Abstract
CD4+FoxP3+ regulatory T (T reg) cells comprise a separate lineage of T cells that are essential for maintaining immunological tolerance to self. The molecular mechanism(s) by which T reg cells mediate their suppressive effects remains poorly understood. One molecule that has been extensively studied in T reg cell suppression is transforming growth factor (TGF)-β, but its importance remains controversial. We found that TGF-β complexed to latency-associated peptide (LAP) is expressed on the cell surface of activated but not resting T reg cells. T reg cell LAP–TGF-β plays an important role in the suppression of the proliferation of activated T cells, but it is not required for the suppression of naive T cell activation. More importantly, T reg cell–derived TGF-β could generate de novo CD4+FoxP3+ T cells in vitro from naive precursors in a cell contact–dependent, antigen-presenting cell–independent and αV integrin–independent manner. The newly induced CD4+FoxP3+ T cells are suppressive both in vitro and in vivo. Transfer of activated antigen-specific T reg cells with naive antigen-specific responder T cells to normal recipients, followed by immunization, also results in induction of FoxP3 expression in the responder cells. T reg cell–mediated generation of functional CD4+FoxP3+ cells via this TGF-β–dependent pathway may represent a major mechanism as to how T reg cells maintain tolerance and expand their suppressive abilities.
Naturally occurring regulatory T (T reg) cells—once a highly controversial topic—have now gained wide acceptance as playing a critical role in the maintenance of self-tolerance. T reg cells suppress immune activation in a dominant manner and are characterized by the expression of the transcription factor FoxP3 (1–3). The importance of T reg cells in immune homeostasis is best illustrated by the development of severe autoimmune diseases in both mice and humans with genetic deficiencies of FoxP3 (4, 5). One question that remains poorly understood is how a very small number of antigen-specific T reg cells can be so efficient in exerting their suppressive effect in vivo (6). One possibility is that T reg cell function can be activated by cytokines such as IL-2 alone (7). Alternatively, T reg cells can induce a suppressive phenotype in potential effector cells thereby expanding the pool of suppressive cells.
The term “infectious tolerance” was originally coined by Gershon and Kondo and referred to suppression of naive lymphocyte populations by cells with regulatory function (8). This concept was later expanded upon by Qin et al. (9) and is now used to describe a process where a tolerance-inducing state is transferred from one cell population to another. Previous studies have demonstrated a role for T reg cells in the long-term acceptance of allogeneic transplants and the induction of infectious tolerance against these grafts (10). Several in vitro studies have demonstrated that T reg cells can induce suppressive CD4+ T cells, but the exact nature of these cells is unclear, including whether they express FoxP3 or not (11–13). The demonstration that TGF-β is a potent inducer of FoxP3 expression (14) and T reg cell function in vitro and in vivo (14, 15), together with recent studies demonstrating that T reg cell production of TGF-β is required for protection against autoimmune disease (16), raised the possibility that one potential mechanism of action of T reg cells is to induce T reg cells de novo from T-naive precursors in a TGF-β–dependent manner, thus generating infectious tolerance. In this study, we demonstrate that T reg cells directly confer infectious tolerance by inducing new CD4+FoxP3+ T cells and define the mechanisms they use to do so.
RESULTS AND DISCUSSION
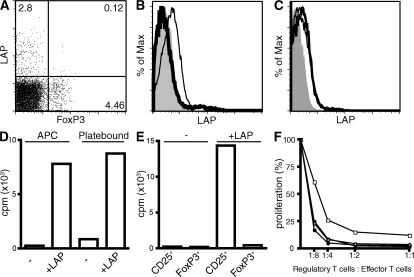
Previous studies have demonstrated that the TGF-β propeptide, latency-associated peptide (LAP), and thus presumably TGF-β are expressed on the surface of T reg cells, but the kinetics of LAP expression remain controversial (17–19). Flow cytometric analysis of mouse lymph node cells demonstrated that FoxP3+ T cells do not express LAP under normal steady-state conditions, whereas a small subpopulation of non-CD4+ T cells stained positive for LAP (Fig. 1 A and unpublished data). After activation, T reg cells but not conventional T cells expressed LAP on their cell surface (Fig. 1 B). To determine if T reg cells also expressed active TGF-β (free from LAP) on their surface, we incubated activated T reg cells with recombinant LAP (rLAP) for 2 h at 4°C and then stained them for LAP. If free TGF-β was present on the surface, it should be able to bind LAP resulting in increased LAP staining. No increase in the intensity of the LAP staining was seen, indicating that there is no active free TGF-β available on the cell surface (Fig. 1 C). Staining of activated T cells with anti–TGF-β antibodies confirmed the lack of free TGF-β on the cell surface of these cells (unpublished data). Similar results were observed with human T reg cells. Freshly explanted human peripheral blood CD4+FoxP3+ T cells did not express LAP (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20080308/DC1), whereas 5% of CD4+FoxP3− T cells appeared to be LAP+. A marked increase of LAP expression was apparent on T reg cells already after 12 h of activation (Fig. S1), and almost all FoxP3+ T cells were LAP+ after 60 h of activation. Thus, it appears that LAP expression correlates with the suppressive function of T reg cells, as the FoxP3+ T cells derived from human CD4+CD25+ T cells are suppressive, whereas the FoxP3+ T cells derived from human CD4+CD25− T cells, in contrast to mouse, fail to express a suppressive phenotype (20–23).
