Abstract
The early pathogen–macrophage interactions that help drive macrophage maturation towards classically or alternatively activated are largely unknown. To examine this question we utilized the immunomodulatory glycan Lacto-N-fucopentaose III (LNFPIII), which contains the Lewis X (LeX) trisaccharide, to activate murine peritoneal macrophages in vivo. Because LNFPIII is known to induce anti-inflammatory responses, we asked if LNFPIII stimulation of macrophages in vivo initiates alternative activation events such as upregulation of Arginase 1, Ym1, FIZZ-1, MGL-1 or macrophage mannose receptor (MMR). Examination of peritoneal exudate cells from mice 20 hr post-LNFPIII injection demonstrated increased Arginase 1 activity, at the mRNA and protein levels, coincident with undetectable inducible nitric oxide synthase expression or nitric oxide production. In addition to Arginase 1, Ym1 expression was also significantly upregulated at 20 and 48 hr after LNFPIII exposure in vivo. However, the expression of FIZZ-1, MGL-1, and MMR was not changed in these macrophages. In an attempt to determine activation requirements for functional activity, we adoptively transferred antigen-pulsed, in vivo LNFPIII activated macrophages into naïve recipients and found that they were capable of triggering recipient T cells to secrete elevated levels of interleukin (IL)-10 and IL-13 compared to mice receiving control macrophages. Together, these data demonstrate that upregulation of expression of Arginase 1 and Ym1 occur very early in activation of macrophages, and can be independent of other alternatively activated (AA) macrophage markers. Importantly, these early events appear to be IL-4/IL-13-independent in our model. In the future we hope to determine if upregulation of these initial AA maturational events is sufficient for these macrophages to exert immunoregulatory activity in vivo.
Keywords: arginase 1, LNFPIII, macrophages
Introduction
Macrophages play significant roles in innate and adaptive immune responses to pathogens. Macrophages can be either classically activated (CAMphs) via signals from interferon-γ (IFN-γ) and interactions with pathogens, or alternatively activated (AAMphs) by interleukin (IL)-4/IL-13 plus pathogen interaction. AAMphs are involved in resolution of inflammation.1–4 Thus, macrophages exhibit significant phenotypic plasticity to control immunological balance in the microenvironment. CAMph are well characterized via expression of inducible nitric oxide synthase (iNOS) and production of oxygen and nitrogen radicals in response to IFN-γ in combination with tumour necrosis factor (TNF) or stimuli that induce TNF.5 AAMphs have been observed in human placenta, in pregnancy, in the lung, and during parasitic and fungal infection.6–14 The presence of AAMph is often associated with T helper 2 (Th2) immune responses to antigen and characterized by upregulation of Arginase 1 expression coincident with downregulation of iNOS expression with respective product release.15 Recently, a third population of macrophages, type II-activated macrophages (II-Mph), activated in the presence of immune complexes and producing IL-2 and IL-10 were described.5 Thus, knowing which type of macrophage phenotype to expand, as well as identifying early activation events that drive expansion of these various types of macrophages, is important in terms of development of vaccines or therapeutic applications for various diseases. Activation plasticity of macrophages has a critical physiological role and might be useful for immunotherapy in the fields of autoimmune diseases and cancer.15–17 Several markers/receptors associated with AA macrophages have been described and include Arginase 1, FIZZ-1, Ym1, MGL-1, and macrophage mannose receptor (MMR).3,7,18–20 Interestingly, these AAMph markers are not each consistently expressed on AAMphs, and their expression appears to be partially dependent on the actual disease, the stage of disease, or differential expression at different sites of diseased mice.10,11,21 Because there is no established definition of which markers constitute an AAMph phenotype, it is still unclear if two or more markers must exist on these macrophages to be considered AAMph.
