Abstract
Macrophage migration inhibitory factor (MIF) plays vital roles in the regulation of responses to stimuli acting via Toll-like receptor (TLR)-4. Recently, a specific small molecule inhibitor of MIF (ISO-1) has been described. We investigated the effects of ISO-1 on TLR responses in primary human monocytes and monocyte-derived macrophages (MDM). In monocytes, ISO-1 caused marked suppression of TLR4-induced proinflammatory cytokine production, and to a lesser extent suppression of TLR2-induced responses. The lipopolysaccharide (LPS)-induced activation of cocultures of monocytes and endothelial cells was strongly inhibited by ISO-1. Suppression of monocyte TLR4 signalling by ISO-1 was associated with alterations in extracellular signal-related kinase (ERK)-1/2 activation status. Previously, regulation of TLR4 signalling by MIF has been noted to be through control of TLR4 expression, but we observed that the actions of ISO-1 were mediated without changes in cell surface TLR4 levels. In contrast, ISO-1 pretreatment did not inhibit responses of MDM to LPS. ISO-1 is a promising parent molecule which inhibits TLR-induced ERK activation and inflammatory cytokine production in monocytes, whose role may be complicated by cell-type specificity.
Keywords: atherosclerosis, inflammation, LPS, MIF, Toll-like receptor
Introduction
Macrophage migration inhibitory factor (MIF) is a pleiotropic cytokine now recognized as a major contributor to acute and chronic components of a diverse range of inflammatory diseases, including atherosclerosis,1 arthritis,2,3 acute respiratory distress syndrome,4 asthma,5–7 and sepsis.8 Binding these observations together are the likely roles for innate immunity in chronic inflammatory diseases, driven by endogenous and pathogen-related stimuli.9
MIF has major roles in the regulation of responses to endotoxin, and can act to amplify responses to lipopolysaccharide (LPS).10 In a model of endotoxaemia, coadministration of MIF and LPS caused increased mortality compared to LPS alone.8 Neutralization of MIF with blocking antibodies or by gene knockout is strongly protective against endotoxic shock.8,11,12 Roles for MIF have also been identified in the development of atheroma and in acute plaque rupture.13 In apolipoprotein E−/− mice, MIF expression is associated with atherosclerotic lesions, and neutralization of MIF reduces local aortic expression of adhesion molecules, matrix metalloproteinases, and proinflammatory cytokines, in addition to reducing circulating fibrinogen and interleukin-6 (IL-6).1 MIF expression has likewise been associated with unstable plaques in human disease.14 For these reasons, inhibition of MIF by a recently-described and specific inhibitor, ISO-1 that is active in vitro and in vivo, has considerable therapeutic potential.15–17
MIF signalling involves a number of proteins whose exact roles are still be elucidated. MIF binds to a cell surface receptor complex including CD74, but roles for CXCR2 and CD44 in the transduction of the MIF signal have been described.18,19 MIF activates extracellular signal-related kinase (ERK) signalling pathways,20 and may achieve this by signalling from the cell surface18 and by binding intracellularly to JAB1/CSN5.21,22 A proposed mechanism by which MIF achieves regulation of LPS responses is through the transcriptional control, and hence decreased expression, of Toll-like receptor-4 (TLR4).23 Downregulation of TLR4 is also a potential explanation for the phenomenon of endotoxin tolerance, but in reality early regulation of signalling is more important than control of TLR4 expression,24 and low levels of TLR4 can enable signalling.25,26 Thus, downregulation of TLR4 may not be the most efficient method of regulating LPS responses. Additionally, whilst generation of tumour necrosis factor-α (TNF-α) is impaired in MIF−/− mice, IL-6 and NO production may be preserved, suggesting that loss of MIF may modify, but not completely inhibit, TLR4 signalling.11
We therefore set out to investigate the effects of ISO-1 on TLR-induced activation of primary human leucocytes, and hypothesized that inhibition of responses would be exerted at levels other than control of TLR4 expression.
Materials and methods
Reagents
Cell culture reagents were purchased from Invitrogen (Paisley, UK) and general laboratory reagents were from Sigma-Aldrich (Poole, UK). Fetal calf serum (FCS; endotoxin levels <0·5 EU/ml) was purchased from BioWhittaker (Verviers, Belgium). Purified LPS from Escherichia coli serotype R515 was from Axxora (Nottingham, UK). (S,R)-3-(4-hydroxyphenyl)-4,5-dihydro-5-isoxazole acetic acid, methyl ester (ISO-1) was obtained from Merck Bioscience (Nottingham, UK) and reconstituted in dimethyl sulphoxide (DMSO). Concentrations of DMSO were held constant in all experiments. ISO-1 was used at a concentration of 50 μm (concentration shown to give just maximal inhibition of MIF responses in RAW264.7 cells15). Independent testing revealed levels of <0·005 EU/ml endotoxin in ISO-1 at the concentrations used. Pam3CSK4 was from EMC Microcollections (Tübingen, Germany). Macrophage-activating lipopeptide 2kDa (MALP-2) was from Axxora (Nottingham, UK). Inhibitors of mitogen-activated protein kinases (MAPK) PD98059, U0126, and the non-inhibiting control molecule SB202474 were from Merck Biosciences. Nitrocellulose membrane and ECL reagent for Western blotting were from GE Healthcare (Chalfont St. Giles, UK). Anti-phospho-ERK1/2 and anti-rabbit secondary antibody were from Cell Signaling Technology (Beverly, MA). Anti-phospho-p38 was from Promega (Southampton, UK). Anti-actin was from Sigma-Aldrich. The phycoerythrin-conjugated anti-TLR4 monoclonal antibody (mAb, clone HTA125) and the appropriate immunoglobulin G2a (IgG2a) isotype control were from eBioscience (San Diego, CA).
Cells and cell culture
Peripheral blood mononuclear cells (PBMC) were isolated from whole blood by density gradient centrifugation over plasma/Percoll or Histopaque as described.25,27 Blood was taken with informed consent from healthy volunteers in accordance with a protocol approved by South Sheffield Local Research Ethics Committee. Monocytes were enriched by negative magnetic selection using Monocyte Isolation Kit II (Miltenyi Biotech, Bergisch Gladbach, Germany) to a typical mean (± SEM) purity of 77·8 ± 1·8% (n = 28) CD14+ cells, and cultured at a density of 2 × 105cells/ml in RPMI-1640 containing 2 mm l-glutamine, supplemented with 10% FCS, 100 U/ml penicillin and 100 μg/ml streptomycin in Falcon Flexiwell plates (Becton Dickinson, UK).