Figure 1.
LAP is expressed by activated T reg cells, and TGF-β is required to suppress CD4+CD25+FoxP3− T cells. (A) Expression of LAP and FoxP3 on freshly isolated lymph node cells from C57BL/6 mice. (B) CD4+CD25− (thick line histogram) and CD4+CD25+ T cells (thin line histogram) were activated for 3 d with plate-bound anti-CD3 and IL-2 and analyzed for LAP expression. Isotype control is depicted with a solid gray histogram. (C) Activated CD4+CD25+ T cells were incubated with (thick line histogram) or without (thin line histogram) 20 μg/ml LAP for 2 h at 4°C and analyzed for LAP expression. Isotype control is depicted with a solid gray histogram. (D) CD4+CD25+ T cells (5 × 104/well) from lymph nodes of C57BL/6 mice were activated with plate-bound anti-CD3 or with irradiated T cell–depleted splenocytes (5 × 104/well) and 0.25 μg/ml of soluble anti-CD3 in the presence or absence of LAP. [3H]TdR incorporation was determined after 72 h of culture. (E) CD4+CD25+ from C57BL/6 mice or CD4+CD25+ GFP+ T cells from C57BL/6 FoxP3-GFP knock-in mice were cultured together with irradiated T cell–depleted splenocytes (5 × 104/well) and 0.25 μg/ml of soluble anti-CD3 in the presence or absence of LAP. [3H]TdR incorporation was determined after 72 h of culture. (F) CD4+CD25− C57BL/6 T cells (5 × 104/well) were co-cultured with CD4+CD25+ T cells from C57BL/6 mice (squares) or with CD4+CD25+GFP+ T cells from C57BL/6 FoxP3-GFP knock-in mice (circles) in the presence of irradiated T cell–depleted splenocytes (5 × 104/well) and 0.25 μg/ml of soluble anti-CD3 with (open symbols) or without (closed symbols) 12.5 μg/ml LAP. [3H]TdR incorporation was determined after 72 h of culture. One representative experiment of at least three is shown (A–F).
The role of TGF-β in T reg cell suppression has been extensively studied, but its importance remains controversial (16–19, 24–26). Our laboratory and others have previously reported that CD4+CD25+ T cells can mediate suppressor function both in vitro and in vivo in the absence of TGF-β (24–26). The results presented above that T reg cells express cell surface LAP in an activation-dependent manner prompted us to reexamine the role of TGF-β in the anergic state of T reg cells and in T reg cell–mediated suppression. To determine if TGF-β plays any role in the anergic state of T reg cells, we used rLAP as a TGF-β inhibitor. We chose LAP as an inhibitor because it directly binds active TGF-β and could also potentially block the binding site of LAP–TGF-β complexes to TGF-β–activating factors. When highly purified CD4+CD25+ T cells (95% FoxP3+) were cultured with APCs and anti-CD3 or plate-bound anti-CD3 in the presence of rLAP, they were no longer completely anergic (Fig. 1 D). However, the addition of rLAP failed to reverse the anergic state of CD4+CD25+FoxP3+ T cells isolated from FoxP3 GFP knock-in mice (Fig. 1 E), suggesting that rLAP allowed CD4+CD25+FoxP3− T cells to produce IL-2 and proliferate even in the presence of 20 times more T reg cells. The specific loss of suppression of CD4+CD25+FoxP3− T cells was also seen when rLAP is added to the standard in vitro suppression assays, which resulted in a modest but reproducible increase in effector cell proliferation only when CD4+CD25+ T cells (Fig. 1 F), but not CD4+CD25+FoxP3+ T cells, were the source of the T reg cells. Thus, membrane-bound or potentially soluble TGF-β plays a role in T reg cell suppression of activated CD4+CD25+FoxP3− T cells, but it is not needed for T reg cells to suppress naive T cells.