Lacto-N-fucopentaose III (LNFPIII) is an immunomodulatory glycan containing the Lewis X (LeX) trisaccharide. Expression of LNFPIII/Lewis X has been associated with immunosuppressive states, being found in breast milk, the urine of pregnant women, on the trophoblast, on various tumours and during numerous parasitic and viral infections.22–29 Previously, we demonstrated that LNFPIII injection induced the expansion of peritoneal macrophages capable of suppressing naïve T-cell proliferation in vitro.30 Further, administration of LNFPIII was shown to prevent development of psoriatic-like lesions in fsn/fsn mice in vivo.31 Understanding the initial activation events that bestow functionality as well as the role(s) alternatively activated macrophages play in vivo are important questions to investigate if the goal of various therapies is to induce their activation. Currently, little is known regarding the early activation events as well as the minimal maturational changes required to bestow functional AAMph activity in vivo.
In this study, we demonstrate that the immunomodulatory glycan LNFPIII rapidly upregulated the expression of Arginase 1 and Ym1 on macrophages. Upregulation of these markers was IL-4/IL-13 independent, as LNFPIII was able to upregulate these markers on macrophages from IL-4Rα knockout (KO) mice. Interestingly, while LNFPIII activated macrophages did not show upregulation of other AAMph markers, FIZZ-1, MGL-1 and MMR 20 hr after LNFPIII injection, adoptive transfer of these macrophages into ovalbumin (OVA)-specific DO.11.10 mice induced naïve T cells to produce significantly elevated levels of IL-10 and IL-13, demonstrating that expression of these other AAMph associated molecules were not required for AAMph function in vivo.
Materials and methods
Mice and injection
Six to 8-week-old female BALB/c, DO.11.10 and IL-4Rαtm1Sz/J KO mice were purchased from Jackson Laboratory (Bar Harbor, ME) and maintained under specific pathogen-free conditions at the animal facility of the Harvard School of Public Health. LNFPIII and the carrier molecule dextran were provided by Neose Technologies Inc., Horsham, PA. The neo-glycoconjugate LNFPIII-dex was prepared by Dr Thomas Norberg. The neo-glycoconjugate consisted on average of eight LNFPIII (853·8 MW) molecules/40 × 103 MW molecule of dextran (Dex). Mice were injected intraperitoneally (i.p.) with 50 μg of LNFPIII-dex or Dex in phosphate-buffered saline.
Cells and cell culture
Peritoneal exudate cells (PEC) were collected as previously described30 20 hr post intraperitoneal injection with LNFPIII-dex or Dex. Collected cells were washed in RPMI-1640 medium supplemented with 10% fetal bovine serum (Hyclone, Logan, UT), 100 U/ml penicillin, 100 μg/ml streptomycin (Sigma, St. Louis, MO), 0·05 mm 2-mercaptoethanol, and 2 mm glutamine (Gibco, Grand Island, NY). Adherent PEC were harvested after 2 hr incubation at 37° in six-well plates and used as peritoneal macrophages.
Adoptive transfer
Groups of mice were injected with 50 μg/ml of Dex or LNFPIII-dex for 20 hr. PEC were isolated, pooled and cultured for 1 hr with 1 μm OVA peptide (OVA223–239 peptide; Sigma-Genosys, Woodlands, TX). Adherent cells were collected and 1 million cells in 200 μl were injected i.p. in two groups of four DO.11.10 mice. Splenocytes from individual recipient mice 5–6 days post-transfer were isolated and plated in the presence of OVA peptide (100 and 1000 nm) for 72 hr. Supernatants from cultured cells were harvested and enzyme-linked immunosorbent assays (ELISA) assays were performed according the manufacturer's protocols (R&D systems, Minneapolis, MN) for each cytokine: IFN-γ, IL-4, IL-10, IL-13 and transforming growth factor-β (TGF-β).
Fluorescence-activated cell sorting (FACS) staining
Cells were stained for 30 min at 4° using conventional protocols. Cells were incubated with anti-Fc-γR antibody (clone 2.4G2) (1 μg/106 cells), and then stained with following antibodies: fluoroscein isothiocyanate (FITC)/antigen-presenting cell (APC)-conjugated F4/80 antibody (Serotec), programmed death ligand 1 (PD-L1)-phycoerythrin (PE), inducible costimulator ligand (ICOS-L)-PE, CD80-PE, CD86-PE, A-I/A-E-PE, intracellular adhesion molecule-1 (ICAM-1)-PE (BD BioSciences, San Jose, CA).