Monocyte derived macrophages (MDM) were prepared from PBMC, plated at 2–3 × 106cells/ml in serum free Iscove's modified Dulbecco's medium (IMDM). After 1 hr, non-adherent cells were removed and adherent cells cultured in IMDM containing 10% FCS for 7 days.
Human umbilical vein endothelial cells (HUVEC) were isolated from umbilical cords, donated with informed consent following a protocol approved by North Sheffield Local Research Ethics Committee and seeded in 24-well tissue culture plates coated with 0·2% gelatin at passage 2–3. Cells were grown to 70–90% confluence, washed and placed into RPMI media supplemented with 2 mm l-glutamine, 0·225% sodium bicarbonate, 100 U/ml penicillin, 100 μg/ml streptomycin and 2% FCS. After 24 hr, cells were again washed and media replaced as above with additional supplements of 20 μg/ml endothelial cell growth supplement and 95 μg/ml heparin. HUVEC/ monocyte cocultures were created through the addition of enriched monocytes (2 × 104/ml) to the HUVEC monolayers, giving a ratio of approximately one monocyte to five HUVEC. Monoculture controls were included in all experiments.
Quantification of cytokines by enzyme-linked immunosorbent assay (ELISA)
Cell free supernatants were collected and stored at −80° until use. Immunoreactive TNF-α, IL-1β and CXCL8 were quantified by ELISA using matched antibody pairs from R&D systems (Abingdon, UK). The detection limits were 78·125, 19·5, and 62·5 pg/ml respectively. Samples whose cytokine levels were undetectable were assigned the detection limit values for graphing and analysis.
Protein detection by Western blot analysis
Whole cell lysates were made by resuspending cells in lysis buffer (50 mm Tris base pH 7·5, 1% Triton-X-100, 50 mm NaF, 50 mmβ-glycerophosphate, 10 mm sodium orthovanadate) for 5 min at 4° before addition of 2× sodium dodecyl sulphate–polyacryalamide gel electrophoresis (SDS–PAGE) loading buffer. Lysates were heated to 95° for 5 min before being resolved by 10% SDS–PAGE and transferred to nitrocellulose membrane. Non-specific binding was blocked with 5% skimmed milk powder and membranes probed with Abs specific to actin (1 : 5000) or phosphorylated forms of ERK1/2 (1 : 1000) and p38 (1 : 1000) overnight. The antigen–antibody complexes were detected using horseradish peroxidase-conjugated anti-rabbit secondary antibody in conjunction with ECL substrate. Densitometry was carried out using NIH Image (version 1.62f).
Flow cytometric analysis of TLR expression
Staining and flow cytometry were carried out as previously described.25,28 Briefly, cells were washed in ice-cold fluorescence-activated cell sorting (FACS) buffer (10 mm phosphate-buffered saline (PBS) without Ca2+ and Mg2+, 10 mm HEPES and 0·25% bovine serum albumin) before non-specific binding was blocked with mouse IgG (50 μg/ml) and cells stained with anti-TLR4 (1 : 25 dilution) or isotype control for 45 min at 4°. Excess antibody was removed by washing, cells fixed in 1× CellFix™ (BD Biosciences, Franklin Lakes, NJ) and cytometry performed on a dual laser FACSCalibur using CellQuest software (BD Biosciences). Data were analysed using FlowJo software v8.5.3 (Tree Star Inc., Ashland, OR).
Statistics
All data are presented as mean ± SEM (where appropriate) of at least three independent experiments on separate donors. Data were analysed using the statistical tests stated, with anova and the indicated post-test being used for multiple comparisons. Data were analysed using Prism (version 4.03; GraphPad, San Diego, CA).
Results
ISO-1 is a recently described specific MIF antagonist that is active in vitro and in vivo.15–17 Previous studies have often focused on aspects of MIF biology in murine cells, lines, and knockouts. We therefore investigated the potential of ISO-1 to modulate TLR-specific responses in primary human monocytes, primary MDM, and simple coculture models of vascular inflammation.
ISO-1 pretreatment inhibits LPS-induced cytokine generation from primary human monocytes
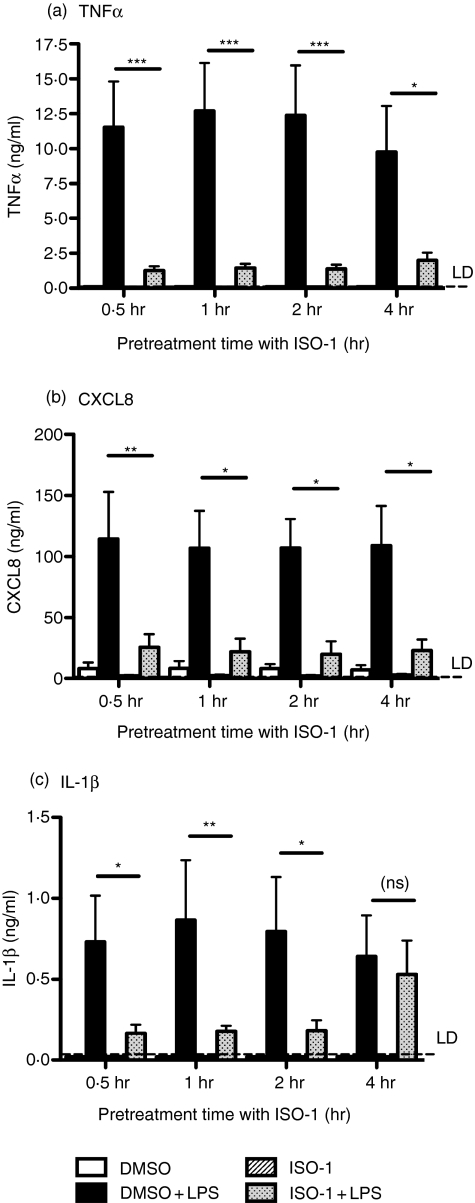
We first investigated the ability of ISO-1 to regulate LPS-induced cytokine release from primary human monocytes. Preliminary data obtained from the study of the THP-1 monocytic line indicated actions of ISO-1 might be transient (data not shown). Monocytes were therefore pretreated for varying lengths of time (≤4 hr) with ISO-1 prior to 24 hr of treatment with LPS, with ISO-1 remaining present throughout the LPS treatment. ISO-1 profoundly inhibited LPS-induced TNF-α, CXCL8, and IL-1β release from freshly-isolated monocytes (Fig. 1). Some temporal regulation of cytokine production was evident, since when the interval between addition of ISO-1 and stimulation of cells with LPS was 4 hr, IL-1β release was no longer inhibited, whilst LPS-induced production of TNF-α and CXCL8 remained blocked (Fig. 1). ISO-1 treatment did not cause monocyte cell death over the time course of these experiments (data not shown).