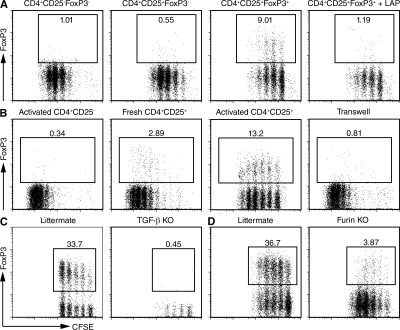
Culture of mouse T cells in the presence of TGF-β and IL-2 results in the induction of FoxP3 expression in a high percentage of the cells, and these induced T reg cells are fully functional in vitro and in vivo (14, 15, 27). Because activated T reg cells express LAP–TGF-β complexes on their cell surface, we asked whether T reg cells would be able to confer infectious tolerance by inducing FoxP3 expression in CD4+FoxP3− T cells. CD4+CD25+FoxP3+ from FoxP3-GFP transgenic mice were activated with plate-bound anti-CD3 and IL-2 for 3 d, followed by 1 d of culture in IL-2–containing media. The activated T cells were then mixed with CFSE-labeled 5CC7 TCR transgenic RAG−/− T cells, which are completely devoid of FoxP3-expressing cells, and co-cultured for 4 d in the presence of IL-2, anti-CD3, and splenic DCs. The addition of IL-2 was necessary for dampening the growth-suppressive effects of the T reg cells and facilitating the recovery of live cells at the end of the co-culture. Co-culture of preactivated FoxP3+ T reg cells, but not preactivated CD4+FoxP3− T cells, with naive CD4+FoxP3− T cells resulted in the induction of FoxP3 expression in 10–30% of the naive T cells (Fig. 2 A). Freshly isolated T reg cells were much less efficient at inducing FoxP3 than previously activated T reg cells (Fig. 2 B), and this correlates with the increased expression of LAP on the preactivated T reg cells. The low number of FoxP3+ cells induced by fresh T reg cells is likely due to the induction of membrane-bound LAP–TGF-β when these cells become activated in the co-culture, but could potentially also be due to subpopulations of T reg cells expressing low levels of TGF-β on their cell surface. Cell contact was required for the T reg cell–dependent induction of FoxP3 because activated T reg cells separated from the naive responders using a transwell system failed to induce FoxP3 (Fig. 2 B). Induction of FoxP3 expression was also seen in the co-cultures of responder TCR transgenic T cells stimulated with their cognate peptide and activated polyclonal T reg cells, indicating that the TCR restimulation of the T reg cells was not required for FoxP3 induction (Fig. S3, available at http://www.jem.org/cgi/content/full/jem.20080308/DC1). T reg cells generated in vitro by TCR stimulation of naive cells in the presence of IL-2 and TGF-β could also induce FoxP3 expression in naive cells, suggesting that the T reg cell–dependent induction of FoxP3 can function in a positive feedback loop capable of inducing more suppressive T reg cells (Fig. S4). It should be noted that we have not seen any differences in FoxP3 induction whether the T reg cells, responder T cells, and APCs are MHC matched or mismatched (unpublished data).
Figure 2.
T reg cells confer infectious tolerance in a TGF-β–dependent manner. (A) Activated CD4+CD25−FoxP3−, CD4+CD25+FoxP3−, and CD4+CD25+FoxP3+ T cells were co-cultured with CFSE-labeled 5CC7 TCR transgenic RAG−/− T cells, 2 μg/ml anti-CD3, splenic DCs, and IL-2 (100 U/ml) in the absence or presence of LAP. (B) Activated CD4+CD25+ and freshly isolated CD4+CD25+ were co-cultured with CFSE-labeled 5CC7 TCR transgenic RAG−/− T cells, 2 μg/ml anti-CD3, splenic DCs, and IL-2 (100 U/ml) (left panels). Activated CD4+CD25+ T cells were co-cultured with the responders or separated from the responders in a transwell (right panel). (C) Activated CD4+CD25+ T cells from the thymus of 2-wk-old TGF-β1−/− mice or from littermate controls were co-cultured with CFSE-labeled 5CC7 TCR transgenic RAG−/− T cells. (D) Activated CD4+FoxP3+ T cells from peripheral lymph nodes of CD4cre-furf/f mice or littermate controls were co-cultured with CFSE-labeled 5CC7 TCR transgenic RAG−/− T cells. Flow cytometric analysis was used to determine the level of FoxP3 expression after 4 d of the co-culture. All dot plots were gated on CFSE+ cells as shown in Fig. S2. One representative experiment out of at least two experiments is shown (A–D). Fig. S2 is available at http://www.jem.org/cgi/content/full/jem.20080308/DC1.
To formally prove that the T reg cell–dependent induction of FoxP3 was dependent on TGF-β, we co-cultured activated T reg cells with naive T cells in the presence of IL-2, anti-CD3, splenic DCs, and rLAP. The addition of rLAP completely inhibited the induction of FoxP3, demonstrating that T reg cell–dependent induction of FoxP3 is TGF-β dependent (Fig. 2 A). To determine the cellular source of TGF-β, we isolated CD4+CD25hi T cells from the thymus of very young TGF-β1−/− mice. The majority of these cells remain FoxP3+ during the co-culture (∼80%) but fail to induce FoxP3 expression (Fig. 2 C). Further evidence that the T reg cells themselves are the source of the TGF-β was obtained by using activated CD4+FoxP3+ T cells from CD4cre-furf/f mice that lack proprotein convertase furin expression in all T cells. These mice phenocopy mice lacking TGF-β1 expression in T cells and are incapable of processing TGF-β. T reg cells from these mice were markedly inefficient in inducing FoxP3 expression in T reg cells (Fig. 2 D and 28).
