Abstract
Oral tolerance is an active process that starts with sampling of luminal antigens by the intestinal epithelial cells (IEC), followed by processing and assembly with major histocompatibility complex class II and subsequently a release of tolerogenic exosomes (tolerosomes) from the IEC. We have previously shown that tolerosomes can be isolated from serum shortly after an antigen feed, and will potently transfer antigen-specific tolerance to naive recipients. Here we study the capacity of the tolerosomes to protect against allergic sensitization in a mouse model of allergic asthma. Serum or isolated serum exosomes from tolerized BALB/c donor mice were transferred to syngeneic recipients followed by sensitization and intranasal exposure to ovalbumin (OVA). Blood, bronchoalveolar lavage (BAL) and lymph nodes were sampled 24 hr after the final exposure. The number of eosinophils was counted in BAL fluid and the levels of immunoglobulin E (IgE) and OVA-specific IgE were measured in serum. Mediastinal and coeliac lymph nodes were analysed by flow cytometry. The animals receiving serum from OVA-fed mice displayed significantly lower numbers of airway eosinophils and lower serum levels of total IgE as well as of OVA-specific IgE compared with controls. Moreover, the tolerant animals showed a significantly higher frequency of activated T cells with a regulatory phenotype in both mediastinal and coeliac lymph nodes. The results show that serum or isolated serum exosomes obtained from OVA-fed mice and administered intraperitoneally to naive recipient mice abrogated allergic sensitization in the recipients.
Keywords: allergy, asthma, exosomes, mouse, tolerance
Introduction
The breakdown of immune regulation of innocuous environmental antigens at mucosal sites can result in a number of different diseases such as allergies and inflammatory bowel disease. Allergy is one of the most common diseases with a prevalence of up to 40% in children from developed countries, and many of these individuals will continue to suffer from allergic manifestations like asthma, rhinitis and food allergy in adulthood.
Mucosal tolerance is an active process that is maintained by the specific recognition of antigens by CD4+ T cells with a down-regulatory function, and is the default response to harmless antigens entering at mucosal sites.1–5 In 1983, Strobel et al.6 showed that serum obtained 1 hr after feeding an antigen, and given intraperitoneally to recipient mice, had the capacity to induce suppression of delayed-type hypersensitivity reactions.
The ‘gut processing’ of the antigen was a requirement to achieve tolerance via serum transfer but the true nature of the tolerogen present in the serum was yet to be discovered.7
The initial step in oral tolerance induction is active sampling by the small intestinal epithelial cells (IECs) of luminal content at the mucosal surface. The antigen is processed and peptides are loaded on major histocompatibility complex (MHC) class II molecules, constitutively present in IECs, exosomes are formed and released at the basolateral side of the IEC.8 Exosomes are small, 40- to 90-nm, membrane-bound vesicles of endocytic origin that are secreted by a variety of cells in culture, e.g. IECs,8,9 T and B lymphocytes, dendritic cells, reticulocytes, mast cells and platelets,10 and can also be isolated in vivo from body fluids such as serum,11,12 bronchoalveolar lavage fluid (BALf)13 and urine.14 After being discharged by the IECs, the exosomes can then either be taken up by local professional antigen-presenting cells or transported across the endothelium into the circulation. The exosomes present in the portal blood may readily be filtered out through the fenestration of the liver. The liver is important because it is the major draining site of the intestinal circulation and contains antigen-presenting cells that effectively clear particulate matter of similar size to the exosomes (≈ 40 nm) from the blood.15 It has also been reported that portal drainage through the liver16,17 is a prerequisite for establishing this form of tolerance.
We have previously shown that exosomes can be isolated from serum 1 hr after an antigen feed and that they transfer antigen-specific tolerance when injected into naive recipients.8 Moreover we have shown that this exosome-mediated tolerance is MHC class II dependent and requires an intact immune system in the fed donor.18 In the present study we wanted to investigate whether serum-derived exosomes from antigen-fed donors could also protect against allergic airway inflammation in a mouse model of allergic asthma.