Figure 1.
TLR4-induced cytokine release in enriched monocytes is inhibited by ISO-1 pretreatment. Monocytes were enriched by negative magnetic selection from human peripheral blood and pretreated with ISO-1 (50 μm) or DMSO for the times shown, prior to addition of LPS (1 ng/ml) for a further 24 hr, with ISO-1 remaining present throughout the time course. Cell-free supernatants were collected and (a) TNF-α, (b) CXCL8, or (c) IL-1β content assayed by ELISA. Data are mean ± SEM from five to six separate experiments from different donors. Significant inhibition of cytokine release is indicated by *P < 0·05; **P < 0·01; ***P < 0·001, analysed by two-way anova with Bonferroni's post-test.
ISO-1 is without effect on primary human MDM
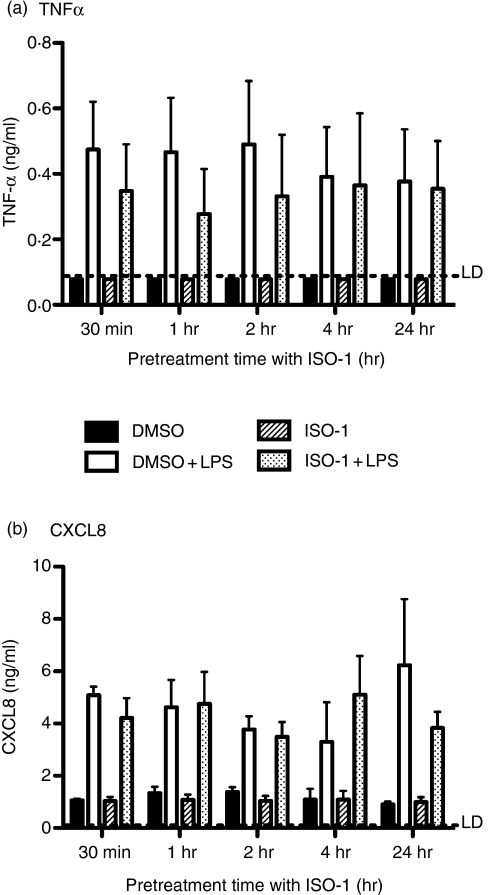
There is now substantial evidence that monocytic cell types can show variable responses to a range of stimuli according to lineage, activation status, and differentiation stage.29 Accordingly, we investigated the consequences of MIF antagonism on cytokine production from primary human MDM. In human MDM, ISO-1 had no significant effect on cytokine (TNF-α and CXCL8) release in response to LPS treatment (Fig. 2).
Figure 2.
ISO-1 has no significant effect on cytokine release from MDMs. Human monocyte-derived macrophages were pretreated with ISO-1 (50 μm) or DMSO for the times shown, prior to addition of LPS (1 ng/ml) for a further 24 hr, with ISO-1 remaining present throughout the time course. Cell free supernatants were collected and (a) TNF-α or (b) CXCL8 content assayed by ELISA. No significant inhibition of cytokine release by ISO-1 was found. Data are mean ± SEM from three separate experiments from different donors and analysed by two-way anova with Bonferroni's post-test.
ISO-1 does not regulate responses to LPS through modulation of TLR4 expression
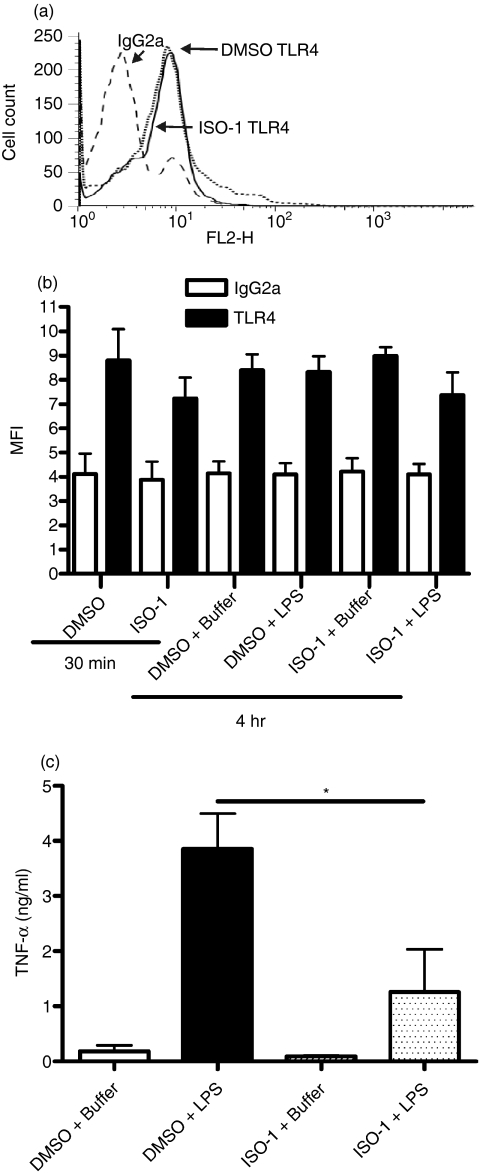
Whilst MIF has been reported to regulate LPS responses by controlling TLR4 cell surface expression via regulation of gene expression,23 the rapid effects of ISO-1 on LPS responses suggested a mechanism involving control of TLR signalling was more likely. We therefore conducted further experiments over shorter time courses to correlate regulation of signalling and TLR4 expression. ISO-1 treatment of primary monocytes for 0·5 or 4·5 hr did not alter TLR4 expression compared to the DMSO vehicle control, whether LPS was present or absent for the latter 4 hr (Fig. 3). However, monocytes released significantly less TNF-α 4 hr post-LPS when in the presence of ISO-1 (Fig. 3), demonstrating that the effect of ISO-1 on cytokine output was not caused by a change in cell-surface TLR4 expression.
