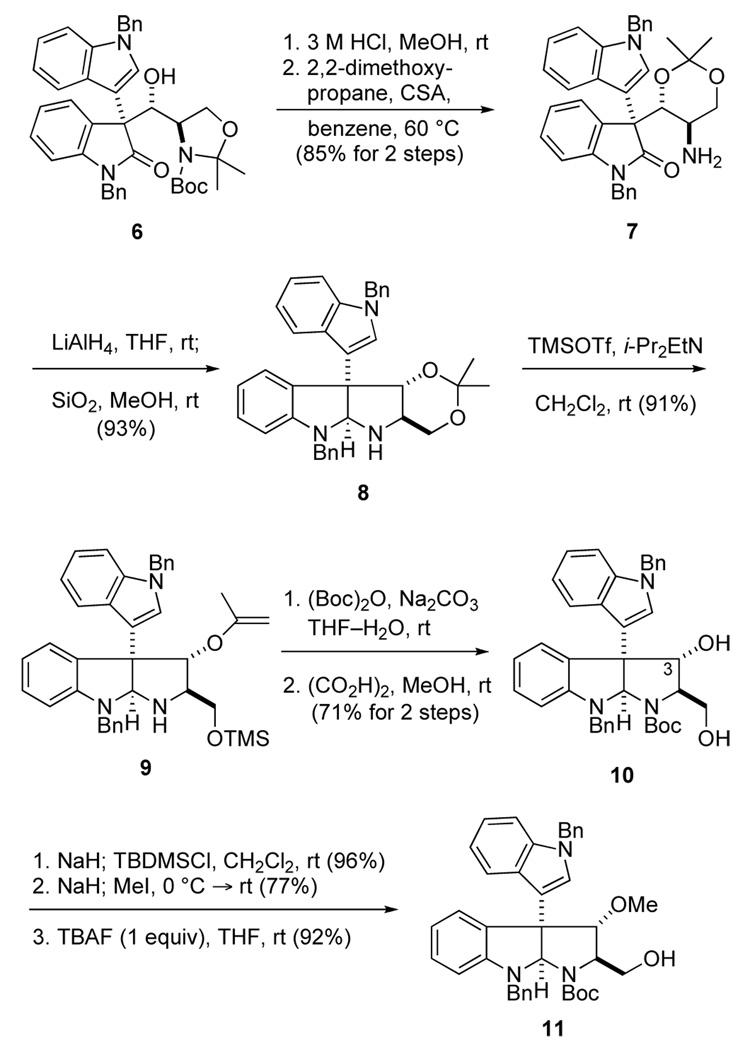
The conversion of Mukaiyama aldol adduct 6 to hydroxymethyl pyrrolidinoindoline 11 is summarized in Scheme 1. This seemingly straightforward elaboration was rendered challenging by the propensity of oxindole 6 to undergo retro-aldol fragmentation under basic conditions,9 and the acid sensitivity of pyrrolidinolindolines having a hydroxyl substituent at C3.12,13 The sequence that ultimately proved successful began by cleavage of the oxazoline and Boc substituents of aldol adduct 6 with 3 M HCl in MeOH, followed by reaction of the resulting amino diol with 2,2-dimethoxypropane, a sequence that delivered 1,3-dioxane 7 in 85% overall yield. The use of formic acid in the first step9 resulted in partial retroaldolization in large-scale reactions when this less volatile acid was removed by evaporation. Reaction of amino oxindole 7 with excess LiAlH4 at room temperature, followed by exposure of the crude product to a slurry of silica gel in MeOH provided pyrrolidinoindoline 8 in 93% yield.
Scheme 1.
Conversion of aldol product 6 to pyrrolidinoindoline alcohol 11
We first became aware of the extreme acid sensitivity of pyrrolidinoindolines containing hydroxyl sustituents at C3 when all standard conditions we surveyed for cleaving the acetonide substituent of intermediate 8 resulted in extensive decomposition. However, using the method developed by Rychnovsky,14 this group was transformed to silyloxy propenyl ether 9 in high yield by exposure to excess TMSOTf and diisopropylethylamine. After introducing a Boc group to protect the pyrrolidine nitrogen, reaction at room temperature with a catalytic amount of oxalic acid in MeOH delivered diol 10 in 71% yield for the two steps. As this intermediate was quite sensitive, all attempts to selectively oxidize the primary alcohol substituent were unsuccessful. Thus, diol 10 was transformed to methoxy derivative 11 by selective protection of the primary alcohol with a TBDMS group, followed by sequential reaction with excess NaH and MeI and then TBAF (1 equiv). This series of three reactions provided intermediate 11 in 68% overall yield from diol precursor 10.15 All steps of this sequence take place under basic conditions, which is likely key to its success.