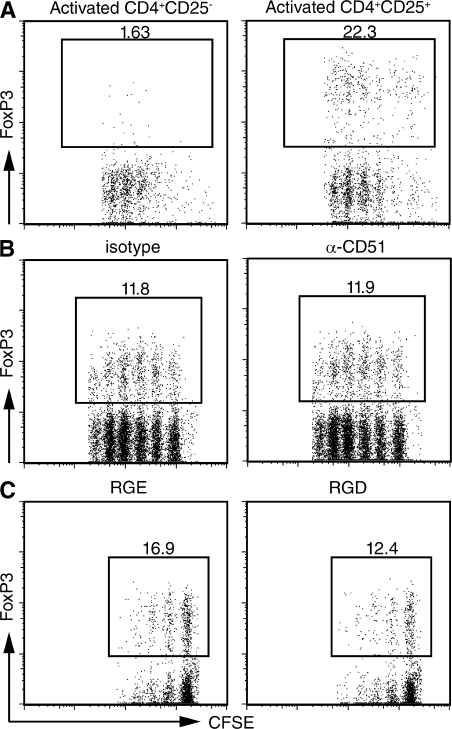
These studies strongly suggest that TCR activation induces the synthesis of LAP–TGF-β that is then secreted and bound to the surface of the T reg cells. αV integrins expressed on DCs play a critical role in the binding of an RGD motif in LAP–TGF-β and in the subsequent activation of latent TGF-β (29). To determine if αV integrins were involved in the binding and/or activation of LAP–TGF-β on T reg cells, we activated T reg cells in the presence of anti-αV or in the presence of the RGDS peptide, both of which would block the binding and subsequent activation of LAP by αV. Addition of these reagents to either the first culture (not depicted) or to both the first and second cultures had no effect on the capacity of activated T reg cells to induce FoxP3 expression in naive responder T cells. Furthermore, the presence of DCs was not required, as T reg cell activation and the induction of FoxpP3 expression could be induced in the presence of plate-bound anti-CD3 in the complete absence of APCs (Fig. 3).
Figure 3.
The induction of FoxP3 expression by activated T reg cells is APC and αv integrin independent. (A) CD4+CD25− (left) and CD4+CD25+ (right) T cells from B10.A mice were activated for 4 d with plate-bound anti-CD3 and IL-2 (100 U/ml). The activated T cells were then co-cultured for an additional 4 d with an equal number of CFSE-labeled CD4+ T cells from 5CC7 TCR transgenic RAG−/− mice in the presence of plate-bound anti-CD3 and IL-2. (B and C) CD4+CD25+ T cells were activated with plate-bound anti-CD3 and IL-2 for 4 d in the absence (left) or the presence (right) of blocking anti-αv monoclonal antibodies (50 μg/ml), RGE peptide, or RGD peptide (10 μg/ml). The activated cells were then co-cultured with CFSE-labeled 5CC7 TCR transgenic T cells co-cultured in the presence or absence of the same inhibitors used in the first culture. FoxP3 expression was determined on day 4 of the co-culture. One representative experiment out of at least three is shown (A–C).
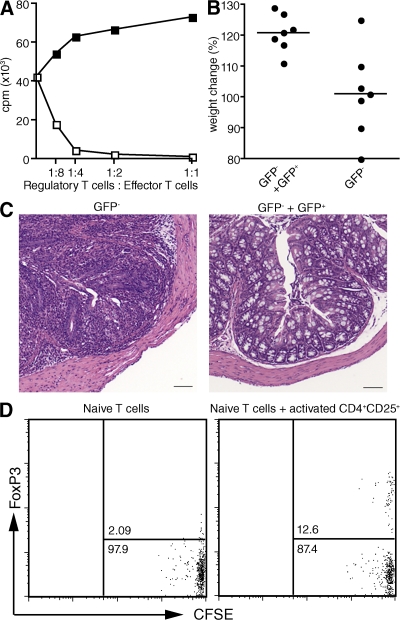
FoxP3 expression in mouse CD4+ T cells correlates with a suppressive phenotype. To determine if CD4+FoxP3+ T cells converted from naive T cells by co-culture with T reg cells were suppressive, we isolated CD4+FoxP3− T cells from FoxP3-GFP knock-in mice, co-cultured them for 4 d with activated CD4+CD25+ T cells from wild-type mice in the presence of plate-bound anti-CD3 and IL-2, and then isolated GFP+ T cells (Fig. S5, available at http://www.jem.org/cgi/content/full/jem.20080308/DC1). The induced CD4+GFP+ T cells were markedly suppressive when mixed with naive responders and stimulated with anti-CD3 (Fig. 4 A). More importantly, the induced CD4+FoxP3+ CD4+ T cells could inhibit the development of colitis when cotransferred into RAG−/− mice with CD4+CD25−FoxP3− T cells (Fig. 4, B and C). To evaluate whether activated FoxP3+ T reg cells are capable of inducing FoxP3 expression in naive T cells in a more physiological setting in vivo, we transferred naive, CFSE-labeled CD4+CD25−CD44− (<0.5% FoxP3+) TCR transgenic T cells that recognize hemagglutinin (HA)110–119 in association with I-Ed into wild-type syngeneic mice with or without preactivated HA-specific activated T reg cells. 48 h after immunization, ∼10% of the CFSE-labeled naive CD4+CD25−CD44− T cells expressed FoxP3 (Fig. 4 D).