Figure 3.
ISO-1 modulation of TNF-α production is not through control of TLR4 expression. Enriched human monocytes were pretreated with ISO-1 (50 μm) or DMSO vehicle control for 0·5 hr before addition of LPS (1 ng/ml) or media control for 4 hr, with ISO-1 remaining present throughout the time course. TLR4 expression was measured by flow cytometry either immediately after the 0·5 hr treatment with ISO-1 or DMSO, or after the addition of LPS or buffer for a further 4 hr. (a) A representative histogram of IgG2a control staining versus TLR4 staining in cells treated with vehicle control (DMSO) or ISO-1 for a total of 4·5 hr is shown. Mean data for four experiments are shown in panel (b). In panel (c), cell-free supernatants were collected after the 4 hr LPS/media treatment and TNFα content measured by ELISA. Data are mean ± SEM of four separate experiments from different donors. Significant differences are indicated by *P < 0·05 analysed by one-way anova with Tukey's multiple comparison post-test.
LPS-induced ERK1/2 MAPK activation is inhibited by ISO-1 in primary human monocytes
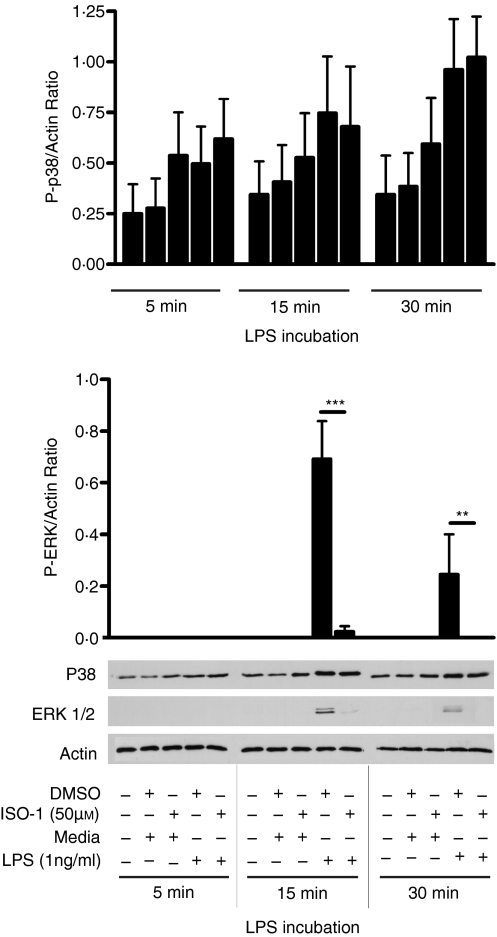
The data above indicated that the rapid ability of ISO-1 to inhibit LPS-induced TNF-α release was the result of the modulation of signalling events downstream of TLR4. MIF has been shown to regulate ERK signalling20 both through engagement of elements of a cell surface receptor complex17–19 and by binding to an intracellular partner, JAB1/CSN5, which exerts multiple actions on ERK.21,22 Accordingly, further experiments determined whether ISO-1 treatment caused inhibition of LPS-induced ERK activation. In primary, freshly-isolated monocytes, ERK1/2 activation peaked 15 min after LPS treatment, and ISO-1 significantly inhibited this response (Fig. 4). The actions of ISO-1 were specific to the ERK pathway, because ISO-1 pretreatment did not modulate activation of p38 MAPK (Fig. 4) at any tested time in these cells.
Figure 4.
LPS induced ERK activation is inhibited by ISO-1. Enriched monocytes were untreated or treated with ISO-1 (50 μm) or DMSO for 0·5 hr followed by LPS (1 ng/ml) for times shown, with ISO-1 remaining present throughout the time course. Whole cell lysates were analysed by western blot using Abs specific to the phosphorylated forms of p38 or ERK1/2. Blots were densitometrically analysed and normalized to β-actin to control for loading. Data are presented as mean ± SEM of four separate experiments from different donors with representative western blots shown below. Significant decrease in activation by ISO-1 treatment is shown by ***P < 0·001 analysed by two-way anova with Bonferroni's post-test.
Inhibition of TLR signalling by ISO-1 is in part mimicked by inhibitors of ERK
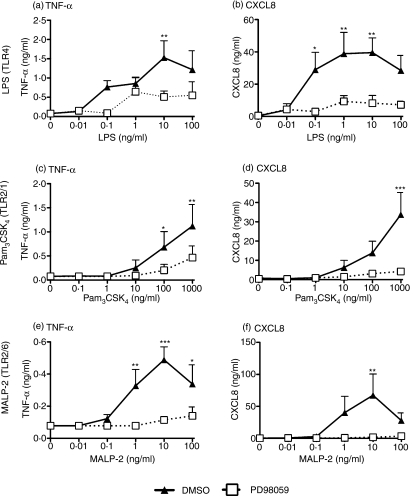
In further experiments, we sought to compare the actions of ISO-1 with well-recognized inhibitors of ERK signalling (Fig. 5), and also studied the activation of monocytes by TLR2 agonists, using Pam3CSK4 (which activates TLR2/1 heterodimers) and MALP-2 (which activates TLR2/6 heterodimers). Inhibition of ERK by PD98059 inhibited TNF-α and CXCL8 production induced by LPS or TLR2 agonists. A non-inhibiting control molecule, SB202474, failed to inhibit TLR signalling (data not shown), whilst another ERK inhibitor, U0126, showed equal and similar efficacy to PD98059 (data not shown).
Figure 5.
Inhibition of TLR signalling by inhibitors of ERK. Enriched human monocytes were pretreated with PD98059 (50 μg/ml) or DMSO vehicle control for 0·5 hr prior to the addition of LPS (a & b), Pam3CSK4 (c & d), or MALP-2 (e & f) at the concentrations indicated for a further 24 hr. Cell-free supernatants were collected and TNF-α (a, c, e) or CXCL8 (b, d, f) content assayed by ELISA. Data are mean ± SEM from three to four separate experiments from different donors. Significant inhibition of cytokine release is indicated by *P < 0·05; **P < 0·01; ***P < 0·001; analysed by two-way anova with Bonferroni's post-test.