Materials and methods
Animals
This study was approved by the Animals Ethics Committee in Göteborg, Sweden. Male BALB/c donor mice 8–10 weeks of age and male BALB/c recipient mice 6–8 weeks of age were purchased from Scanbur AB (Sollentuna Sweden). The animals were kept in the animal facilities of The Department of Rheumatology and Inflammation Research, University of Göteborg under standard conditions of temperature and light.
Transfer of serum from ovalbumin-fed mice
The mice were starved overnight and fed by gavage with 50 mg ovalbumin (OVA) (grade V; Sigma-Aldrich, St Louis, MO), dissolved in phosphate-buffered saline (PBS). Each mouse received a total volume of 300 μl. One hour after feeding the mice were bled by cardiac puncture, the blood samples were pooled and allowed to clot. To obtain the serum the blood was centrifuged twice at 3000 g for 10 min at room temperature. The serum was then administered by intraperitoneal (i.p.) injections to naive recipient mice. Each mouse received 1 ml serum from OVA-fed mice or 1 ml serum from PBS-fed mice as control.
Isolation and transfer of serum exosomes from OVA-fed mice
The sera were collected as described above and were further centrifuged at 10 000 g for 30 min at 4° to remove cell debris, followed by an ultracentrifugation at 100 000 g for 1 hr. The pellet fraction was resuspended in PBS and washed once. After collection of the pellet, the supernatant was centrifuged for a second time to remove any remaining exosomes. The pellet fraction was ultimately collected and resuspended in PBS. Naive recipient mice received exosome pellet corresponding to 1 ml serum dissolved in 1 ml PBS.
Sensitization and allergen exposure
All mice were sensitized twice with an interval of 10 days by i.p. injections of 10 μg OVA (grade V; Sigma-Aldrich) in 100 μl aluminium hydroxide gel, Al(OH)3, diluted with PBS to a total volume of 150 μl. Seven to nine days after the second immunization, the mice were briefly anaesthetized with Isoflurane and given an intranasal administration of 100 μg OVA in 25 μl PBS on three consecutive days. Twenty-four hours after the last OVA exposure the mice were killed and blood, BALf cells, and mediastinal and coeliac lymph nodes were collected.
Cell collection and sample processing
Twenty-four hours after the final OVA exposure the animals were lethally anaesthetized with a mixture of xylazine (130 mg/kg, Rompun; Bayer, Gothenburg, Sweden) and ketamine (670 mg/kg, Ketalar; Pfizer, Täby, Sweden) and BALf, blood samples and lymph nodes were collected. To obtain BALf, the animals were tracheostomized, and bronchoalveolar lavage was performed by instilling 0·4 ml PBS through the tracheal cannula, followed by gentle aspiration and then a repeat with a second volume of 0·4 ml PBS. Blood samples were taken by heart puncture at the time of killing and mediastinal and coeliac lymph nodes were removed and kept in Iscove's complete medium on ice for further preparation and analysis by flow cytometry as described below.
Blood and BALf
Serum was obtained from blood after centrifugation for 10 min at 3000 g. Serum samples were stored at −80° until analyzed. Total immunoglobulin E (IgE) was measured by enzyme-linked immunosorbent assay (ELISA) and OVA-specific IgE was estimated by passive cutaneous anaphylaxis (PCA) as described below.
The BALf samples were centrifuged at 400 g for 10 min at 4°, the cells were then resuspended and washed. The total number of cells was estimated by counting a sample in a Bürker chamber. The rest of the cells were subjected to cytospin followed by staining with May–Grünwald–Giemsa. Between 250 and 300 cells were differentially counted in a light microscope.
