Abstract
Growth of Salmonella enterica in mammalian tissues results from continuous spread of bacteria to new host cells. Our previous work indicated that infective S. enterica are liberated from host cells via stochastic necrotic burst independently of intracellular bacterial numbers. Here we report that liver phagocytes can undergo apoptotic caspase-3-mediated cell death in vivo, with apoptosis being a rare event, more prevalent in heavily infected cells. The density-dependent apoptotic cell death is likely to constitute an alternative mechanism of bacterial spread as part of a bet-hedging strategy, ensuring an ongoing protective intracellular environment in which some bacteria can grow and persist.
Keywords: apoptosis, caspase, necrosis, Salmonella typhimurium, systemic infection
Introduction
Salmonella enterica is a facultative intracellular pathogen that causes a spectrum of diseases in humans and animals. S. enterica serovar Typhi causes approximately 22 million cases of typhoid fever and over 200 000 deaths annually while serovar Paratyphi causes about 5·5 million illnesses in humans.1 Other non-typhoidal Salmonella serovars cause gastroenteritis in humans and animals and can spread from animals to humans via contaminated food. Non-typhoidal Salmonella serovars are a common cause of bacteraemia and sepsis in immunocompromised individuals and in children, especially in developing countries where they constitute a major cause of death.2,3
A well-established and tractable mouse typhoid model has been used extensively to study mechanisms of pathogenesis and immunity to typhoidal S. enterica infections. During systemic infections the liver is an important site of intracellular bacterial replication and persistence. The ability of S. enterica to resist and evade the antimicrobial armoury of phagocytes is a prerequisite for virulence. Recruitment of phagocytes to the sites of infection, and their activation are dependent on release of local and systemic inflammatory cytokines such as tumour necrosis factor-α, interferon-γ, and interleukins 12, 15 and 18. This results in the retardation of growth of intracellular S. enterica, which is dependent on reactive oxygen intermediates, reactive nitrogen intermediates, lysosomal enzymes and defensins.4–6
Our previous studies, based on direct in vivo, post-mortem, microscopic observation of individual infected cells within the liver, have shown that virulent bacteria, in addition to resisting intracellular killing, must escape from infected phagocytes and disseminate to other uninfected cells. We have shown that variations in the number of infected phagocytes parallel the net rate of bacterial growth in the tissues. This indicates that escape from infected cells and from already-formed pathological lesions is essential for infections to progress, and possibly enables bacteria to evade the local activation of immune defences.6,7 The continuous redistribution of bacteria from infected to uninfected cells leads to a heterogeneous numerical distribution of intracellular bacteria with low bacterial numbers seen at any one time in the majority of infected phagocytes.7 The integration of mathematical modelling with microscopy data has indicated that cell-to-cell spread of S. enterica is likely to occur via necrotic cell death and release of individual bacteria into the extracellular space.8 This analysis also predicted cell death to be a stochastic process, not dependent on intracellular bacterial density.8 Validation and improvement of our novel mathematical models of S. enterica infection require their continuous challenge in the light of new experimental data. In this context, a better understanding of macrophage cell death during S. enterica infection is central to unravelling the dynamics of pathogen spread and distribution in host organs, and may also provide new paradigms for understanding the dynamics of diseases with other intracellular pathogens.
Phagocyte death may proceed via apoptosis, autophagy, oncosis, necrosis or pyroptosis.9 The contributions of different forms of cell death to the pathophysiology of infections caused by S. enterica are still relatively poorly understood. Mechanisms of S. enterica-induced macrophage cell death have largely been inferred from in vitro cell culture studies and direct extrapolation to in vivo infection processes may be inappropriate. Investigations using animal models have been confined to the early stages of infection in the gut, where caspase-1-mediated cell death is prevalent,10–13 with little analysis of the systemic phase of disease. In this study we focused on the systemic compartment in a murine model of typhoidal infection. We employed multicolour fluorescence microscopy to: (1) visualize the presence of cells undergoing cell death in the livers of infected animals; (2) determine the level of colocalization between S. enterica and cells undergoing death; and (3) quantify intracellular bacterial numbers within these cells.
Materials and methods
Animals
Female C57BL/6 and BALB/c mice were purchased from Harlan Olac Ltd., (Blackthorn, Bicester, UK), tlr4−/− mice are described elsewhere14 and were bred at Harlan. Mice were used when older than 8 weeks.
Bacteria
Salmonella enterica serovar Typhimurium strain C5 is a virulent wild-type strain with 50% lethal dose of < 1·87 Log10 colony-forming units (CFU) for BALB/c mice.15 The bacteria were grown for 16 hr as a stationary culture at 37° in Luria–Bertani broth. Bacteria were diluted in phosphate-buffered saline (PBS) before intravenous or oral inoculation.
Enumeration of viable Salmonella in the tissue
Mice were killed by cervical dislocation, half of the liver was homogenized in a Seward Stomacher 80 Biomaster (Seward, Worthing, UK) in 10 ml distilled water and viable bacterial counts were assayed on pour plates of Luria–Bertani agar.