Figure 4.
Induced FoxP3+ T cells are suppressive in vitro and in vivo. (A) T reg cell–induced CD4+FoxP3+ T cells (open squares) were isolated as in Fig. S5 and tested for suppressive ability by co-culturing them with CD4+CD25− T cells (5 × 104/well) in the presence of irradiated T cell–depleted splenocytes (5 × 104/well) and 0.25 μg/ml of soluble anti-CD3. Activated CD4+CD25−GFP− T cells (closed squares) from C57BL/6 FoxP3-GFP mice were used as controls. Proliferation was measured by the incorporation of [3H]TdR after 72 h of culture. (B) CD4+GFP− T cells (5 × 105) from C57BL/6 FoxP3-GFP knock-in mice were transferred into C57BL/6 RAG−/− mice with or without 1.5 × 105 T reg cell–induced FoxP3+ T cells isolated as described in Fig. S5. The weight of all recipients was measured at 6 wk after cell transfer. P < 0.006 using Student's t test. (C) Hematoxylin and eosin–stained tissue sections from the colon of C57BL/6 RAG−/− mice that received CD4+FoxP3− T cells with (right) or without (left) the addition of T reg cell–induced CD4+FoxP3+ T cells. Bar, 100 μm. (D) CFSE-labeled CD4+CD25−CD44− HA TCR transgenic T cells (0.5 × 106) were transferred into B10.D2 mice with or without activated HA-specific TCR transgenic T reg cells (2.5 × 106). 1 d later, all recipients were immunized with HA peptide in IFA, and draining lymph node cells were analyzed for FoxP3 expression 48 h after transfer. One representative experiment out of at least two is shown (A–D). Fig. S5 is available at http://www.jem.org/cgi/content/full/jem.20080308/DC1.
Although TGF-β can be produced by many different cell types and has pleiotropic effects on many different tissues, recent studies suggest that TGF-β production by FoxP3+ T reg cells plays a unique role in the maintenance of self-tolerance (16). Although TGF-β secreted by T reg cells might suppress immune responses in an endocrine fashion, our studies and those of others strongly suggest that TGF-β is synthesized during T reg cell activation, processed by furin, secreted complexed to LAP, and maintained on the surface of the T reg cells as a LAP–TGF-β complex (18, 19). Although some studies suggested that free TGF-β or LAP–TGF-β complexes could be detected on the surface of resting T reg cells (19), we were only able to detect LAP on the surface of either activated mouse or human FoxP3+ T reg cells. Technical differences in the purification and assays for cell surface LAP may account for the differences between these studies.
The contribution of T reg cell–produced TGF-β to the inhibition of the proliferation of CD4+CD25− T cells in co-cultures in vitro has remained controversial (17–19, 24). We and others have previously demonstrated that CD4+CD25+ T cells from TGF-β−/− mice or mice with a T cell–specific deletion of TGF-β are fully competent suppressors in vitro (16, 18, 24), whereas others have claimed that T reg cell suppression in vitro can be reversed by anti–TGF-β (19) or LAP (18). Our results demonstrate that T reg cell–produced TGF-β selectively inhibits the activation of CD4+CD25+FoxP3− T cells, but it is not needed for inhibiting the activation of CD4+CD25−FoxP3− T cells. During an immune response, many effector cells express CD25 and it is thus possible that TGF-β plays an important role as a suppressor effector mechanism at sites of inflammation. As preparations of CD4+CD25+ T cells, particularly from the spleen, contain variable numbers of CD4+CD25+FoxP3− T cells, it remains possible that the partial reversal of the suppression mediated by CD4+CD25+Foxp3+ in the presence of anti–TGF-β (19) or LAP (18) is secondary to reversal of the suppression of CD4+CD25+Foxp3− T cells by T reg cell–produced TGF-β. It is unclear why partially activated CD4+CD25+FoxP3− T cells would be uniquely susceptible to T reg cell–produced TGF-β, as they have similar levels of TGF-β receptors when compared with naive CD4+ T cells (unpublished data). We also believe that this study strongly favors the view that T reg cells use more than one mechanism of suppression (30), and it appears that the activation state of the responder T cells may also influence the specific suppressor pathway used.