Passive cutaneous anaphylaxis
The levels of circulating anti-OVA IgE antibodies were assayed by PCA in Sprague-Dawley rats. The rats were briefly anaesthetized with Isoflurane followed by i.p. injections of 8 mg/kg xylazine and 40 mg/kg ketamine. Mouse sera were serially diluted in PBS, and 50 μl of each dilution was injected intradermally into the shaved dorsal skin of the rats. Seventy-two hours later, the rats were challenged intravenously with 5 mg OVA diluted in 1 ml PBS containing 1% Evans blue (Sigma-Aldrich). One hour later the rats were killed and the skin areas were examined for the appearance of a blue dot. The PCA titres were defined as the reciprocal of the highest dilution of serum giving a readable (> 2 mm in diameter) blue spot.
Fluorescence-activated cell sorter (FACS) analysis
Mediastinal lymph nodes and coeliac lymph nodes were collected and single-cell suspensions were prepared. For analysis in the flow cytometer the following monoclonal antibodies against the following markers were used; CD4, CD25, CD103, CD69, CTLA-4 (BD Biosciences, Stockholm, Sweden) and FoxP3 (eBioscience, BioSite, Stockholm, Sweden) along with their corresponding isotype controls. Three-colour cytometry was performed on a FACSCalibur equipped with dual lasers (BD Biosciences). A total of 0·5 × 106 to 1·0 × 106 cells from either mediastinal or coeliac lymph nodes were incubated with Fc-block for 10 min at room temperature, and then stained with either phycoerythrin (PE), peridinin chlorophyll protein (PerCP) or allophycocyanin (APC) -conjugated monoclonal antibody for 20 min at 4°. For the analysis of the intracellular CTLA-4 and FoxP3 the cells were surface stained as described above, followed by fixation and permeabilization for 1 hr at 4° using the FoxP3 staining kit from (eBioscience). The cells were then incubated with Fc-block for 10 min at room temperature, and then stained with PE-labelled antibody for 20 min at 4°. The samples were washed twice in either FACS buffer or Perm Wash before analysis.
Statistical analysis
Experiments were performed at least twice with reproducible results. The results were compared statistically using the Mann–Whitney U-test.
Results
Transfer of serum after an antigen feed protects against allergic airway sensitization
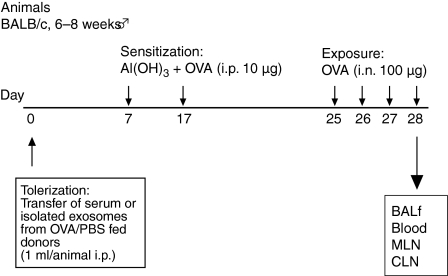
To study whether serum or isolated serum-derived exosomes could protect against an allergic airway sensitization, mice were given serum from OVA-fed donor mice and, as a control, serum from PBS-fed donors followed by an allergic airway sensitization protocol. The donor mice were starved overnight and fed OVA by gavage in the morning. The animals were bled and the serum was isolated and transferred to recipient mice. One week after serum transfer the mice were subjected to the airway OVA sensitization protocol as described in the Materials and methods and overviewed in Fig. 1.
Figure 1.
Overview of the protocol. The serum transfer followed by the allergic airway sensitization protocol. BALf, bronchoalveolar lavage fluid; CLN, coeliac lymph nodes; i.p., intraperitoneal; MLN, mediastinal lymph nodes; OVA, ovalbumin; PBS, phosphate-buffered saline.
Analysis of cells in BALf
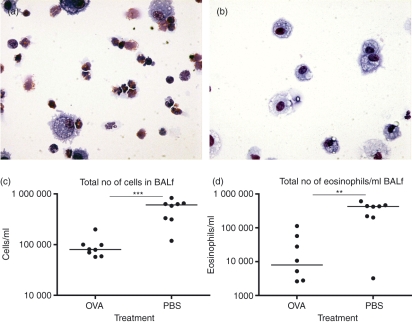
Following the serum transfer and the allergic sensitization the animals were killed and bronchoalveolar lavage was performed twice with 0·4 ml PBS on each animal. The total number of cells in the BALf was counted followed by May–Grünwald–Giemsa staining to count the number of eosinophils, which is a measurement of the severity of an allergic reaction. The number of eosinophils in BALf was greatly reduced in the group receiving serum from OVA-fed donors and the difference was clearly visible by microscopy (Fig. 2a,b). The results showed a significant reduction of both total number of cells (Fig. 2c, P = 0·0003) and eosinophils (Fig. 2d, P = 0·003), the percentage of eosinophils was reduced from 65·5% to 12%, in BALf from mice that received serum from OVA-fed donors compared to mice receiving serum from PBS-fed donors.