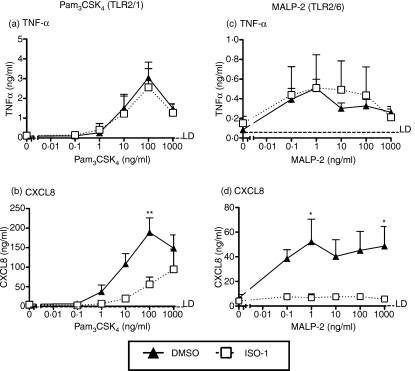
Signalling events induced by agonists of TLR2 are only partially inhibited by ISO-1 pretreatment
Previous reports have primarily considered the effects of manipulating MIF on TLR4 expression and function. The marked effects of ISO-1 on ERK signalling, and the evidence that an ERK inhibitor, PD98059, also inhibited TLR2 responses in monocytes, led us to determine whether ISO-1 would also affect signalling of TLR2. Similarly to effects on LPS activation, production of CXCL8 by both TLR2 agonists was inhibited by ISO-1 pretreatment (Fig. 6). In contrast, production of TNF-α in response to these agonists was not inhibited by ISO-1 (Fig. 6).
Figure 6.
TLR2-induced cytokine release in enriched monocytes is in part inhibited by ISO-1 pretreatment. Monocytes were enriched by negative magnetic selection from human peripheral blood and pretreated with ISO-1 (50 μm) or DMSO for 1 hr, prior to addition of Pam3CSK4 (a & b) or MALP-2 (c & d) at the concentrations indicated for a further 24 hr, with ISO-1 remaining present throughout the time course. Cell-free supernatants were collected and TNF-α (a & c) or CXCL8 (b & d) production determined by ELISA. Data are mean ± SEM from four to five separate experiments from different donors. Significant inhibition of cytokine release is indicated by *P < 0·05; **P < 0·01; analysed by two-way anova with Bonferroni's post-test.
ISO-1 inhibits cytokine production in a model of vascular inflammation
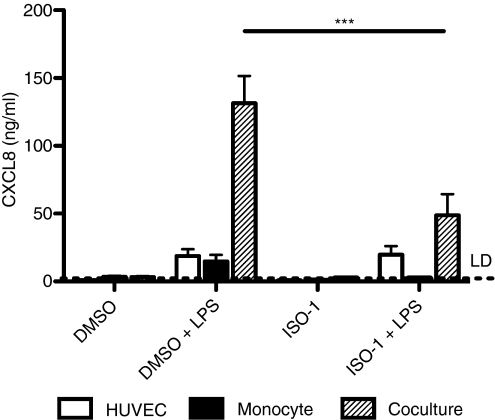
We have recently shown that responses to TLR agonists such as LPS can be most effectively generated when leucocytes and tissue cells have the opportunity to co-operate. We have shown that activation of monocytes by LPS results in production of mediators, including IL-1β, that cause secondary activation of tissue cells and marked amplification of proinflammatory cytokine production.30,31 These systems have the potential to contribute substantially to vascular inflammation.30 We therefore established cocultures of primary HUVEC and monocytes and stimulated these with LPS in the presence or absence of ISO-1.
In these cocultures, monocytes were added at much lower numbers than in the studies above examining responses of monocytes alone (see Materials and methods). Thus, relatively little CXCL8 was produced from the wells containing monocytes alone, but when in coculture with HUVEC, there was a marked upregulation of CXCL8 production in keeping with other work from our group (manuscript in preparation).30 We found that pretreatment with ISO-1 for 1 hr caused a significant reduction in CXCL8 production from cocultures (Fig. 7), showing that this prototypic inhibitor might exert clinically important effects during acute periods of inflammation, reducing the risk of vascular inflammation and atherosclerotic plaque rupture or disease progression.
Figure 7.
ISO-1 inhibits HUVEC/monocyte co-culture responses to LPS. Cocultures were prepared as described in Materials and methods and pretreated with ISO-1 (50 μm) or DMSO vehicle control for 1 hour prior to addition of LPS (1 ng/ml) for 24 hr, with ISO-1 remaining present throughout the time course. Cell-free supernatants were collected and CXCL8 content assayed by ELISA. Data are mean ± SEM from three separate experiments. Significant inhibition of cytokine release is indicated by ***P < 0·001, analysed by two-way anova with Bonferroni's post-test.
Discussion
Targeting of MIF has the potential to be of considerable therapeutic benefit in septic and proinflammatory conditions, and a recent description of a MIF-specific inhibitor, ISO-1, has brought clinical targeting nearer.15–17 To date, much of our understanding of MIF biology has been derived from mouse models and systems, and data obtained from cell lines. Murine monocytic cells have been identified as important producers and targets of MIF,32 and recent studies have shown that human dendritic cells may be sources of MIF, and may produce more MIF when isolated from patients with inflammatory diseases such as rheumatoid arthritis.33
Accordingly, we sought to explore the actions of ISO-1 on acute responses of primary human monocytic cells. In this study, we have revealed that ISO-1 inhibits responses of human monocytes, but not MDM, to TLR4 agonists, and such regulation occurs independently of control of TLR4 expression. Furthermore, we have shown that responses of monocytes to TLR2 agonists are also in part impaired. These actions of ISO-1 showed partial equivalence to those of specific inhibitors of ERK, consistent with the potential for MIF to regulate ERK activation.
In initial experiments, we found that the recently-identified MIF-specific inhibitor, ISO-1,16 caused a striking suppression of LPS-induced proinflammatory cytokine production in primary human monocytes. We therefore investigated the mechanisms involved in the inhibition of TLR signalling by ISO-1. In MIF−/− mice, impaired TLR signalling has been linked to loss of TLR4 expression.23 Preservation of selected aspects of the LPS response has, however, been observed in MIF−/− mice by some, though not all studies,11,23,34 suggesting that TLR4 function may be maintained in some settings. We also observed that ISO-1 was effective even when added only very shortly before the TLR agonist, strongly suggesting that actions of ISO-1 were unlikely to be mediated by control of TLR4 expression. When directly tested, our data showed that TNF-α production could be suppressed by ISO-1 in these cells even when TLR4 expression was maintained. Furthermore, impairment of TLR responses extended to an ability of ISO-1 to impair production of CXCL8, but not TNF-α, in response to TLR2 agonists, suggesting regulation of TLR signalling by this molecule.