The potential contribution of T reg cell–produced TGF-β to suppression in vivo has also been difficult to dissect. We and others (25, 26) have shown that T reg cells from TGF-β−/− mice are fully capable of suppressing the induction of inflammatory bowel disease by CD45RBhi or CD4+CD25− T cells in vivo. On the other hand, more recent studies have shown that mice with T cell–specific deficiencies in TGF-β production (16) or processing (unpublished data) gradually lose self-tolerance and develop inflammatory bowel disease. At present, we can only speculate on the differences between these studies, but they may be related to differences in the bacterial flora in different colonies and the severity of the inflammatory milieu in the gut. T reg cell–mediated FoxP3 induction appears to be tightly regulated and dependent on T reg cell activation. It is therefore likely that T reg cell–mediated FoxP3 induction would be most prominent at sites of significant inflammation such as the gastrointestinal tract or in allografts undergoing chronic rejection. In addition, the microenvironment may play an important role in controlling the balance between T reg cell induction and the induction of Th17 cells, as T reg cell production of TGF-β in association with IL-6 may induce effector Th17 cells (31). Although we could not define a role for DCs in the induction of FoxP3 in vitro, in vivo, simultaneous stimulation of both the T reg cells and antigen-specific T cells likely occurs on the platform of a DC in the form of a three-cell interaction. As a single DC can present more than one organ-derived antigen, it is possible that the T reg cell–TGF-β pathway of FoxP3 induction can result in expansion of T reg cells of broad antigen specificities and thus serve as an important adjuvant in the use of T reg cells for cellular biotherapy in autoimmunity and transplantation.
MATERIALS AND METHODS
Mice.
C57BL/6, C57BL/6 Rag1−/−, B10.A, B10D.2, HA TCR transgenic B10D2, and B10.A 5CC7 TCR transgenic Rag2−/− mice were obtained from Taconic Farms. C57BL/6 FoxP3-GFP knock-in mice, TGF-β1–deficient mice, and Furin-loxp crossed with CD4-cre mice were provided by Y. Belkaid, W. Chen, and M. Pesu, respectively (all at NIH, Bethesda, MD). The mice were maintained under specific pathogen-free conditions in the NIAID animal facility and used at 4–10 wk of age. TGF-β1–deficient mice were used at 2 wk of age. All animal experiments were approved by the NIAID animal care and use committee.
Media and cytokines.
Cells were grown in RPMI 1640 supplemented with 10% heat-inactivated FCS, 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM l-glutamine, 10 mM Hepes, 0.1 mM nonessential amino acids, 1 mM sodium pyruvate (all from Biofluids), and 50 μM 2-ME (Sigma-Aldrich). Recombinant mouse IL-2 was purchased from PeproTech and used at 100 U/ml. Recombinant human LAP was purchased from R&D Systems.
T cell isolation.
Axillary, inguinal, cervical, mandibular, and mesenteric lymph nodes were harvested and passed over a cell strainer to prepare single cell suspensions, followed by lysis of red blood cells using ACK lysis buffer. The cells were then incubated with PE-labeled anti-CD25, followed by magnetic anti-PE beads. The cells were then separated into a CD25+ and CD25− population using an AutoMACS. Both cell populations were then labeled with anti-CD4 tricolor antibodies and CD4+CD25− and CD4+CD25hi, and the appropriate cell populations were isolated using a FACStar Cell Sorter (Becton Dickinson).
Isolation of splenic DCs.
Spleen tissue was fragmented and digested for 30 min at 37°C in the presence of liberase blendzyme II (Roche) and 2 μg/ml DNase (Roche) in complete medium. Undigested fibrous material was removed by filtration through a 40-μm cell strainer. Red blood cells were lysed using an ACK lysis buffer, and CD11c+ cells were then isolated by positive selection using anti-CD11c–coated microbeads and autoMACS separation.
FoxP3 FACS analysis.
Cells were then washed with PBS-BSA and permeabilized overnight in Fix/perm buffer (eBioscience). Nonspecific binding sites were blocked with a 1/100 dilution of rat Ig (Jackson ImmunoResearch Laboratories) for 15 min at 4°C, and then the cells were stained with the anti-Foxp3 clone FJK-16s at a final dilution of 1/200 for 45 min at 4°C. The cells were then washed, resuspended in PBS, and analyzed on a FACSCalibur (BD Biosciences).
In vitro proliferation assays.
5 × 104 CD4+ T cells from C57BL/6 mice were cultured with 5 × 104 irradiated T-depleted spleen cells and 0.25 μg/ml anti-CD3 for 3 d in the presence of varying numbers of T reg cells. Proliferation was measured in triplicates by the incorporation of [3H]TdR during the last 6–8 h of the co-culture. Anergy was assessed using the same setup without the addition of T reg cells.
T cell transfer model of colitis.
4.5 × 105 naive T cells and 1.5 × 105 T reg cells were transferred alone or in combination with Rag1−/− mice. After T cell reconstitution, mice were weighted weekly and monitored for signs of disease.