Figure 2.
Serum from donors fed ovalbumin (OVA) or phosphate-buffered saline (PBS) was transferred to syngeneic recipient mice (BALB/c to BALB/c) followed by allergic sensitization of the recipient. Bronchoalveolar lavage was performed on the animals and cells from the bronchoalveolar lavage fluid (BALf) were counted, followed by staining with May–Grünwald–Giemsa to distinguish the eosinophils. (a) Microscopic image of cells in BALf after allergen exposure from animals transferred with serum isolated from PBS-fed donors and (b) animals receiving serum from the OVA-fed donors. (c) The total number of cells in the BALf. (d) The number of eosinophils/ml of BALf. All data are from one representative experiment out of three.
Tolerant animals have lower levels of serum IgE
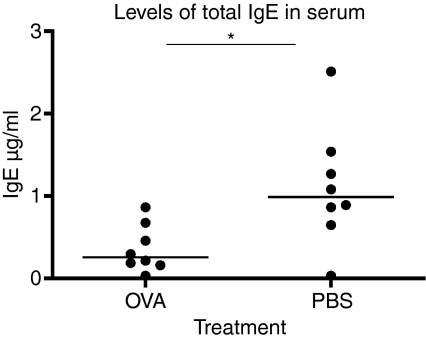
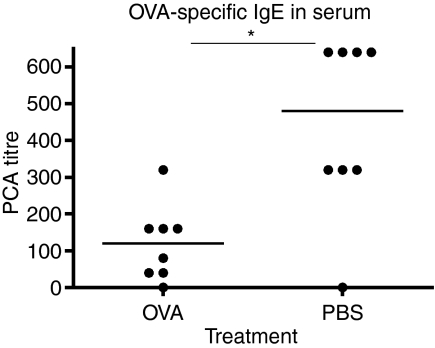
To further analyse the allergic response we measured the serum levels of total IgE as well as OVA-specific IgE by PCA. Total IgE was measured with a standard ELISA and the results showed significantly lower levels of IgE in the animals that had received serum from OVA-fed donors (Fig. 3, P = 0·0207). The levels of OVA-specific IgE were consistent with the results from the analyses of total IgE and showed a significant reduction in the group of animals administered serum from OVA-fed donors (Fig. 4, P = 0·0148). Taken together both analyses showed a significant decrease in IgE levels among the mice given serum from OVA-fed mice as compared to controls.
Figure 3.
Total immunoglobulin E (IgE) levels in serum from mice receiving serum from donors fed either ovalbumin (OVA) or phosphate-buffered saline (PBS) followed by allergic sensitization of the recipient. The animals receiving serum from OVA-fed (OVA) donors had significantly lower levels of IgE in serum compared to PBS-fed donors (PBS). The serum levels of IgE in the recipient animals were measured with standard enzyme-linked immunosorbent assay. One representative experiment out of three is shown.
Figure 4.
Ovalbumin (OVA) -specific immunoglobulin E (IgE) antibody levels in serum from mice receiving serum from donors fed either OVA or phosphate-buffered saline (PBS) followed by allergic sensitization of the recipient. OVA-specific IgE was measured with passive cutaneus anaphylaxis (PCA) in rats. One representative experiment out of three is shown.