TLR2 and TLR4 use signalling pathways that have many common features with that of the IL-1R, and it is interesting to note that fibroblasts from MIF−/− mice also show impaired responses to IL-1, though whether this is mediated by regulation of intracellular signalling or effects on IL-1R expression remains unclear.35 Similarly, induction of fibroblast proliferation by IL-1 is inhibited by neutralization of MIF.36 Our data strongly implied that TLR responses must be regulated by ISO-1 at an intracellular level, and identify a broader interaction with innate immune signals than was previously known. TLR2 and TLR4 both couple to MyD88 and Mal to initiate signalling (with TLR4 additionally coupling to TRIF and TRAM), and the differences in actions of ISO-1 on TNF-α production induced by activation of these receptors suggested that the points of regulation would lie below these adapters.
Signalling pathways of MIF are complex. It can activate CXCL8 production in fibroblasts via nuclear factor-κB and tyrosine-kinase dependent signalling,37 and contributes to the regulation of fibroblast proliferation via ERK signalling.36 Various data have also described MIF signalling via a pathway involving activation of Ras/Raf/MEK/ERK,20,38 whilst in monocytic cell lines regulation of the p38 MAPK pathway by MIF is mediated through regulation of MAPK phosphatase-1 (MKP-1).39 MIF binds to an intracellular partner, JAB1/CSN5, which exerts both positive and negative regulation of ERK signalling.22 Accordingly, we compared the actions of ISO-1 with two well-characterized inhibitors of ERK, PD98059 and U0126. We observed that PD98059 also inhibited TLR signalling in freshly-isolated human monocytes, although unlike ISO-1, it was able to inhibit TNF-α production in response to MALP-2 and Pam3CSK4. The mechanisms underpinning these differences between ISO-1 and PD98059 actions are not clear and are currently the subject of ongoing work in our laboratory. Other groups have also shown that inhibition of ERK leads to reduced TNF-α expression in human monocytic cells stimulated with LPS.40,41 We directly tested the hypothesis that ISO-1 inhibited TLR-induced activation of ERK by examination of MAPK phosphorylation patterns. We found that, in freshly-isolated primary human monocytes, ISO-1 inhibited LPS-induced ERK1/2 MAPK activation but did not inhibit activation of p38 MAPK. These data are in keeping with a hypothesis that, in monocytes, MIF regulation of ERK signalling may be as a high-level permissive regulator of TLR4 signalling. Alternatively, despite the specific binding of ISO-1 to MIF,16 and the failure of ISO-1 to inhibit LPS-induced TNF-α production in MIF−/− mice,16 our data might be revealing an off-target but relatively selective inhibition of ERK in human cells by ISO-1, a possibility we in part addressed by examining responses of MDM.
In contrast to the results observed in primary monocytes, we observed that cytokine release from primary MDM was unaffected by exposure to ISO-1 over the time course tested. These cells show induction of TNF-α production in response to LPS that has been shown to be inhibited by PD98059.41 Thus, if ISO-1 were exerting its actions exclusively through off-target effects on ERK signalling, inhibition of TNF-α production by MDM may also have been expected. Existing data from other studies predicts that prolonged ISO-1 exposure may result in a later phase of regulation of TLR4 expression.23,34 ISO-1 could therefore still exert activity on these cells through regulation of TLR4, or indeed through the modification of downstream signalling molecules via control of MKP-1 expression,39 but over a different time course to that observed in the monocytes. Such variations in responses to ISO-1 between primary human cells, coupled with evidence noted above describing different potential pathways by which MIF might interact with signalling pathways, reinforce the need to study MIF biology, and the role of new candidate inhibitors, in relevant primary cells.
Treatment with ISO-1 or derivatives might therefore exert complex effects on the local immune response. We have shown previously that responses to LPS are most effectively mediated by cooperative signalling between leucocytes and tissue cells.30,31 In these settings, low numbers of monocytes can act upon tissue cells to amplify their responses significantly: evidence from in vivo models of lung inflammation suggest that these models are biologically significant.42 We observed that treatment of cocultures of endothelial cells and monocytes with ISO-1 resulted in a dramatic inhibition of coculture activation as determined by CXCL8 production. Our previous observations show that IL-1 production by LPS-activated monocytes is important for the activation of tissue cells in cocultures.30,31 In this study, we have shown that ISO-1 pretreatment delays IL-1 production from LPS-activated monocytes, and thus the decreased activation of LPS-stimulated cocultures which have also been treated with ISO-1 may well be predominantly by inhibition of IL-1β production. Thus, ISO-1 may have important roles in regulating the production of mediators directly involved in leucocyte recruitment (such as CXCL8), and also mediators acting to amplify inflammatory responses (such as IL-1β). These data demonstrate that the ability of ISO-1 to inhibit activation of TLR signalling may result in a rapid control of inflammatory responses in the vasculature and other tissues, where inflammation involves monocytes.
In conclusion, we have defined a novel mechanism by which the inhibitor, ISO-1, appears to regulate inflammatory responses to TLR agonists such as LPS. This involves regulation of ERK signalling in a manner potentially consistent with the MIF signalling pathway, but the possibility of off-target effects of ISO-1 on ERK activation need to be borne in mind. This mechanism shows variations between monocytic cells of different differentiation state and perhaps between species, implying that the therapeutic use of small molecule inhibitors based on this prototypic drug will require careful thought with respect to the model system utilized and the length of exposure to the drug.
Acknowledgments
We would like to thank Dr Kathryn Vaughan and Ms Emily Dick for preparation of the primary human monocytes, and Professors Steven K. Dower and Moira K. Whyte for helpful discussions.
P.W.W. is funded by a British Heart Foundation studentship (ref: FS/05/031/19423). J.R.W. is funded by a British Heart Foundation grant (ref: FS/06/004). I.S. is funded by a Medical Research Council Senior Clinical Fellowship (ref: G116/170), which also supports L.C.P.