Adoptive transfer.
CFSE-labeled CD4+CD25+CD44− (<0.5% Foxp3+) HA TCR transgenic T cells were transferred along with activated CD4+CD25+ HA TCR transgenic T cells. 24 h after transfer, the mice were immunized with HA peptide followed by analysis of FoxP3 expression after an additional 48 h.
Online supplemental material.
Fig. S1 shows LAP expression on human CD4+ T cells. Fig. S2 illustrates the gating on CFSE+ T cells used in co-culture assays. Fig. S3 demonstrates that TCR restimulation of T reg cells is not necessary for FoxP3 induction. Fig. S4 shows that TGF-β–induced T reg cells are capable of inducing FoxP3 expression. Fig. S5 shows the purity of induced CD4+FoxP3+ T cells after isolation using cell sorting. The online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20080308/DC1.
Supplementary Material
Acknowledgments
We thank Wanjun Chen and Pin Zhang for supplying TGF-β–deficient mice and Sarah Tanksley for cell sorting.
The NIAID Intramural Research Program has supported this work.
The authors have no conflicting financial interests.
References
- 1.Khattri, R., T. Cox, S.A. Yasayko, and F. Ramsdell. 2003. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat. Immunol. 4:337–342. [DOI] [PubMed] [Google Scholar]
- 2.Hori, S., T. Nomura, and S. Sakaguchi. 2003. Control of regulatory T cell development by the transcription factor Foxp3. Science. 299:1057–1061. [DOI] [PubMed] [Google Scholar]
- 3.Fontenot, J.D., M.A. Gavin, and A.Y. Rudensky. 2003. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 4:330–336. [DOI] [PubMed] [Google Scholar]
- 4.Brunkow, M.E., E.W. Jeffery, K.A. Hjerrild, B. Paeper, L.B. Clark, S.A. Yasayko, J.E. Wilkinson, D. Galas, S.F. Ziegler, and F. Ramsdell. 2001. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat. Genet. 27:68–73. [DOI] [PubMed] [Google Scholar]
- 5.Bennett, C.L., J. Christie, F. Ramsdell, M.E. Brunkow, P.J. Ferguson, L. Whitesell, T.E. Kelly, F.T. Saulsbury, P.F. Chance, and H.D. Ochs. 2001. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat. Genet. 27:20–21. [DOI] [PubMed] [Google Scholar]
- 6.Shevach, E.M., R.A. DiPaolo, J. Andersson, D.M. Zhao, G.L. Stephens, and A.M. Thornton. 2006. The lifestyle of naturally occurring CD4+ CD25+ Foxp3+ regulatory T cells. Immunol. Rev. 212:60–73. [DOI] [PubMed] [Google Scholar]
- 7.Andersson, J., I. Stefanova, G.L. Stephens, and E.M. Shevach. 2007. CD4+CD25+ regulatory T cells are activated in vivo by recognition of self. Int. Immunol. 19:557–566. [DOI] [PubMed] [Google Scholar]
- 8.Gershon, R.K., and K. Kondo. 1971. Infectious immunological tolerance. Immunology. 21:903–914. [PMC free article] [PubMed] [Google Scholar]
- 9.Qin, S., S.P. Cobbold, H. Pope, J. Elliott, D. Kioussis, J. Davies, and H. Waldmann. 1993. “Infectious” transplantation tolerance. Science. 259:974–977. [DOI] [PubMed] [Google Scholar]
- 10.Cobbold, S.P., R. Castejon, E. Adams, D. Zelenika, L. Graca, S. Humm, and H. Waldmann. 2004. Induction of foxP3+ regulatory T cells in the periphery of T cell receptor transgenic mice tolerized to transplants. J. Immunol. 172:6003–6010. [DOI] [PubMed] [Google Scholar]
- 11.Jonuleit, H., E. Schmitt, H. Kakirman, M. Stassen, J. Knop, and A.H. Enk. 2002. Infectious tolerance: human CD25+ regulatory T cells convey suppressor activity to conventional CD4+ T helper cells. J. Exp. Med. 196:255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qiao, M., A.M. Thornton, and E.M. Shevach. 2007. CD4+ CD25+ regulatory T cells render naive CD4+ CD25− T cells anergic and suppressive. Immunology. 120:447–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dieckmann, D., C.H. Bruett, H. Ploettner, M.B. Lutz, and G. Schuler. 2002. Human CD4+CD25+ regulatory, contact-dependent T cells induce interleukin 10–producing, contact-independent type 1–like regulatory T cells. J. Exp. Med. 196:247–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen, W., W. Jin, N. Hardegen, K.J. Lei, L. Li, N. Marinos, G. McGrady, and S.M. Wahl. 2003. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J. Exp. Med. 198:1875–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DiPaolo, R.J., C. Brinster, T.S. Davidson, J. Andersson, D. Glass, and E.M. Shevach. 2007. Autoantigen-specific TGFbeta-induced Foxp3+ regulatory T cells prevent autoimmunity by inhibiting dendritic cells from activating autoreactive T cells. J. Immunol. 179:4685–4693. [DOI] [PubMed] [Google Scholar]