The tolerogenic effect of the serum is mediated by exosomes
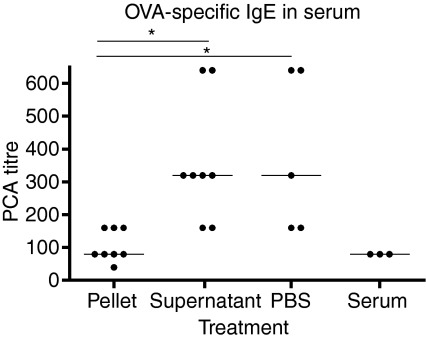
We have previously isolated the serum exosomes and shown that the tolerogenic effect is mediated by exosomes. The tolerance in these experiments was measured by delayed-type hypersensitivity reaction, and the results showed that the serum-derived exosomes were capable of suppressing a T helper type 1 (Th1) dominated response. In the present study we show that transfer of serum from tolerized mice suppress a Th2 response. To confirm that the tolerance induction and the absence of allergic inflammation are actually mediated by exosomes we isolated and transferred exosomes. To isolate exosomes the serum was subjected to a series of centrifugations with a final ultracentrifugation of 100 000 g to sediment the serum exosomes. The exosome pellet was given in volumes corresponding to 1 ml of whole serum. Serum supernatant as well as PBS alone were given as controls. This was followed by the same allergic sensitization protocol as previously described. The results showed that both the group of animals given PBS and the group of animals given serum supernatant had significantly higher levels of OVA-specific IgE than the group of animals given the pellet fraction (Fig. 5, P = 0·0109). These results confirm that the exosomes were responsible for the induction of tolerance also in this model.
Figure 5.
Exosomes were isolated from donor animal serum after feeding them ovalbumin (OVA). Recipient animals were given pellet or serum supernatant, phosphate-buffered saline (PBS) and whole serum as controls. The OVA-specific immunoglobulin E (IgE) in serum was measured with passive cutaneous anaphylaxis (PCA). One representative experiment out of three is shown.
Regulatory T-cell populations in lung and liver draining lymph nodes
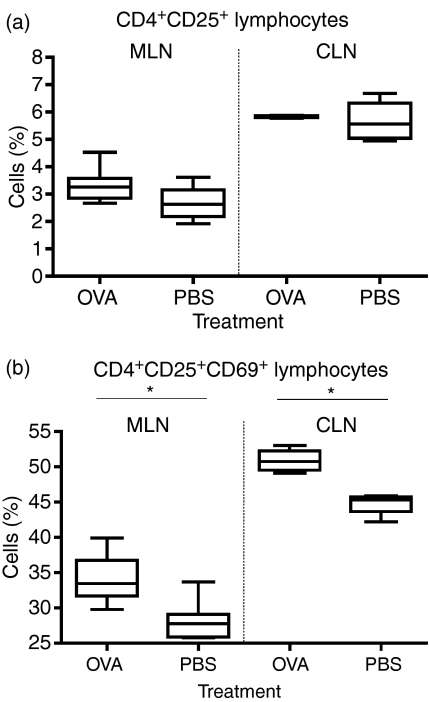
To study the effect of the transfer of serum exosomes followed by allergic airway sensitization on the potentially regulatory T-cell population we performed FACS analysis on the mediastinal lymph nodes as well as the coeliac lymph nodes. Analysis was made of the CTLA-4, CD103, CD69 and FoxP3 expression on T cells with CD4+ CD25high phenotype. There was no difference in the total CD4+ CD25+ T-cell population in the OVA-fed animals compared with the controls (Fig. 6a), but further analysis of this population showed a difference in the expression of the activation marker CD69. The analysis of the CD4+ CD25+ T-cell population and the expression of CD69 showed a significant increase of activated, CD69-positive, CD4+ CD25+ T cells in the animals receiving serum from OVA-fed donors. This was true for both mediastinal and coeliac lymph nodes (Fig. 6b, P = 0·0019 and P = 0·0286). Given this result we expected to find a higher overall proportion of T cells with a regulatory phenotype in the tolerant animals. However, when we analysed the CD4+ CD25+ T-cell population we could not detect any difference in the CTLA-4, or FoxP3 expression between the groups in either mediastinal or celiac lymph nodes (not shown).
Figure 6.
Analysis of T cells in MLN and CLN after serum transfer followed by allergic sensitization. The total CD4+ CD25+ T cell population (a). Analysis of the CD4+ CD25+ T cell population and the expression of the activation marker CD69 (b, P = 0·0019 and P = 0·0286). One representative experiment out of three.