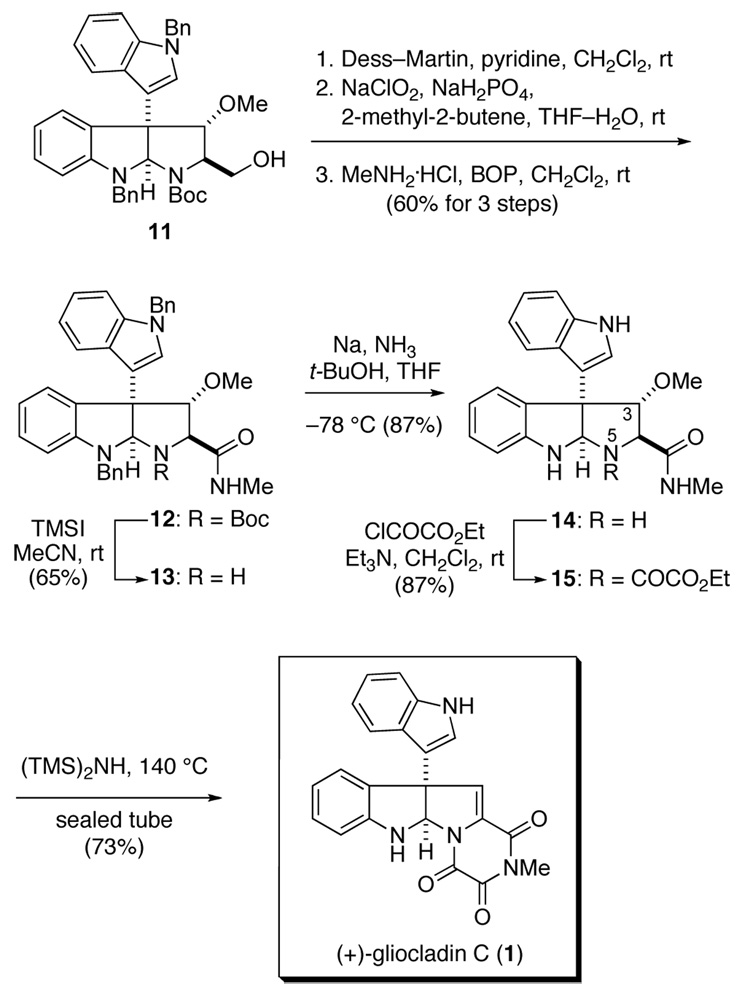
The trioxopiperazine ring of (+)-gliocladin C was assembled, and the Δ11,12-unsaturation introduced, by the series of transformations summarized in Scheme 2. The primary alcohol substituent of alcohol 11 was first oxidized to give the corresponding acid without effecting the indole substituent by a two-step sequence involving initial reaction with Dess-Martin periodinane16 to give the corresponding aldehyde, followed by sodium chlorite oxidation.17 Coupling of the crude acid product with methylamine using the BOP reagent18 then delivered amide 12 in 60% overall yield form hydroxymethyl pyrrolidinoindoline 11. To set the stage for assembling the trioxopiperazine ring, the Boc group was cleaved by reaction of 12 with TMSI to give secondary amine 13 in 65% yield.19,20 A preliminary survey of the reactivity of the pyrrolidine nitrogen of congeners of 1321 had shown that acylation of the hindered and inductively deactivated secondary amine was problematic; thus, the benzyl protecting group of the adjacent nitrogen and that of the indole substituent were removed at this stage by the reaction of 13 at −78 °C with excess Na and t-BuOH in THF–NH3. This deprotection was remarkably clean, providing the secondary triamine 14 in 87% yield. Although several potential approaches for fashioning the trioxopiperazine ring in one step were unsuccessful,22 reaction of 14 with ethyl chlorooxoacetate in the presence of Et3N took place cleanly at N5 to give oxalyl half-ester half-amide 15 in 87% yield. To our initial dismay, attempts to cyclize this intermdiate by reaction with a variety of bases (e.g., DBU, i-Pr2EtN, Et3N, or NaH) led to extensive decomposition. Fortunately, a method developed by Mulliez to form peptide-derived trioxopiperazines proved successful.23 Thus, when a solution of 15 and 1,1,1,3,3,3-hexamethyldisilazane was heated at 140 °C in a sealed tube, cyclization to form the trioxopiperazine and elimination of the methoxy group both took place to give (+)-gliocladin C (1), a pale yellow solid, in 73% yield. Comparison of 1H and 13C NMR data24,25 of synthetic 1 with those of the natural product confirmed their identity. The optical rotation of synthetic 1, [α]23D +116, (c 0.02 CHCl3), compared well with that reported for the natural sample, [α]D +131, (c 0.07 CHCl3). Because the relative and absolute configuration of the Fmoc derivative of synthetic precursor 7 had been determined by single-crystal X-ray analysis,9 this comparison establishes the absolute configuration of (+)-gliocladin C (1) to be as depicted.