Immunostaining for fluorescence microscopy
Tissues were fixed overnight in 4% paraformaldehyde diluted in PBS, washed for 1 hr in two changes of PBS and then immersed in 20% sucrose (in PBS) for 16 hr at 4° before freezing at −80° in Cryo-Gel embedding medium (Instrumedics Inc., Hackensack, NJ). Thirty-micrometre sections were cut, blocked and rendered permeable for 10 min in a solution containing 10% normal goat serum diluted in PBS containing 0·2% Saponin (Sigma, Poole, UK) and then incubated in primary antibody (diluted in 10% normal goat serum/Saponin/PBS) for 16 hr at 4°. Subsequently, sections were washed in PBS for an hour (two 30-min washes) then incubated in secondary antibody (at a dilution of 1/200) at room temperature for 30 min, followed by two 15-min washes in PBS. Sections were mounted on vector bond treated slides (Vectabond reagent; Vector Labs, Burlingame, CA) using ProLong Gold antifade reagent supplemented with DAPI, (Molecular Probes, Invitrogen, Paisley, UK). The analysis of tissue sections was by multicolour fluorescence microscopy using a Leica DM6000B fluorescence microscope running FW4000 acquisition software. Dual staining of S. typhimurium and apoptotic cells was used to ascertain the degree of colocalization between cells targeted for death and bacterial load. Salmonella were immunostained using a monoclonal antibody directed against S. typhimurium lipopolysaccharide (LPS) O4 antigen (Biogenesis, Poole, UK), directly conjugated to Alexa Fluor 488 (Custom conjugation, Molecular Probes, Invitrogen). Anti-O4 (unconjugated) rabbit-polyclonal Salmonella agglutinating serum (Remel Europe Ltd, Dartford, UK) was also used for the visualization of Salmonella. Phagocytes were stained using an anti-CD18 monoclonal antibody followed by Cy5-conjugated polyclonal goat anti-rat serum. Cell nuclei were visualized using DAPI. Cell death was monitored by TUNEL (terminal deoxynucleotidyl transferase mediated dUTP nick end labelling) and by staining cells for cleaved caspase-3 and caspase-1. TUNEL-positive cells were detected using a TUNEL ApopTag kit (ApopTag kit; Intergen Company, Purchase, NY). Caspase-1-positive cells were visualized by rabbit polyclonal antisera to caspase-1 (Abcam, Cambridge, UK) or caspase-1 FLICA reagent (Immunochemistry Technologies, Bloomington, MN). We confirmed that the two substrates were able to detect caspase-1-positive cells, and importantly the same cells, by orally infecting C57BL/6 with C5, C5 sipB−/− or PBS and assaying the terminal ileum, in particular the Peyer's patches, for caspase-1-positive cells 24 hr postinfection (p.i.) (data not shown). To determine whether the number of caspase-1-positive cells increases in the liver during infection, the number of caspase-1-positive phagocytes in the liver at 72 and 96 hr p.i. was determined by staining the tissues with rabbit polyclonal antibody to caspase-1 and by using the caspase-1 FLICA reagent (FAM-YVAD-7MK). Tissue from caspase-1−/− mice was used as a negative control and gave negative staining using either the rabbit polyclonal anti-caspase-1 antibody or the caspase-1 FLICA reagent (FAM-YVAD-7MK) (data not shown). Caspase-3-positive cells were detected by anti-caspase-3 antibody (Cell Signalling Technology, Danvers, MA) or caspase-3 PhiPhiLux®-G2D2 reagent (OncoImmunin, Inc., Gaithersburg, MD), on fresh tissue samples. Both methods of detection identified similar numbers of caspase-3-positive cells in the tissues and, importantly, dual labelling studies indicated that the methods identified the same positive cells. Tissue from caspase-3−/− mice was used as a negative control and showed no positive cells (data not shown). Rat placental tissue was used as a positive control and contained a high frequency of caspase-3-positive cells (data not shown). The filter set was DAPI (Nuclei), fluorescein isothiocyanate (anti-O4 LPS Alexa Fluor 488 antibody, caspase-1 FLICA reagent, caspase-3, PhiPhiLux®-G2D2 reagent, TUNEL, ApopTag kit) and Cy3·5 (polyclonal anti-O4 LPS antibody, anti-caspase-1 antibody or anti-caspase-3 antibody, which were sequentially conjugated with goat anti-rabbit Alexa Fluor 568 antibody). The ApopTag, FLICA and PhiPhiLux kits were used according to the manufacturer's instructions, before the application of primary antibody. Intracellular analysis was carried out using imaris3d render software (Bitplane, Zurich, Switzerland). Images were manipulated in Adobe Photoshop 6.0/7.0.
Statistical analysis
Infected phagocytes in the liver were counted using a 63 × magnification oil-immersion objective. Three random samples of 3000 cells each were taken at 72 and 96 hr p.i. from a pool of liver tissue obtained from four mice. The bacterial load per cell was noted, and then the infected cells were scored independently for the presence or absence of cell death according to caspase-1, caspase-3 and TUNEL markers. The tests for each of the three comparisons were carried out as follows; note that we denote the number of cells within each of the positive and negative groups, SP and SN, respectively. First, the proportions of cells which contained one bacterium in the positive and negative groups, PP and PN, respectively, were calculated. The difference between these proportions, D0 = PP – PN, was then determined. Second, the observations for the positive and negative groups were pooled, and then randomly assigned to two groups of size SP and SN. Next, the proportion of cells that contained one bacterium per cell in each of the two randomly assigned groups of size SP and SN, PP and PN, respectively, were also calculated. Subsequently the difference between these proportions, D = PP – PN, was calculated and recorded. This second step was carried out 106 times to obtain a non-parametric estimate of the distribution of D under the null hypothesis that there was no difference between the proportions of positive and negative cells which had one bacterium per cell. Finally, a two-sided P-value was obtained by considering the proportion of random assignments that resulted in |D| > |D0|. Statistical analysis and programming was carried out using R Version 2.2.1,16 and minitab 14 software (Minitab Inc., State College, PA).