References
- 1.Burger-Kentischer A, Gobel H, Kleemann R, et al. Reduction of the aortic inflammatory response in spontaneous atherosclerosis by blockade of macrophage migration inhibitory factor (MIF) Atherosclerosis. 2006;184:28–38. doi: 10.1016/j.atherosclerosis.2005.03.028. [DOI] [PubMed] [Google Scholar]
- 2.Leech M, Metz C, Hall P, et al. Macrophage migration inhibitory factor in rheumatoid arthritis: evidence of proinflammatory function and regulation by glucocorticoids. Arthritis Rheum. 1999;42:1601–8. doi: 10.1002/1529-0131(199908)42:8<1601::AID-ANR6>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 3.Morand EF, Bucala R, Leech M. Macrophage migration inhibitory factor: an emerging therapeutic target in rheumatoid arthritis. Arthritis Rheum. 2003;48:291–9. doi: 10.1002/art.10728. [DOI] [PubMed] [Google Scholar]
- 4.Donnelly SC, Haslett C, Reid PT, Grant IS, Wallace WA, Metz CN, Bruce LJ, Bucala R. Regulatory role for macrophage migration inhibitory factor in acute respiratory distress syndrome. Nat Med. 1997;3:320–3. doi: 10.1038/nm0397-320. [DOI] [PubMed] [Google Scholar]
- 5.Mizue Y, Ghani S, Leng L, et al. Role for macrophage migration inhibitory factor in asthma. Proc Natl Acad Sci U S A. 2005;102:14410–5. doi: 10.1073/pnas.0507189102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Magalhaes ES, Mourao-Sa DS, Vieira-de-Abreu A, et al. Macrophage migration inhibitory factor is essential for allergic asthma but not for Th2 differentiation. Eur J Immunol. 2007;37:1097–106. doi: 10.1002/eji.200635968. [DOI] [PubMed] [Google Scholar]
- 7.Rossi AG, Haslett C, Hirani N, Greening AP, Rahman I, Metz CN, Bucala R, Donnelly SC. Human circulating eosinophils secrete macrophage migration inhibitory factor (MIF). Potential role in asthma. J Clin Invest. 1998;101:2869–74. doi: 10.1172/JCI1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernhagen J, Calandra T, Mitchell RA, et al. MIF is a pituitary-derived cytokine that potentiates lethal endotoxaemia. Nature. 1993;365:756–9. doi: 10.1038/365756a0. [DOI] [PubMed] [Google Scholar]
- 9.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–95. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 10.Kudrin A, Scott M, Martin S, et al. Human macrophage migration inhibitory factor (MIF): A proven immunomodulatory cytokine? J Biol Chem. 2006;281:29641–51. doi: 10.1074/jbc.M601103200. [DOI] [PubMed] [Google Scholar]
- 11.Bozza M, Satoskar AR, Lin G, Lu B, Humbles AA, Gerard C, David JR. Targeted disruption of migration inhibitory factor gene reveals its critical role in sepsis. J Exp Med. 1999;189:341–6. doi: 10.1084/jem.189.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calandra T, Echtenacher B, Roy DL, et al. Protection from septic shock by neutralization of macrophage migration inhibitory factor. Nat Med. 2000;6:164–70. doi: 10.1038/72262. [DOI] [PubMed] [Google Scholar]
- 13.Burger-Kentischer A, Goebel H, Seiler R, et al. Expression of macrophage migration inhibitory factor in different stages of human atherosclerosis. Circulation. 2002;105:1561–6. doi: 10.1161/01.cir.0000012942.49244.82. [DOI] [PubMed] [Google Scholar]
- 14.Kong YZ, Huang XR, Ouyang X, et al. Evidence for vascular macrophage migration inhibitory factor in destabilization of human atherosclerotic plaques. Cardiovasc Res. 2005;65:272–82. doi: 10.1016/j.cardiores.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 15.Lubetsky JB, Dios A, Han J, Aljabari B, Ruzsicska B, Mitchell R, Lolis E, Al-Abed Y. The tautomerase active site of macrophage migration inhibitory factor is a potential target for discovery of novel anti-inflammatory agents. J Biol Chem. 2002;277:24976–82. doi: 10.1074/jbc.M203220200. [DOI] [PubMed] [Google Scholar]
- 16.Al-Abed Y, Dabideen D, Aljabari B, et al. ISO-1 binding to the tautomerase active site of MIF inhibits its pro-inflammatory activity and increases survival in severe sepsis. J Biol Chem. 2005;280:36541–4. doi: 10.1074/jbc.C500243200. [DOI] [PubMed] [Google Scholar]
- 17.Meyer-Siegler KL, Iczkowski KA, Leng L, Bucala R, Vera PL. Inhibition of macrophage migration inhibitory factor or its receptor (CD74) attenuates growth and invasion of DU-145 prostate cancer cells. J Immunol. 2006;177:8730–9. doi: 10.4049/jimmunol.177.12.8730. [DOI] [PubMed] [Google Scholar]
- 18.Shi X, Leng L, Wang T, et al. CD44 is the signaling component of the macrophage migration inhibitory factor-CD74 receptor complex. Immunity. 2006;25:595–606. doi: 10.1016/j.immuni.2006.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bernhagen J, Krohn R, Lue H, et al. MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat Med. 2007;13:587–96. doi: 10.1038/nm1567. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell RA, Metz CN, Peng T, Bucala R. Sustained mitogen-activated protein kinase (MAPK) and cytoplasmic phospholipase A2 activation by macrophage migration inhibitory factor (MIF). Potential role in asthma. J Biol Chem. 1999;274:18100–6. doi: 10.1074/jbc.274.25.18100. [DOI] [PubMed] [Google Scholar]
- 21.Lue H, Kapurniotu A, Fingerle-Rowson G, et al. Rapid and transient activation of the ERK MAPK signalling pathway by macrophage migration inhibitory factor (MIF) and dependence on JAB1/CSN5 and Src kinase activity. Cell Signal. 2006;18:688–703. doi: 10.1016/j.cellsig.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 22.Lue H, Thiele M, Franz J, et al. Macrophage migration inhibitory factor (MIF) promotes cell survival by activation of the Akt pathway and role for CSN5/JAB1 in the control of autocrine MIF activity. Oncogene. 2007;26:5046–59. doi: 10.1038/sj.onc.1210318. [DOI] [PubMed] [Google Scholar]