Arginase activity
Arginase activity in cell lysates of PEC was measured based on the conversion of l-arginine to l-ornithine and urea according to the technique described by Corraliza et al., 1994.32 Briefly, cells were lysed for 30 min with 40 μl of 0·1% Triton-X-100. Thirty μl of 25 mm Tris-HCl, pH 7·4 and 10 μl of 10 mm MnCl2 were added, and the enzyme was heat-activated for 10 min at 56°. Similar amounts of sample (40 μl) and 0·5 m l-arginine (pH 9·7) were mixed and incubated for 1 hr at 37°. The reaction was stopped with 400 μl of H2SO4 (96%), H3PO4 (85%), H2O (1/3/7, v/v/v). The urea concentration was measured at 540 nm after the addition of 8 μl of α-isonitropropiophenone, followed by heating at 95° for 20 min. One unit of enzyme activity is defined as the amount of enzyme that catalyses the formation of 1 μmol urea per 1 hr.
Reverse transcription–polymerase chain reaction (RT–PCR) assay
RNA was purified using TRIzol Reagent (Invitrogen, Carlsbad, CA) according to manufacturer's instructions. One μg of total RNA from each sample was used to synthesize cDNA using the ThermoScript Kit (Invitrogen). Gene expression of Arginase 1, iNOS and β-actin was determined via multiplex RT–PCR using the primer pairs listed in Table 1. Expression levels of YM1, FIZZ1, MMR, MGL-1 and β-actin were determined by standard RT–PCR using primers listed in Table 1. Genes were amplified using the AccuPrime Taq DNA polymerase (Invitrogen) for 35 cycles (95° for 1 min, 55° for 1 min and 68° for 1 min) which were proceeded with 10 min incubation at 68°. PCR products were separated onto 2% agarose gels, images were acquired using Kodak Image Station 440FC, and band intensities were determined using the associated Kodak software. Band intensities were normalized to β-actin, and fold increase/decrease in gene expression over the control (uninjected) was calculated.
Table 1.
Invitrogen primers
| Primers | Primer sequence | Product size (bp) | |
|---|---|---|---|
| β-Actin | S | 5′-TGG AAT CCT GTG GCA TCC ATG AAA C-3′ | 349 |
| AS | 5′-TAA AAC GCA GCT CAG TAA CAG TCC G-3′ | ||
| MGL-1 | S | 5′-ATG ATG TCT GCC AGA GAA CC-3′ | 252 |
| AS | 5′-ATC ACA GAT TTC AGC AAC CTT A-3′ | ||
| MMR | S | 5′-GCA AAT GGA GCC GTC TGT GC-3′ | 280 |
| AS | 5′-CTC GTG GAT CTC CGT GAC AC-3′ | ||
| iNOS | S | 5′-CCC TTC CGA AGT TTC TGG CAG C-3′ | 497 |
| AS | 5′-GGC TGT CAG AGC CTC GTG GCT TTG G-3′ | ||
| Arginase 1 | S | 5′-ATG GAA GAG ACC TTC AGC TAC-3′ | 220 |
| AS | 5′-GCT GTC TTC CCA AGA GTT GGG-3′ | ||
| FIZZ-1 | S | 5′-TCC CAG TGA ATA CTG ATG AGA-3′ | 213 |
| AS | 5′-CCA CTC TGG ATC TCC CAA GA-3′ | ||
| Ym1 | S | 5′-GGG CAT ACC TTT ATC CTG AG-3′ | 305 |
| AS | 5′-CCA CTG AAG TCA TCC ATG TC-3′ | ||
S, sense; AS, antisense.
Quantitative RT–PCR
RNA was purified using RNeasy Kit according to the manufacturer's instructions (Qiagen, Valencia, CA). cDNA was synthesized using 1 μg of RNA and iScript Kit according to manufacturer's instructions (Bio-Rad, Hercules, CA). Real-time PCR primers for Arginase 1, Ym1, FIZZ-1, and reduced glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were purchased from Applied Biosystems (Foster City, CA). Real-time PCR was conducted with the ABI 7300 sequence detection system using TaqMan Master Mix (Applied Biosystems) following manufacturer's instructions. For data analysis, the comparative threshold cycle (Ct) value for GAPDH was used to normalize loading variations in the real-time PCRs. A ΔΔCt value was then obtained by subtracting control ΔCt values from the corresponding experimental ΔCt. The ΔΔCt values were converted to fold difference compared with the control by raising two to the ΔΔCt power.