Results
Using the detection of DNA fragments as a marker of apoptotic activation, apoptotic nuclei are found in the infected liver
Systemic infection was followed in BALB/c mice injected intravenously with Log10 3·17 CFU of S. enterica serovar Typhimurium strain C5.15 Bacterial numbers were counted in individual liver phagocytes using microscopy and the total numbers of viable bacteria in the tissues were determined 72 and 96 hr p.i. These data confirmed that S. enterica growth in the liver resulted in an increase in the number of infected phagocytes and in the number of multicellular pathological lesions, with the majority of infected cells containing low bacterial numbers. The distribution of the number of bacteria per cell was similar to our previously reported findings.7 Apoptosis is a regulated active process controlled by signal transduction pathways, generating fragmented DNA,17 which can be detected by TUNEL.18 We determined that uninfected livers, and livers at 72 hr p.i. (bacterial load per organ, Log10 6·10 ± 0·16 CFU), were largely devoid of TUNEL-positive cells. However, we detected TUNEL-positive cells in the livers of infected mice 96 hr p.i. (bacterial load per organ, Log10 7·79 ± 0·27 CFU) (Fig. 1). This confirmed19 that host cells with fragmentation of nuclear DNA could be found during systemic S. enterica infection. However, TUNEL positivity is not always suitable for detecting early apoptotic events and the TUNEL assay does not definitively discriminate between apoptotic and necrotic nuclei.20 Another major limitation of TUNEL is its inability to provide information on the mechanisms of cell death that can potentially occur as a consequence of S. enterica infection (e.g. caspase-1-mediated death versus caspase-3-mediated death). Caspases are evolutionarily conserved cysteine proteases that share similarities in amino acid sequence and structure but differ in their physiological roles. The caspases can be broadly divided into two groups; those that are centrally involved in apoptosis (caspase-2, -3, -6, -7, -8, -9 and -10) and those related to caspase-1 (caspase-1, -4, -5, -13 and -14, as well as murine caspase-11 and -12), whose primary role is in cytokine processing during inflammatory responses.9 We therefore proceeded to assess the relative incidence of caspase-1 and caspase-3-positive cells in the livers of infected mice.
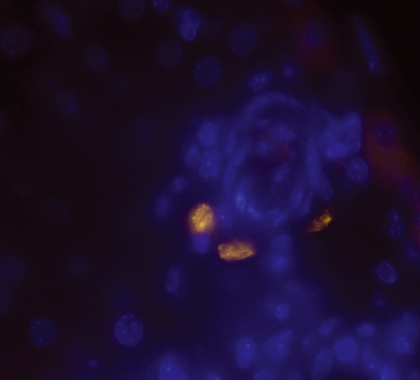
Figure 1.
Multicolour fluorescence microscopy showing TUNEL-positive cells detected 96 hr postinfection in BALB/c mice infected with Salmonella Typhimurium C5. (TUNEL-positive cells appear yellow/green, nuclei were stained with DAPI and appear blue. Images were taken at magnification × 630).
Salmonella-induced, caspase-3-mediated cell death is observed during the systemic infection of mice
Caspase-1-mediated cell death occurs during the early part of S. enterica infections in the gastrointestinal tract,10–13 but its role in the pathogenesis of salmonellosis remains uncertain.21 We observed no detectable increase in the frequency of cells positive for caspase-1 in the livers of BALB/c mice undergoing a systemic S. enterica infection. The frequency of caspase-1-positive cells in the tissues of uninfected BALB/c mice was one caspase-1-positive cell in 100 fields (magnification × 63) with no significant increase seen in tissues from infected animals. The lack of an increase in such cells in infected animals suggested that other forms of cell death might be occurring in the systemic compartment of infected mice. We therefore tested whether caspase-3-dependent programmed cell death was detectable in the liver during the systemic phase of a S. enterica infection. Previous studies have reported Salmonella-induced caspase-3 activation, but these have been in in vitro models of infection,22–25 in a murine endotoxin shock model26 and in a porcine jejunal loop model.27 We observed an increase in the numbers of caspase-3-positive cells in the livers of infected versus uninfected control animals at both 72 and 96 hr p.i. (Fig. 2). To our knowledge this is the first direct evidence that host cell death with the features of classical caspase-3-dependent apoptosis can be detected in response to S. enterica in the livers of mice during a typhoidal infection. The confinement of caspase-1-positive cells to the gut tissues,10–13 and the presence of caspase-3-mediated cell death in the systemic compartment of infected mice indicates that S. enterica can cause cell death via different mechanisms in different anatomical sites.
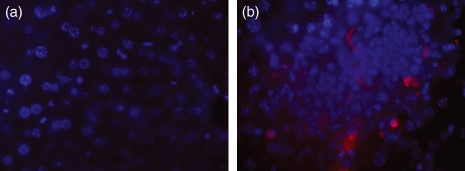
Figure 2.
Multicolour fluorescence microscopy showing (a) the absence of caspase-3-positive cells in uninfected tissue, (b) a caspase-3-positive cell within a focus of infection 96 hr postinfection in BALB/c mice infected with Salmonella Typhimurium C5. (Caspase-3-positive cells were detected with Anti-Caspase-3 antibody/Alexa Fluor 568 and appear red, nuclei are stained with DAPI and appear blue. Images were taken at magnification × 630).