- 23.Roger T, David J, Glauser MP, Calandra T. MIF regulates innate immune responses through modulation of Toll-like receptor 4. Nature. 2001;414:920–4. doi: 10.1038/414920a. [DOI] [PubMed] [Google Scholar]
- 24.Medvedev AE, Henneke P, Schromm A, Lien E, Ingalls R, Fenton MJ, Golenbock DT, Vogel SN. Induction of tolerance to lipopolysaccharide and mycobacterial components in Chinese hamster ovary/CD14 cells is not affected by overexpression of Toll-like receptors 2 or 4. J Immunol. 2001;167:2257–67. doi: 10.4049/jimmunol.167.4.2257. [DOI] [PubMed] [Google Scholar]
- 25.Sabroe I, Jones EC, Usher LR, Whyte MKB, Dower SK. Toll-like receptor (TLR)2 and TLR4 in human peripheral blood granulocytes: a critical role for monocytes in leukocyte lipopolysaccharide responses. J Immunol. 2002;168:4701–10. doi: 10.4049/jimmunol.168.9.4701. [DOI] [PubMed] [Google Scholar]
- 26.Visintin A, Mazzoni A, Spitzer JH, Wyllie DH, Dower SK, Segal DM. Regulation of Toll-like receptors in human monocytes and dendritic cells. J Immunol. 2001;166:249–55. doi: 10.4049/jimmunol.166.1.249. [DOI] [PubMed] [Google Scholar]
- 27.Sabroe I, Prince LR, Jones EC, Horsburgh MJ, Foster SJ, Vogel SN, Dower SK, Whyte MKB. Selective roles for Toll-like receptor (TLR)2 and TLR4 in the regulation of neutrophil activation and life span. J Immunol. 2003;170:5268–75. doi: 10.4049/jimmunol.170.10.5268. [DOI] [PubMed] [Google Scholar]
- 28.Parker LC, Whyte MKB, Vogel SN, Dower SK, Sabroe I. Toll-like receptor (TLR)2 and TLR4 agonists regulate CCR expression in human monocytic cells. J Immunol. 2004;172:4977–86. doi: 10.4049/jimmunol.172.8.4977. [DOI] [PubMed] [Google Scholar]
- 29.Stout RD, Suttles J. Functional plasticity of macrophages: reversible adaptation to changing microenvironments. J Leukoc Biol. 2004;76:509–13. doi: 10.1189/jlb.0504272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morris GE, Parker LC, Ward JR, et al. Cooperative molecular and cellular networks regulate Toll-like receptor-dependent inflammatory responses. FASEB J. 2006;20:2153–5. doi: 10.1096/fj.06-5910fje. [DOI] [PubMed] [Google Scholar]
- 31.Morris GE, Whyte MKB, Martin GF, Jose PJ, Dower SK, Sabroe I. Agonists of Toll-like receptors 2 and 4 activate airway smooth muscle via mononuclear leukocytes. Am J Respir Crit Care Med. 2005;171:814–22. doi: 10.1164/rccm.200403-406OC. [DOI] [PubMed] [Google Scholar]
- 32.Calandra T, Bernhagen J, Mitchell RA, Bucala R. The macrophage is an important and previously unrecognized source of macrophage migration inhibitory factor. J Exp Med. 1994;179:1895–902. doi: 10.1084/jem.179.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Popa C, van Lieshout AW, Roelofs MF, Geurts-Moespot A, van Riel PL, Calandra T, Sweep FC, Radstake TR. MIF production by dendritic cells is differentially regulated by Toll-like receptors and increased during rheumatoid arthritis. Cytokine. 2006;36:51–6. doi: 10.1016/j.cyto.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 34.Roger T, Froidevaux C, Martin C, Calandra T. Macrophage migration inhibitory factor (MIF) regulates host responses to endotoxin through modulation of Toll-like receptor 4 (TLR4) J Endotoxin Res. 2003;9:119–23. doi: 10.1179/096805103125001513. [DOI] [PubMed] [Google Scholar]
- 35.Toh ML, Aeberli D, Lacey D, et al. Regulation of IL-1 and TNF receptor expression and function by endogenous macrophage migration inhibitory factor. J Immunol. 2006;177:4818–25. doi: 10.4049/jimmunol.177.7.4818. [DOI] [PubMed] [Google Scholar]
- 36.Lacey D, Sampey A, Mitchell R, Bucala R, Santos L, Leech M, Morand E. Control of fibroblast-like synoviocyte proliferation by macrophage migration inhibitory factor. Arthritis Rheum. 2003;48:103–9. doi: 10.1002/art.10733. [DOI] [PubMed] [Google Scholar]
- 37.Onodera S, Nishihira J, Koyama Y, Majima T, Aoki Y, Ichiyama H, Ishibashi T, Minami A. Macrophage migration inhibitory factor up-regulates the expression of interleukin-8 messenger RNA in synovial fibroblasts of rheumatoid arthritis patients: common transcriptional regulatory mechanism between interleukin-8 and interleukin-1beta. Arthritis Rheum. 2004;50:1437–47. doi: 10.1002/art.20190. [DOI] [PubMed] [Google Scholar]
- 38.Onodera S, Nishihira J, Iwabuchi K, Koyama Y, Yoshida K, Tanaka S, Minami A. Macrophage migration inhibitory factor up-regulates matrix metalloproteinase-9 and -13 in rat osteoblasts. Relevance to intracellular signaling pathways. J Biol Chem. 2002;277:7865–74. doi: 10.1074/jbc.M106020200. [DOI] [PubMed] [Google Scholar]
- 39.Roger T, Chanson AL, Knaup-Reymond M, Calandra T. Macrophage migration inhibitory factor promotes innate immune responses by suppressing glucocorticoid-induced expression of mitogen-activated protein kinase phosphatase-1. Eur J Immunol. 2005;35:3405–13. doi: 10.1002/eji.200535413. [DOI] [PubMed] [Google Scholar]
- 40.Guha M, O’Connell MA, Pawlinski R, Hollis A, McGovern P, Yan SF, Stern D, Mackman N. Lipopolysaccharide activation of the MEK-ERK1/2 pathway in human monocytic cells mediates tissue factor and tumor necrosis factor alpha expression by inducing Elk-1 phosphorylation and Egr-1 expression. Blood. 2001;98:1429–39. doi: 10.1182/blood.v98.5.1429. [DOI] [PubMed] [Google Scholar]
- 41.MacKenzie S, Fernandez-Troy N, Espel E. Post-transcriptional regulation of TNF-alpha during in vitro differentiation of human monocytes/macrophages in primary culture. J Leukoc Biol. 2002;71:1026–32. [PubMed] [Google Scholar]
- 42.Hollingsworth JW, Chen BJ, Brass DM, Berman K, Gunn MD, Cook DN, Schwartz DA. The critical role of hematopoietic cells in lipopolysaccharide induced airway inflammation. Am J Respir Crit Care Med. 2005;171:806–13. doi: 10.1164/rccm.200407-953OC. [DOI] [PubMed] [Google Scholar]