Discussion
In this study we wanted to examine whether serum obtained shortly after an antigen (OVA) feed or isolated serum-derived exosomes have the capacity to impair allergic sensitization in a mouse model of allergic asthma. The results clearly show that both the total number of cells and the number of infiltrating eosinophils were markedly reduced in BALf of mice receiving serum from OVA-fed mice as compared to control-fed mice, after sensitization and challenge with OVA. The effect on the eosinophil infiltration was most pronounced with an almost 10-fold reduction of the eosinophil counts. Accordingly the lowered serum levels of both total and OVA-specific IgE revealed that the animals which received either whole serum or the exosome fraction isolated from serum of OVA-fed mice were protected from allergic sensitization to the fed antigen. This is the first time that exosomes have been shown to prevent allergic sensitization and these results confirm our previous results where the exosome fraction from serum of OVA-fed donors were effective in preventing a Th1-dominated sensitization.19,20 The tolerogenic effect of serum obtained shortly after an antigen feed was first described by Strobel et al.6 using a Th1 sensitization as read-out; however, despite great efforts the nature of the tolerizing serum factor was not revealed until recently.18,20,21
We also monitored the involvement of regulatory T cells in the induction of tolerance in the present model. As described in our results we found a higher frequency of activated CD4+ T cells with a regulatory phenotype in the group receiving the tolerogenic serum. This was true for both the local mediastinal lymph nodes and the liver-draining coeliac lymph nodes. Our results are therefore consistent with our previous findings and point to the importance of the liver-draining lymph nodes in this experimental model in two ways.3 First, the number of activated regulatory CD4+ T cells in coeliac lymph nodes is higher in the group that received exosomes from OVA-fed donors, and second, the overall frequency of T cells with a regulatory phenotype is greater in CLN compared to the local peripheral lymph nodes. This strengthens the idea of a central role for the coeliac lymph nodes in tolerance induced by feeding an antigen, which suggest that they function as a ‘boot camp for regulatory T cells’.
The preventive effect of treatment with tolerogenic serum or serum-derived exosomes seen in this study implies that the exosomes induce antigen-specific regulatory T cells in the recipients. It is well known that oral administration of an antigen gives rise to a CD4+ T-cell population that down-regulates the immune response and maintains tolerance to the fed antigen.22–24
We have previously proposed that oral tolerance is dependent on exosomes produced by IECs, which can be isolated from serum shortly after an antigen feed.8,19,20 We have also shown that after oral administration of an antigen there is an induction of regulatory T cells in the liver-draining coeliac lymph nodes and that these lymph nodes rapidly become engaged in the response to fed antigens.3 The T cells become activated within 6 hr and later develop into a distinct antigen-specific T-cell population with a regulatory phenotype and a suppressive function.3 This is in line with the results from the present study where we can see an elevated population of putative regulatory T cells in the coeliac lymph nodes.
Both human and murine IEC lines produce exosomes in culture, and our previous studies on biopsies from human small intestine showed that the human small intestinal epithelial cells constitutively express the key elements for antigen processing and the production of exosomes.8,9,25 Furthermore, it was recently shown in humans that antigen-loaded exosomes are formed in colonic epithelial cells and released in the basolateral compartment of the IECs.26 It is likely that these exosomes play an important role in rendering the mucosal interface capable of effectively presenting luminal antigens to the immune system. This will enable an effective monitoring and an appropriate response to luminal antigens. Moreover, it has recently been shown that exosomes from B cells, isolated and cultured from human peripheral blood mononuclear cells, can present allergen peptides and activate allergen specific T cells to proliferate and produce Th2 cytokines.27 Taken together these findings suggest that exosomes from different sources may play a role in the development of asthma and allergy in at least two ways, either as a failure to induce effective tolerance or as enhancers of an already established response. This makes exosomes highly interesting as therapeutic targets in anti-allergy treatment.