Scheme 2.
Construction of the trioxopiperazine ring to form (+)-gliocladin C (1)
In summary, the first total synthesis of the structurally novel marine alkaloid (+)-gliocladin C (1) was completed in ~4% overall yield and 21 steps from isatin. A central step in this sequence is asymmetric construction of the quaternary carbon stereocenter by a Mukaiyama aldol reaction of siloxyindole 4 and enantiopure aldehyde 5.9 Knowledge gained during the latter stages of this synthesis could potentially allow the synthetic sequence to be streamlined. Of more importance, a better appreciation of the acid sensitivity of pyrrolidinoindolines containing oxygen substituents at C3 should assist in the design of synthetic approaches to related, more complex and biologically more potent, alkaloids.26
Supplementary Material
Experimental procedures, tabulated 1H and 13C NMR spectra of natural and synthetic (+)-gliocladin C, copies of 1H and 13C NMR spectra of new compounds, and the X-ray model of the C3 acetate analog of 13 (34 pages). This material is available free of charge via the Internet at http://pubs.acs.org).
Acknowledgment
This research was supported by grant GM-30859 from the National Institutes of General Medical Sciences. We also thank Amgen, Merck, Pfizer and Roche Palo Alto for unrestricted support. We particularly thank Professor Y. Usami (Osaka University of Pharmaceutical Sciences) for providing copies of NMR spectra of natural gliocladin C and for useful discussion. We also thank Dr. Young Ho Rhee for optimizing the synthesis of 7 and Dr. Sébastien Caillé for first preparing compounds 8 and 9. NMR and mass spectra were determined at UC Irvine with instruments purchased with the assistance of the NSF and NIH shared instrumentation programs.
References
- 1.Blunt JW, Copp BR, Munro MHG, Northcote PT, Prinsep MR. Nat. Prod. Rept. 2006;23:26–78. doi: 10.1039/b502792f. and earlier reviews in this series. [DOI] [PubMed] [Google Scholar]
- 2.Usami Y, Yamaguchi J, Numata A. Heterocycles. 2004;63:1123–1129. [Google Scholar]
- 3.Takahashi C, Numata A, Ito Y, Matsumura E, Araki H, Iwaki H, Kushida K. J. Chem. Soc., Perkin Trans. 1. 1994:1859–1864. [Google Scholar]
- 4.Dong J-Y, He H-P, Shen Y-M, Zhang K-Q. J. Nat. Prod. 2005;68:1510–1513. doi: 10.1021/np0502241. [DOI] [PubMed] [Google Scholar]
- 5.Feng Y, Blunt JW, Cole ALJ, Munro MHG. J. Nat. Prod. 2004;67:2090–2092. doi: 10.1021/np030326w. [DOI] [PubMed] [Google Scholar]
- 6.A trioxopiperazine ring is found in dithiosecoemestrin7a and neoechinulin.7b
- 7.(a) Seya H, Nozawa K, Udagawa S, Nakajima S, Kawai K. Chem. Pharm. Bull. 1986;34:2411–2416. doi: 10.1248/cpb.34.2411. [DOI] [PubMed] [Google Scholar]; (b) Casnati G, Pochini A, Ungaro R. Gazz. Chim. Ital. 1973;103:141–151. [Google Scholar]
- 8.Julian PL, Pikl J, Boggess D. J. Am. Chem. Soc. 1934;56:1797–1801. [Google Scholar]
- 9.Adhikari S, Caillé S, Hanbauer M, Ngo VX, Overman LE. Org. Lett. 2005;7:2795–2798. doi: 10.1021/ol051172+. [DOI] [PubMed] [Google Scholar]
- 10.Mukaiyama T, Banno K, Narasaka K. J. Am. Chem. Soc. 1974;96:7503–7509. [Google Scholar]
- 11.Garner P, Park JM. Org. Synth., Coll. Vol. 1999;9:300–305. [Google Scholar]
- 12.(a) The acid sensitivity of 3-hydroxypyrrolidinoindolines is believed to derive from acid-catalyzed ring opening of the aminal functionality. Iminium species generated in this way could degrade by multiple pathways, for example, by retroaldol-type cleavage. (b) Degradative studies of verticillin A13a and leptosin B13b demonstrated the instability of the 3-hydroxypyrrolidinoindoline subunit under strongly basic conditions or upon reaction with triphenylphosphine.