- 16.Li, M.O., Y.Y. Wan, and R.A. Flavell. 2007. T cell-produced transforming growth factor-beta1 controls T cell tolerance and regulates Th1- and Th17-cell differentiation. Immunity. 26:579–591. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura, K., A. Kitani, and W. Strober. 2001. Cell contact–dependent immunosuppression by CD4+CD25+ regulatory T cells is mediated by cell surface–bound transforming growth factor β. J. Exp. Med. 194:629–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakamura, K., A. Kitani, I. Fuss, A. Pedersen, N. Harada, H. Nawata, and W. Strober. 2004. TGF-beta 1 plays an important role in the mechanism of CD4+CD25+ regulatory T cell activity in both humans and mice. J. Immunol. 172:834–842. [DOI] [PubMed] [Google Scholar]
- 19.Oida, T., L. Xu, H.L. Weiner, A. Kitani, and W. Strober. 2006. TGF-beta-mediated suppression by CD4+CD25+ T cells is facilitated by CTLA-4 signaling. J. Immunol. 177:2331–2339. [DOI] [PubMed] [Google Scholar]
- 20.Allan, S.E., S.Q. Crome, N.K. Crellin, L. Passerini, T.S. Steiner, R. Bacchetta, M.G. Roncarolo, and M.K. Levings. 2007. Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int. Immunol. 19:345–354. [DOI] [PubMed] [Google Scholar]
- 21.Tran, D.Q., H. Ramsey, and E.M. Shevach. 2007. Induction of FOXP3 expression in naive human CD4+FOXP3 T cells by T-cell receptor stimulation is transforming growth factor-beta dependent but does not confer a regulatory phenotype. Blood. 110:2983–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morgan, M.E., J.H. van Bilsen, A.M. Bakker, B. Heemskerk, M.W. Schilham, F.C. Hartgers, B.G. Elferink, L. van der Zanden, R.R. de Vries, T.W. Huizinga, et al. 2005. Expression of FOXP3 mRNA is not confined to CD4+CD25+ T regulatory cells in humans. Hum. Immunol. 66:13–20. [DOI] [PubMed] [Google Scholar]
- 23.Wang, J., A. Ioan-Facsinay, E.I. van der Voort, T.W. Huizinga, and R.E. Toes. 2007. Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur. J. Immunol. 37:129–138. [DOI] [PubMed] [Google Scholar]
- 24.Piccirillo, C.A., J.J. Letterio, A.M. Thornton, R.S. McHugh, M. Mamura, H. Mizuhara, and E.M. Shevach. 2002. CD4+CD25+ regulatory T cells can mediate suppressor function in the absence of transforming growth factor β1 production and responsiveness. J. Exp. Med. 196:237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kullberg, M.C., V. Hay, A.W. Cheever, M. Mamura, A. Sher, J.J. Letterio, E.M. Shevach, and C.A. Piccirillo. 2005. TGF-beta1 production by CD4+ CD25+ regulatory T cells is not essential for suppression of intestinal inflammation. Eur. J. Immunol. 35:2886–2895. [DOI] [PubMed] [Google Scholar]
- 26.Fahlen, L., S. Read, L. Gorelik, S.D. Hurst, R.L. Coffman, R.A. Flavell, and F. Powrie. 2005. T cells that cannot respond to TGF-β escape control by CD4+CD25+ regulatory T cells. J. Exp. Med. 201:737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davidson, T.S., R.J. DiPaolo, J. Andersson, and E.M. Shevach. 2007. Cutting edge: IL-2 is essential for TGF-beta-mediated induction of Foxp3+ T regulatory cells. J. Immunol. 178:4022–4026. [DOI] [PubMed] [Google Scholar]
- 28.Pesu, M., W.T. Watford, L. Wei, L. Xu, I. Fuss, W. Strober, J. Andersson, E.M. Shevach, M. Quezado, N. Bouladoux, et al. 2008. T-cell-expressed proprotein convertase furin is essential for maintenance of peripheral immune tolerance. Nature. In press. [DOI] [PMC free article] [PubMed]
- 29.Travis, M.A., B. Reizis, A.C. Melton, E. Masteller, Q. Tang, J.M. Proctor, Y. Wang, X. Bernstein, X. Huang, L.F. Reichardt, et al. 2007. Loss of integrin alpha(v)beta8 on dendritic cells causes autoimmunity and colitis in mice. Nature. 449:361–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang, Q., and J.A. Bluestone. 2008. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat. Immunol. 9:239–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu, L., A. Kitani, I. Fuss, and W. Strober. 2007. Cutting edge: regulatory T cells induce CD4+CD25−Foxp3− T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J. Immunol. 178:6725–6729. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.