Not all TUNEL-positive or caspase-3-positive cells contain intracellular Salmonella
To determine whether caspase-3-dependent apoptosis requires the presence of intracellular bacteria, liver sections were double-stained using anti-caspase-3 and anti-S. enterica antibodies (Fig. 3). Not all caspase-3-positive apoptotic cells contained intracellular S. enterica, showing that caspase-3-mediated cell death does not necessarily require the presence of intracellular bacteria and can occur in uninfected bystander cells. We obtained similar results using the TUNEL reaction, in agreement with previous reports.19 We also detected that not all TUNEL-positive phagocytes were caspase-3-positive, suggesting that caspase-3-dependent and -independent pathways of cell death increase during infection.
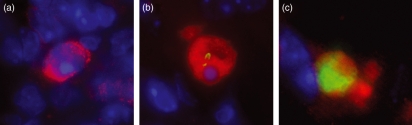
Figure 3.
Multicolour fluorescence microscopy images showing caspase-3-positive cells detected 96 hr postinfection in BALB/c mice infected with Salmonella Typhimurium C5. (a) A caspase-3-positive cell containing no intracellular bacteria, (b) a caspase-3-positive cell harbouring an intracellular bacterium, (c) a caspase-3-positive cell containing many intracellular bacteria. (S. enterica were detected with Anti-Salmonella O4-LPS Antibody Congjugated to Alexa Fluor 488 and appear green, caspase-3-positive cells were detected with Anti-Caspase-3 antibody/Alexa Fluor 568 and appear red, nuclei are stained with DAPI and appear blue. Images were taken at magnification × 630).
The number of intracellular bacteria per infected phagocyte varies between TUNEL-positive or caspase-3-positive and non-apoptotic cells
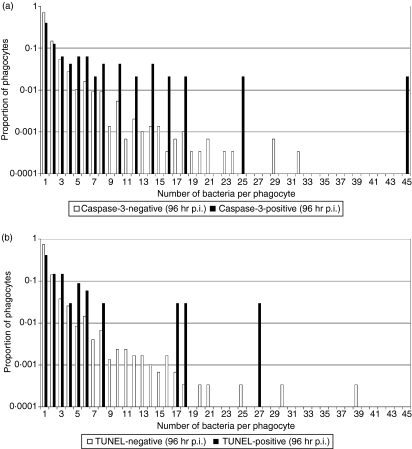
Salmonella enterica release from cells following necrotic burst appears to be a stochastic process and occurs independently of intracellular bacterial numbers.8 To test whether caspase-3-dependent cell death is related to the intracellular bacterial load we acquired microscopy data on the correlation between the number of intracellular bacteria and the presence of cell death markers in individual phagocytes in the livers of mice infected with S. enterica (Figs 3 and 4, and Table 1). This is particularly relevant in the light of suggestions that S. enterica-induced apoptosis could be a bacterial response to nutrient deprivation that is likely to occur when high bacterial numbers are present inside a phagosome.28 Our data indicate that apoptotic (defined as TUNEL/caspase-3-positive) phagocytes contain higher bacterial numbers than non-apoptotic (defined as TUNEL/caspase-3-negative) phagocytes (Fig. 4 and Table 1). Although apoptosis is a rare event, with only around 5% of all infected cells being apoptotic, it becomes increasingly common in heavily infected cells. For both caspase-3 and TUNEL markers, the rate of apoptosis goes from around 1% in (common) singly-infected cells to more than 20% in (rare) heavily infected cells. Three independent non-parametric randomization tests were carried out to compare the bacterial counts per cell in marker-positive and marker-negative cells (explained in detail in Materials and methods, summarized in Table 2). Randomization tests were used because they make no distributional assumptions about the test statistic and do no require a large sample size to be valid. For all three cases, (caspase-3 at 72 and 96 hr, and TUNEL at 96 hr), the proportion of infected cells containing one bacterium was significantly lower for the marker-positive groups compared with their relevant control group. Therefore, from the randomization tests there was very strong evidence for a difference between the distributions of Salmonella within cells for the caspase-3-positive and -negative phagocytes, and the TUNEL-positive and -negative phagocytes.
Figure 4.
Variation of intracellular bacterial numbers in cell death marker-positive and –negative phagocytes as a proportion of each subset of data. (a) Infected caspase-3-positive/negative phagocytes, (b) infected TUNEL-positive/negative phagocytes, in the livers of BALB/c mice infected with Salmonella Typhimurium C5, 96 hr postinfection (p.i.).
Table 1.
Correlation between the number of intracellular bacteria and the presence of cell death markers in individual phagocytes in the livers of mice infected with Salmonella enterica: the mean intracellular bacterial load and [range], per marker-positive and -negative cell at 72 and 96 hr postinfection
| Mean intracellular bacterial load per cell | |
|---|---|
| BALB/c caspase-3-negative (72 hr) | 1·47 [1…23] (n = 2819) |
| BALB/c caspase-3-positive (72 hr) | 1·54 [1…6] (n = 181) |
| BALB/c caspase-3-negative (96 hr) | 1·81 [1…33] (n = 2952) |
| BALB/c caspase-3-positive (96 hr) | 5·60 [1…45] (n = 48) |
| BALB/c TUNEL-negative (96 hr) | 1·70 [1…39] (n = 2966) |
| BALB/c TUNEL-positive (96 hr) | 4·24 [1…27] (n = 34) |
BALB/c mice were infected with S. typhimurium C5 (Log10 3·17 CFU).
Table 2.