- 13.(a) Minato H, Matsumoto M, Katayama T. J. Chem. Soc., Perkin Trans 1. 1973:1819–1825. doi: 10.1039/p19730001819. [DOI] [PubMed] [Google Scholar]; (b) Takahashi C, Numata A, Ito Y, Matsumura E, Araki H, Iwaki H, Kushida K. J. Chem. Soc., Perkin Trans 1. 1994:1859–1864. [Google Scholar]
- 14.(a) Rychnovsky SD, Hoye RC. J. Am. Chem. Soc. 1994;116:1753–1765. [Google Scholar]; (b) Rychnovsky SD, Kim J. Tetrahedron Lett. 1991;32:7219–7222. [Google Scholar]
- 15.The use of excess TBAF resulted in lower yields.
- 16.(a) Dess DB, Martin JC. J. Org. Chem. 1983;48:4155–4156. [Google Scholar]; (b) Meyer SD, Schreiber SL. J. Org. Chem. 1994;59:7549–7552. [Google Scholar]
- 17.(a) Bal BS, Childers WE, Jr, Pinnick HW. Tetrahedron. 1981;37:2091–2096. [Google Scholar]; (b) Kraus GA, Taschner MJ. J. Org. Chem. 1980;45:1175–1176. [Google Scholar]
- 18.BOP = benzotriazole-1-yloxy-tris(dimethylamino)phosphonium hexafluorophosphate.
- 19.Depew KM, Marsden SP, Zatorska D, Zatorski A, Bornmann WG, Danishefsky SJ. J. Am. Chem. Soc. 1999;121:11953–11963. [Google Scholar]
- 20.The C3 acetate analog of 13 provided single crystals allowing the relative configuration of this intermediate to be confirmed by X-ray crystallography.
- 21.The substituent was OAc or OTIPS instead of OMe.
- 22.(a) Makino S, Nakanishi E, Tsuji T. Synlett. 2003;6:817–820. [Google Scholar]; (b) Bailey PD, Bannister N, Bernad M, Blanchard S, Boa AN. J. Chem. Soc., Perkin Trans. 1. 2001:3245–3251. [Google Scholar]
- 23.Mulliez M, Royer J. Tetrahedron. 1984;40:5143–5151. [Google Scholar]
- 24.The small signal for the quaternary carbon C3' at 116.7 ppm, which is seen in the 13C NMR spectrum of natural gliocladin C, was not reported in reference 2. Assignments reported in this paper for signals at 122.7 and 120.11/120.13 ppm should be changed to C6' and C4'/C5'.25 A summary of peak assignments for synthetic gliocladin C, which were established by HMQC and HMBC experiments, can be found in Supporting Information.
- 25.Usami Y. Personal communication. 2006. Aug 8,
- 26.Anthoni U, Christophersen C, Nielsen PH. Naturally Occurring Cyclotryptophans and Cyclotryptamines. In: Pelletier SW, editor. Alkaloids: Chemical and Biological Perspectives. Vol. 13. New York: Pergamon; 1999. pp. 163–236. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental procedures, tabulated 1H and 13C NMR spectra of natural and synthetic (+)-gliocladin C, copies of 1H and 13C NMR spectra of new compounds, and the X-ray model of the C3 acetate analog of 13 (34 pages). This material is available free of charge via the Internet at http://pubs.acs.org).