Western blotting
Freshly isolated macrophages were lysed with 1% Triton-X-100 buffer. Samples (20 μg protein/lane) were subjected to electrophoresis in 12% sodium dodecyl sulphate–polyacrylamide gels, and then blotted onto 0·45-μm polyvinylidene difluoride membranes. Membranes were blocked for 1 hr at room temperature with 5% dry skimmed milk in Tris-buffered saline (20 mm Tris-HCl, pH 7·6, 137 mm NaCl plus 0·05% (v/v) Tween 20) and then probed with rabbit anti-Arginase 1 antibody (1 : 1000; BD-Pharmingen) overnight at 4°. Membranes were washed and incubated for 2 hr at room temperature with secondary goat anti-rabbit antibody conjugated with horseradish peroxidase (1 : 2000; Cell Signaling). Loading controls were determined by stripping the same membrane and reprobing with anti-extracellular signal-related kinase (ERK) antibody. Results were visualized by chemiluminescence detection using SuperSignal West Pico Chemiluminescent System (Pierce, Rockford, CA).
Statistical analysis
Statistical significance of differences among groups was determined by Student's t-test.
Results
Arginase 1 expression and activity of PEC from LNFPIII-injected mice
Previous studies documented that a single intraperitoneal injection of LNFPIII-dex for 20 hr was sufficient to induce the expansion of F4/80+ cells capable of suppressing naïve T-cell proliferation in response to anti-CD3/CD28 stimulation in vitro.30 In this study, we decided to further analyse both phenotypically and functionally, the peritoneal macrophages activated via LNFPIII-dex injection. We initially investigated the release of products of activated enzymes iNOS and Arginase 1 as they share l-arginine as a common substrate and are differentially expressed in CA versus AAMphs.
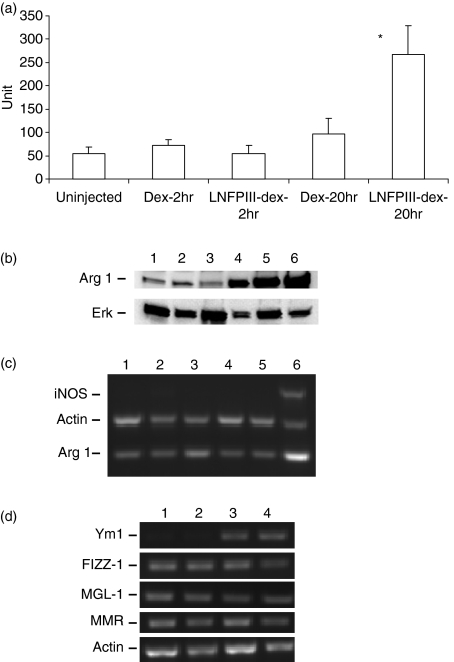
Adherent PEC collected 2 hr after LNFPIII-dex or Dex injection showed low levels of Arginase 1 activity. However, at 20 hr post-injection, while Arginase 1 remained low in PEC from Dex injected mice, the Arginase 1 activity of PEC from LNFPIII-Dex injected mice was significantly increased (P < 0·001) (Fig. 1a). Furthermore, Western blot analysis showed an upregulation of Arginase 1 in PEC from mice injected with LNFPIII-dex for 20 hr (Fig. 1b). On the other hand, nitric oxides were not detectable in the media of PEC obtained from LNFPIII-dex or Dex injected mice cultured for 24–48 hr (data not shown).
Figure 1.