Correlation between the number of intracellular bacteria and the presence of cell death markers in individual phagocytes in the livers of mice infected with Salmonella enterica: the proportion of infected cells containing one bacterium per cell and two-sided P-values for randomization tests for caspase-3-positive cells at 72 hr, caspase-3-positive cells at 96 hr, and TUNEL-positive cells at 96 hr, along with respective control (marker-negative) groups
| Positive group | Negative group | Two-sided P-values (5 d.p.) | |
|---|---|---|---|
| BALB/c caspase-3 (72 hr) | 0·663 | 0·783 | 0·00040 |
| BALB/c caspase-3 (96 hr) | 0·396 | 0·709 | 0·00002 |
| BALB/c TUNEL (96 hr) | 0·412 | 0·745 | 0·00094 |
BALB/c mice were infected with S. typhimurium C5 (Log10 3·17 CFU)
Salmonella-induced activation of Toll-like receptor 4 is responsible for a component of caspase-3-dependent programmed cell death observed during systemic Salmonella infection
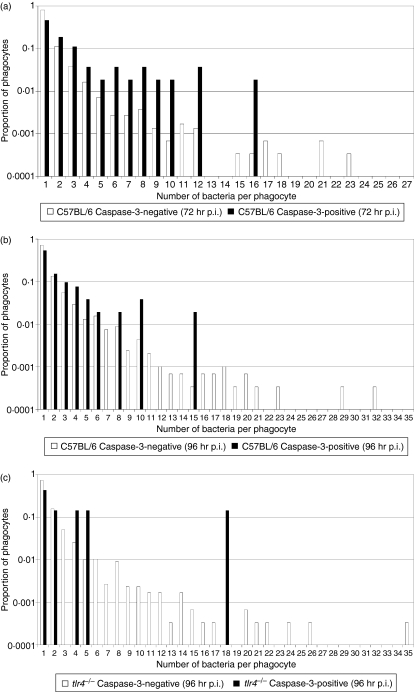
In cell culture, Salmonella strains can induce delayed caspase-3-mediated apoptosis, which is dependent on SPI-2 function and may also require the stimulation of Toll-like receptor-4 (TLR4) by bacterial LPS, and the double-stranded RNA responsive protein kinase (PKR).29–31 To establish whether the Salmonella-induced, density-dependent, caspase-3-mediated cell death was attributable to TLR4 stimulation, systemic infection was followed in tlr4−/− mice and congenic wild-type control mice on a C57BL/6 background. Mice were injected intravenously with Log10 3·11 CFU S. Typhimurium strain C515 and bacterial numbers in the livers of mice were determined at 72 and 96 hr p.i. (Table 3). A non-parametric Kruskal–Wallis test with a Bonferroni step-down correction for multiple testing indicated no statistical difference between the bacterial load in the organs between groups at 72 hr p.i.; however, there was evidence of a statistically significant difference between both the livers and the spleens of C57BL/6 and tlr4−/− mice at 96 hr p.i. (P = 0·021 for both groups), with an increased bacterial burden in the organs of the tlr4−/− mice. We acquired microscopy data on the correlation between the number of intracellular bacteria and the presence of cell death markers (caspase-1- and caspase-3-mediated cell death). The frequency of caspase-1-positive cells in the tissues of uninfected C57BL/6 and tlr4−/− mice was higher than that observed for the BALB/c mice, around one caspase-1-positive cell in 10 fields (magnification × 63) with a slight increase in tissues from infected animals. No intracellular bacteria were found in any caspase-1-positive cells. Our data indicate that, as previously detailed for BALB/c mice, during a systemic S. enterica infection the livers of C57BL/6 mice contained caspase-3-positive phagocytes, not all of which contained bacteria. Furthermore, the mean number of bacteria in infected caspase-3-positive cells was higher than in infected non-apoptotic (defined as caspase-3-negative) phagocytes (Table 4 and Fig. 5). At 96 hr p.i., the tlr4−/− mice also followed this trend, although the number of uninfected caspase-3-positive phagocytes was lower than in the wild-type controls. A non-parametric randomization test did not detect any evidence of a statistically significant difference between the bacterial loads in the caspase-3-positive cells in C57BL/6 versus tlr4−/− mice (P = 0·698); however, it should be noted that the sample size was extremely low for the tlr4−/− group. In the tlr4−/− mice there were no caspase-3-positive phagocytes containing Salmonella at 72 hr p.i. (3000 infected phagocytes counted), although we did observe occasional caspase-3-positive phagocytes that did not harbour bacteria. Collectively these results suggested that Salmonella-induced activation of TLR4 was responsible for a component of caspase-3-dependent programmed cell death observed during systemic Salmonella infection; however, an additional as yet undefined TLR4-independent mechanism must also exist.
Table 3.
Correlation between the number of intracellular bacteria and the presence of cell death markers in individual phagocytes in the livers of wild-type and tlr4−/− mice infected with Salmonella enterica: bacterial counts in the livers of mice infected with S. typhimurium
| Time p.i./organ | C57BL/6 | tlr4−/− | P |
|---|---|---|---|
| 72 hr Liver | 6·00 ± 0·19 | 6·25 ± 0·11 | 0·061 |
| 72 hr Spleen | 6·06 ± 0·06 | 6·05 ± 0·08 | 0·061 |
| 96 hr Liver | 6·80 ± 0·53 | 7·83 ± 0·13 | 0·021 |
| 96 hr Spleen | 7·01 ± 0·19 | 7·89 ± 0·18 | 0·021 |
The tlr4−/− mice and congenic wild-type control mice on a C57BL/6 background were injected intravenously with Log10 3·11 CFU of S. Typhimurium strain C5 and bacterial numbers in the livers of mice (n = 4) were determined at 72 and 96 hr postinfection. Results are expressed as Log10 viable count ± SD (P is derived from a non-parametric Kruskal–Wallis test of the raw data with a Bonferroni step-down correction for multiple testing).