In conclusion, we show here that allergic sensitization, both in terms of eosinophilic infiltration and antigen-specific IgE response, can effectively be reduced by transfer of exosomes present in the circulation of the donor animals shortly after an antigen feed. We believe that this is one of the most important mechanisms that instruct the immune system to avoid adverse reactions to antigens presented to the mucosal immune system.
Acknowledgments
This work has been supported by the Swedish Medical Research Council (grant no. 20066096) and Ernst Collianders foundation.
References
- 1.Challacombe SJ, Tomasi TB., Jr Systemic tolerance and secretory immunity after oral immunization. J Exp Med. 1980;152:1459–72. doi: 10.1084/jem.152.6.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dahlman-Hoglund A, Dahlgren U, Ahlstedt S, Hanson LA, Telemo E. Bystander suppression of the immune response to human serum albumin in rats fed ovalbumin. Immunology. 1995;86:128–33. [PMC free article] [PubMed] [Google Scholar]
- 3.Hultkrantz S, Ostman S, Telemo E. Induction of antigen-specific regulatory T cells in the liver-draining celiac lymph node following oral antigen administration. Immunology. 2005;116:362–72. doi: 10.1111/j.1365-2567.2005.02236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lundin BS, Karlsson MR, Svensson LA, Hanson LA, Dahlgren UI, Telemo E. Active suppression in orally tolerized rats coincides with in situ transforming growth factor-beta (TGF-beta) expression in the draining lymph nodes. Clin Exp Immunol. 1999;116:181–7. doi: 10.1046/j.1365-2249.1999.00834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mowat AM. Anatomical basis of tolerance and immunity to intestinal antigens. Nat Rev Immunol. 2003;3:331–41. doi: 10.1038/nri1057. [DOI] [PubMed] [Google Scholar]
- 6.Strobel S, Mowat AM, Drummond HE, Pickering MG, Ferguson A. Immunological responses to fed protein antigens in mice. II. Oral tolerance for CMI is due to activation of cyclophosphamide-sensitive cells by gut-processed antigen. Immunology. 1983;49:451–6. [PMC free article] [PubMed] [Google Scholar]
- 7.Bruce MG, Ferguson A. Oral tolerance to ovalbumin in mice: studies of chemically modified and ‘biologically filtered’ antigen. Immunology. 1986;57:627–30. [PMC free article] [PubMed] [Google Scholar]
- 8.Karlsson M, Lundin S, Dahlgren U, Kahu H, Pettersson I, Telemo E. ‘Tolerosomes’ are produced by intestinal epithelial cells. Eur J Immunol. 2001;31:2892–900. doi: 10.1002/1521-4141(2001010)31:10<2892::aid-immu2892>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 9.van Niel G, Raposo G, Candalh C, Boussac M, Hershberg R, Cerf-Bensussan N, Heyman M. Intestinal epithelial cells secrete exosome-like vesicles. Gastroenterology. 2001;121:337–49. doi: 10.1053/gast.2001.26263. [DOI] [PubMed] [Google Scholar]
- 10.Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–79. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 11.Janiszewski M, Do Carmo AO, Pedro MA, Silva E, Knobel E, Laurindo FR. Platelet-derived exosomes of septic individuals possess proapoptotic NAD(P)H oxidase activity: a novel vascular redox pathway. Crit Care Med. 2004;32:818–25. doi: 10.1097/01.ccm.0000114829.17746.19. [DOI] [PubMed] [Google Scholar]
- 12.Caby MP, Lankar D, Vincendeau-Scherrer C, Raposo G, Bonnerot C. Exosomal-like vesicles are present in human blood plasma. Int Immunol. 2005;17:879–87. doi: 10.1093/intimm/dxh267. [DOI] [PubMed] [Google Scholar]
- 13.Admyre C, Grunewald J, Thyberg J, et al. Exosomes with major histocompatibility complex class II and co-stimulatory molecules are present in human BAL fluid. Eur Respir J. 2003;22:578–83. doi: 10.1183/09031936.03.00041703. [DOI] [PubMed] [Google Scholar]