Table 4.
Correlation between the number of intracellular bacteria and the presence of cell death markers in individual phagocytes in the livers of wild-type and tlr4−/− mice infected with Salmonella enterica: the mean intracellular bacterial load and [range], per marker-positive and marker-negative cell (C57BL/6 and tlr4−/−) at 72 and 96 hr postinfection (p.i.)
| Mean intracellular bacterial load per cell | |
|---|---|
| C57BL/6 caspase-3-negative (72 hr) | 1·42 [1…21] (n = 2946) |
| C57BL/6 caspase-3-positive (72 hr) | 3·15 [1…16] (n = 54) |
| C57BL/6 caspase-3-negative (96 hr) | 1·79 [1…29] (n = 2948) |
| C57BL/6 caspase-3-positive (96 hr) | 2·58 [1…15] (n = 52) |
| tlr4−/− caspase-3-negative (72 hr) | 1·44 [1…27] (n = 3000) |
| tlr4−/− caspase-3-positive (72 hr) | Not detected |
| tlr4−/− caspase-3-negative (96 hr) | 1·68 [1…35] (n = 2993) |
| tlr4−/− caspase-3-positive (96 hr) | 4·57 [1…18] (n = 7) |
Figure 5.
Variation of intracellular bacterial numbers in cell death marker-positive and -negative phagocytes as a proportion of each subset of data. Infected caspase-3-positive/negative phagocytes in the livers of mice infected with Salmonella Typhimurium C5 (a) C57BL/6 [72 hr postinfection (p.i.)], (b) C57BL/6 (96 hr p.i.) and (c) tlr4−/− (96 hr p.i.).
Discussion
A recent study reports that the specific nature of the initial interactions of Salmonella with host cells during internalization determines the development of the intracellular niche and bacterial response.32 Our previous research has indicated that the spread and distribution of S. enterica in the tissues is underlain by a branching process where intracellular growth is followed by stochastic lysis of phagocytes and redistribution of individual bacteria to new phagocytic cells.8 The data presented here provide continued refinement to our understanding of the variables that govern the dynamics of S. enterica spread in the tissues. We show that liver phagocytes can undergo apoptotic caspase-3-mediated cell death in vivo, with apoptosis being more prevalent in heavily infected cells and a proportion being dependent on LPS-induced activation of TLR4. We have evidence for two distinct mechanisms of S. enterica-induced phagocyte death operating in parallel but governed by different dynamics. Caspase-3-mediated cell death is rare and correlates with intracellular bacterial density, whereas the more common necrotic lysis and extracellular release of individual bacteria is independent of intracellular bacterial numbers.8
Previous reports, using in vitro tissue culture models, have indicated that delayed, SipB-independent, macrophage apoptosis by Salmonella requires activation via TLR4, leading to the triggering of signal transduction pathways mediated by adapter proteins and cellular kinases.29,31 Once activated, the macrophage is in a balance of opposing effects of pro- and anti-apoptotic factors, with Salmonella altering the balance in favour of apoptosis by the action of virulence effector proteins secreted through type III secretion systems.29,30 TLR4 ligation by LPS also leads to activation of the double-stranded RNA responsive protein kinase PKR, which phosphorylates elongation factor eukaryotic initiation factor-2 (eIF2-α) resulting in the inhibition of protein synthesis of anti-apoptotic proteins.29,30 Moreover, PKR and type I interferons can activate interferon response factor 3 (IRF3), which also induces pro-apoptotic factors in macrophages.29 This investigation showed that caspase-3-mediated programmed cell death is more prevalent in heavily infected cells with a component that is dependent on LPS-induced activation of TLR4; however, the precise details of the downstream adapter molecules involved cannot be extrapolated from this study. Interestingly, a recent study confirming and extending observations by Hsu et al.,29 suggests that, at least in tissue culture, S. Typhimurium induces SipB-independent cell death through TLR4 signalling via the adapter proteins Tram and Trif and not through the adapter proteins Mal or MyD88.31
It is generally believed that apoptotic cells with intact membranes are engulfed by phagocytes in vivo, whereas breakup of the membrane of apoptotic cells is occasionally seen in vitro.33 Therefore, it is reasonable to assume that infected apoptotic host cells would die without releasing their load of bacteria extracellularly, thus allowing S. enterica to spread intercellularly via apoptotic bodies, potentially carrying multiple bacteria. This scenario might appear incompatible with our current model for the spread and distribution of S. enterica in mammalian tissues8 where extracellular release of individual bacteria following cell lysis is assumed to be the basis of the observed increase in the number of infected cells during infection. However, careful analysis illustrates that phagocytosis of infected apoptotic bodies would lead formally within the model to a situation where a new infected host cell simply replaces the previous host cell containing the same set of bacteria, allowing neither the redistribution of single bacteria nor an increase in the number of foci of infection. The ingestion of an infected apoptotic body by a new host cell would simply allow the progression of the division of that set of bacteria within a single host cell without violating the model assumptions.
Several pathogens (e.g. Listeria and Shigella) have evolved sophisticated mechanisms of cell-to-cell spread that allow the bacteria to distribute in the tissues within an exclusively intracellular environment. Caspase-3-dependent apoptosis is likely to be of relevance to the biology of systemic S. enterica infections by providing a proportion of the bacteria with an intracellular niche where they can spread and persist in the tissues with minimal triggering of inflammatory responses. Intracellular and extracellular routes of spread in the tissues can also have vastly different consequences on bacterial susceptibility to established immune responses (e.g. humoral versus cell-mediated immunity) or antimicrobial intervention strategies. Therefore an ability to ‘bet-hedge’34,35– to mix these two strategies of cell-to-cell transfer – may broaden the persistence of this pathogen in the face of an ever changing host environment. Indeed, bacterial persistence via apoptotic bodies may explain why antibiotics that poorly penetrate host cells, e.g. gentamicin, fail to completely clear all bacteria during an infection.36 The existence of extracellular bacterial spread (following necrosis) and intracellular spread (following apoptosis) provide a rationale for the requirement for both antibodies and T cells in immunity to S. enterica and for the limited efficacy of those S. enterica vaccines that elicit only one of the above mentioned branches of the immune response.
Our work suggests that S. enterica uses a fine balance between multiple mechanisms to disseminate in the body and optimally avoid the host immune response. Clearly, a greater appreciation of the mechanisms and consequences of pathogen-induced host cell death in infected animals is an important research priority, not only for our understanding of the dynamics and determinants of bacterial growth and distribution during infection, but also for the continued development of novel therapeutic strategies.
Acknowledgments
We thank Richard Flavell for the kind donation of C57BL/6 caspase-1−/− and caspase-3−/− liver samples. We thank Richard Clarkson for useful discussions and we are grateful to Anjam Khan for supplying the C5 sipB−/− strain. This work was supported by BBSRC grant BBS/B/02266 awarded to P.M. and D.J.M. The authors have no conflicting financial interests.
References
- 1.Crump JA, Luby SP, Mintz ED. The global burden of typhoid fever. Bull World Health Organ. 2004;82:346–53. [PMC free article] [PubMed] [Google Scholar]
- 2.Bahwere P, Levy J, Hennart P, Donnen P, Lomoyo W, Dramaix-Wilmet M, Butzler JP, De Mol P. Community-acquired bacteremia among hospitalized children in rural central Africa. Int J Infect Dis. 2001;5:180–8. doi: 10.1016/s1201-9712(01)90067-0. [DOI] [PubMed] [Google Scholar]
- 3.Graham SM. Salmonellosis in children in developing and developed countries and populations. Curr Opin Infect Dis. 2002;15:507–12. doi: 10.1097/00001432-200210000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Mastroeni P, Vazquez-Torres A, Fang FC, Xu Y, Khan S, Hormaeche CE, Dougan G. Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. II. Effects on microbial proliferation and host survival in vivo. J Exp Med. 2000;192:237–48. doi: 10.1084/jem.192.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vazquez-Torres A, Jones-Carson J, Mastroeni P, Ischiropoulos H, Fang FC. Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. I. Effects on microbial killing by activated peritoneal macrophages in vitro. J Exp Med. 2000;192:227–36. doi: 10.1084/jem.192.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mastroeni P, Sheppard M. Salmonella infections in the mouse model: host resistance factors and in vivo dynamics of bacterial spread and distribution in the tissues. Microbes Infect. 2004;6:398–405. doi: 10.1016/j.micinf.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 7.Sheppard M, Webb C, Heath F, Mallows V, Emilianus R, Maskell D, Mastroeni P. Dynamics of bacterial growth and distribution within the liver during Salmonella infection. Cell Microbiol. 2003;5:593–600. doi: 10.1046/j.1462-5822.2003.00296.x. [DOI] [PubMed] [Google Scholar]
- 8.Brown SP, Cornell SJ, Sheppard M, Grant AJ, Maskell DJ, Grenfell BT, Mastroeni P. Intracellular demography and the dynamics of Salmonella enterica infections. PLoS Biol. 2006;4:2091–8. doi: 10.1371/journal.pbio.0040349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fink SL, Cookson BT. Apoptosis, pyroptosis and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun. 2005;73:1907–16. doi: 10.1128/IAI.73.4.1907-1916.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hersh D, Monack DM, Smith MR, Ghori N, Falkow S, Zychlinsky A. The Salmonella invasion SipB induces macrophage apoptosis by binding to Caspase-1. Proc Natl Acad Sci USA. 1999;96:2396–401. doi: 10.1073/pnas.96.5.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monack DM, Hersh D, Ghori N, Bouley D, Zychlinsky A, Falkow S. Salmonella exploits Caspase-1 to colonize Peyer's patches in a murine typhoid model. J Exp Med. 2000;192:249–58. doi: 10.1084/jem.192.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Monack DM, Navarre WW, Falkow S. Salmonella-induced macrophage death: the role of Caspase-1 in death and inflammation. Microbes Infect. 2001a;3:1201–12. doi: 10.1016/s1286-4579(01)01480-0. [DOI] [PubMed] [Google Scholar]