- 14.Pisitkun T, Shen RF, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci USA. 2004;101:13368–73. doi: 10.1073/pnas.0403453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsuno K, Ezaki T, Kudo S, Uehara Y. A life stage of particle-laden rat dendritic cells in vivo: their terminal division, active phagocytosis, and translocation from the liver to the draining lymph. J Exp Med. 1996;183:1865–78. doi: 10.1084/jem.183.4.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Callery MP, Kamei T, Flye MW. The effect of portacaval shunt on delayed-hypersensitivity responses following antigen feeding. J Surg Res. 1989;46:391–4. doi: 10.1016/0022-4804(89)90208-4. [DOI] [PubMed] [Google Scholar]
- 17.Yang R, Liu Q, Grosfeld JL, Pescovitz MD. Intestinal venous drainage through the liver is a prerequisite for oral tolerance induction. J Pediatr Surg. 1994;29:1145–8. doi: 10.1016/0022-3468(94)90297-6. [DOI] [PubMed] [Google Scholar]
- 18.Ostman S, Taube M, Telemo E. Tolerosome-induced oral tolerance is MHC dependent. Immunology. 2005;116:464–76. doi: 10.1111/j.1365-2567.2005.02245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karlsson MR, Kahu H, Hanson LA, Telemo E, Dahlgren UI. Tolerance and bystander suppression, with involvement of CD25-positive cells, is induced in rats receiving serum from ovalbumin-fed donors. Immunology. 2000;100:326–33. doi: 10.1046/j.1365-2567.2000.00050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karlsson MR, Kahu H, Hanson LA, Telemo E, Dahlgren UI. An established immune response against ovalbumin is suppressed by a transferable serum factor produced after ovalbumin feeding: a role of CD25+ regulatory cells. Scand J Immunol. 2002;55:470–7. doi: 10.1046/j.1365-3083.2002.t01-1-01079.x. [DOI] [PubMed] [Google Scholar]
- 21.Rask C, Evertsson S, Telemo E, Wold AE. A full flora, but not monocolonization by Escherichia coli or lactobacilli, supports tolerogenic processing of a fed antigen. Scand J Immunol. 2005;61:529–35. doi: 10.1111/j.1365-3083.2005.01598.x. [DOI] [PubMed] [Google Scholar]
- 22.Chen Y, Kuchroo VK, Inobe J, Hafler DA, Weiner HL. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994;265:1237–40. doi: 10.1126/science.7520605. (New York, NY) [DOI] [PubMed] [Google Scholar]
- 23.Groux H, O’Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–42. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 24.Karlsson MR, Kahu H, Hanson LA, Telemo E, Dahlgren UI. Neonatal colonization of rats induces immunological tolerance to bacterial antigens. Eur J Immunol. 1999;29:109–18. doi: 10.1002/(SICI)1521-4141(199901)29:01<109::AID-IMMU109>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 25.Lin XP, Almqvist N, Telemo E. Human small intestinal epithelial cells constitutively express the key elements for antigen processing and the production of exosomes. Blood Cells Mol Dis. 2005;35:122–8. doi: 10.1016/j.bcmd.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 26.Hundorfean G, Zimmer KP, Strobel S, Gebert A, Ludwig D, Buening J. Luminal antigens access late endosomes of intestinal epithelial cells enriched in MHC I and MHC II molecules –in vivo study in Crohn's ileitis. Am J Physiol Gastrointest Liver Physiol. 2007;293:6798–808. doi: 10.1152/ajpgi.00135.2007. [DOI] [PubMed] [Google Scholar]
- 27.Admyre C, Bohle B, Johansson SM, Focke-Tejkl M, Valenta R, Scheynius A, Gabrielsson S. B cell-derived exosomes can present allergen peptides and activate allergen-specific T cells to proliferate and produce T(H)2-like cytokines. J Allergy Clin Immunol. 2007;120:1418–24. doi: 10.1016/j.jaci.2007.06.040. [DOI] [PubMed] [Google Scholar]