- 13.Monack DM, Detweiler CS, Falkow S. Salmonella pathogenicity island 2-dependent macrophage death is mediated in part by the host cysteine protease Caspase-1. Cell Microbiol. 2001b;3:825–37. doi: 10.1046/j.1462-5822.2001.00162.x. [DOI] [PubMed] [Google Scholar]
- 14.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–52. [PubMed] [Google Scholar]
- 15.Hormaeche CE. Natural resistance to Salmonella Typhimurium in different inbred mouse strains. Immunology. 1979;37:311–8. [PMC free article] [PubMed] [Google Scholar]
- 16.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2005. ISBN 3-900051-07-0 URL http://www.R-project.org. [Google Scholar]
- 17.Wyllie AH. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980;284:555–6. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- 18.Ben-Sasson SA, Sherman Y, Gavrieli Y. Identification of dying cells –in situ staining. Methods Cell Biol. 1995;46:29–39. [PubMed] [Google Scholar]
- 19.Richter-Dahlfors A, Buchan AMJ, Finlay BB. Murine Salmonellosis studied by confocal microscopy: Salmonella Typhimurium resides intracellularly inside macrophages and exerts a cytotoxic effect on phagocytes in vivo. J Exp Med. 1997;186:569–80. doi: 10.1084/jem.186.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boise LH, Collins CM. Salmonella-induced cell death: apoptosis, necrosis or programmed cell death? Trends Microbiol. 2001;9:64–7. doi: 10.1016/s0966-842x(00)01937-5. [DOI] [PubMed] [Google Scholar]
- 21.Lara-Tejero M, Sutterwala FS, Ogura Y, Grant EP, Bertin J, Coyle AJ, Flavell RA, Galan JE. Role of the Caspase-1 inflammasome in Salmonella Typhimurium pathogenesis. J Exp Med. 2006;203:1407–12. doi: 10.1084/jem.20060206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jesenberger V, Procyk KJ, Yuan J, Reipert S, Baccarini M. Salmonella-induced Caspase-2 activation in macrophages: a novel mechanism in pathogen-mediated apoptosis. J Exp Med. 2000;192:1035–45. doi: 10.1084/jem.192.7.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paesold G, Guiney DG, Eckmann L, Kagnoff MF. Genes in the Salmonella pathogenicity island 2 and the Salmonella virulence plasmid are essential for Salmonella-induced apoptosis in intestinal epithelial cells. Cell Microbiol. 2002;4:771–81. doi: 10.1046/j.1462-5822.2002.00233.x. [DOI] [PubMed] [Google Scholar]
- 24.Valle E, Guiney DG. Characterization of Salmonella-induced cell death in human macrophage-like THP-1 cells. Infect Immun. 2005;73:2835–40. doi: 10.1128/IAI.73.5.2835-2840.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takaya A, Suzuki A, Kikuchi Y, Eguchi M, Isogai E, Tomoyasu T, Yamamoto T. Derepression of Salmonella pathogenicity island 1 genes within macrophages leads to rapid apoptosis via caspase-1- and caspase-3-dependent pathways. Cell Microbiol. 2005;7:79–90. doi: 10.1111/j.1462-5822.2004.00435.x. [DOI] [PubMed] [Google Scholar]
- 26.Jaeschke H, Fisher MA, Lawson JA, Simmons CA, Farhood A, Jones DA. Activation of Caspase 3 (CPP32)-like protease is essential for TNF-α-induced hepatic parenchymal cell apoptosis and neutrophil-mediated necrosis in a murine endotoxin shock model. J Immunol. 1998;160:3480–6. [PubMed] [Google Scholar]
- 27.Schauser K, Olsen JE, Larsson L-E. Salmonella Typhimurium infection in the porcine intestine: evidence for caspase-3-dependent and -independent programmed cell death. Histochem Cell Biol. 2005;123:43–50. doi: 10.1007/s00418-004-0731-8. [DOI] [PubMed] [Google Scholar]
- 28.Lundberg U, Vinatzer U, Berdnik D, von Gabain A, Baccarinim M. Growth phase-regulated induction of Salmonella-induced macrophage apoptosis correlates with transient expression of SPI-1 genes. J Bacteriol. 1999;181:3433–7. doi: 10.1128/jb.181.11.3433-3437.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsu L-C, Park JM, Zhang K, et al. The protein kinase PKR is required for macrophage apoptosis after activation of Toll-like receptor 4. Nature. 2004;428:341–5. doi: 10.1038/nature02405. [DOI] [PubMed] [Google Scholar]
- 30.Guiney DG. The role of host cell death in Salmonella infections. Curr Top Microbiol Immunol. 2005;289:131–50. doi: 10.1007/3-540-27320-4_6. [DOI] [PubMed] [Google Scholar]
- 31.Cook P, Totemeyer S, Stevenson C, Fitzgerald KA, Yamamoto M, Akira S, Maskell DJ, Bryant CE. Salmonella-induced SipB-independent cell death requires Toll-like receptor-4 signalling via the adapter proteins Tram and Trif. Immunology. 2007;122:222–9. doi: 10.1111/j.1365-2567.2007.02631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drecktrah D, Knodler L, Ireland R, Steele-Mortimer O. The mechanism of Salmonella entry determines the vacuolar environment and intracellular gene expression. Traffic. 2006;7:39–51. doi: 10.1111/j.1600-0854.2005.00360.x. [DOI] [PubMed] [Google Scholar]
- 33.Edinger AL, Thompson CB. Death by design: apoptosis, necrosis and autophagy. Curr Opin Cell Biol. 2004;16:663–9. doi: 10.1016/j.ceb.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 34.Jansen VAA, Stumpf MPH. Making sense of evolution in an uncertain world. Science. 2005;309:2005–7. doi: 10.1126/science.1118711. [DOI] [PubMed] [Google Scholar]
- 35.Kussell E, Leibler S. Phenotypic diversity, population growth, and information in fluctuating environments. Science. 2005;309:2075–8. doi: 10.1126/science.1114383. [DOI] [PubMed] [Google Scholar]
- 36.Bonina L, Costa GB, Mastroeni P. Comparative effect of gentamicin and pefloxacin treatment on the late stages of mouse typhoid. Microbiologica. 1998;21:9–14. [PubMed] [Google Scholar]