LNFPIII-dex upregulates Arginase 1 and Ym1 of PEC in vivo. Groups of five to six mice were left uninjected or injected with 50 μg of Dex or LNFPIII-dex. Mice were killed at indicated time points and peritoneal macrophages were isolated. (a) Arginase 1 activity was determined in PEC lysates from all mice as described in Materials and methods. One unit of enzyme activity is defined as the amount of enzyme that catalyzes the formation of 1 μmol urea per 1 hr. All data of four experiments are summarized and expressed as the mean ± SD. A statistical significance between groups was determined by Student's t-test (P < 0·001), and significant differences are indicated with asterisk. (b) Western blot analysis of Arginase 1 in PEC obtained from uninjected mice (lane 1), 2 hr Dex-injected mice (lane 2), 2 hr LNFPIII-dex injected mice (lane 3), 20 hr LNFPIII-dex injected mice (lane 4). PEC stimulated with 20 ng/ml rmIL-4 (lane 5) and 1 μg/ml lipopolysaccharide (LPS) + 20 ng/ml IFN-γ (lane 6) for 20 hr were used as positive controls. The same blot was stripped and reprobed with anti-ERK antibody. (c) Expression levels of Arginase 1 and iNOS were determined by multiplex RT–PCR for PEC from uninjected mice (lane 1), mice injected with Dex for 20 hr (lane 2) or 48 hr (lane 4), LNFPIII-dex for 20 (lane 3), or LNFPIII-dex for 48 hr (lane 5), positive control represents the PEC stimulated with LPS + IFN-γ (lane 6). (d) Gene expression of Ym1, FIZZ1, MMR and MGL-1 in PEC from unjected mice (lane 1), injected with 50 μg/ml of Dex (lane 2), LNFPIII-dex for 20 hr (lane 3) or LNFPIII-dex for 48 hr (lane 4) was determined by RT–PCR. The RT–PCR figures are representative of three independent experiments.
Because at 2 hr after LNFPIII-dex injection we did not find any differences in Arginase 1 expression but did see Arginase 1 activity increased at 20 hr, we decided to determine Arginase 1 and iNOS expression at 20 and 48 hr after LNFPIII-dex injection. Gene expression of Arginase 1 and iNOS was analysed by multiplex RT–PCR analysis using gene specific primers for β-actin (as an endogenous control for normalization) iNOS and Arginase 1. We found upregulation of Arginase 1 expression at 20 hr after LNFPIII-dex mice injection and no iNOS expression at any time points (Fig. 1c).
AAmacrophage markers expression following LNFPIII-dex stimulation
Upregulation of Arginase 1 in LNFPIII-treated macrophages led us to further investigate LNFPIII-elicited macrophages in regards to other markers associated with alternatively activated macrophages. Besides Arginase 1, the expression of genes coding for MMR (CD206), MGL-1, FIZZ-1, and Ym1 have been shown to be upregulated in AAMPh during parasite infections.3,18,33–36
We analysed expression of the genes encoding MGL-1, MMR, FIZZ-1 and Ym1 by RT–PCR. We found that similar to Arginase 1, Ym1 expression was significantly upregulated 20 hr after LNFPIII injection. Ym1 expression was continuously upregulated at 48 hr after LNFPIII-dex injection (Fig. 1d). As compared with Dex-injected mice, MGL-1, FIZZ-1, and MMR mRNA expression was not increased in LNFPIII-stimulated macrophages neither at 20 nor at 48 hr after injection.
Taken together, these data indicate that peritoneal macrophages from LNFPIII-dex injected mice demonstrate a unique phenotype we would associate with AAMph.
Upregulation of maturation markers on macrophages after LNFPIII-dex injection
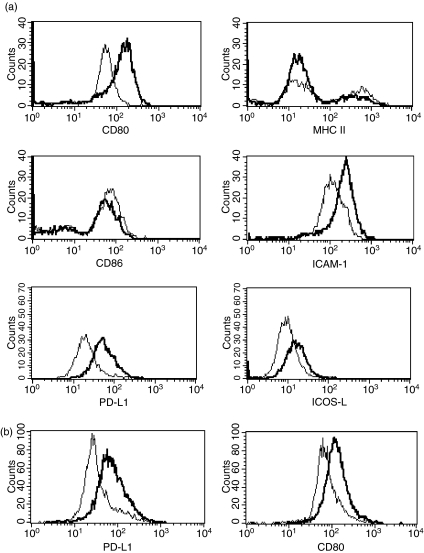
To examine the ability of LNFPIII-dex to induce maturation of PEC, we injected BALB/c mice with 50 μg of LNFPIII-dex or Dex for 20 hr. PEC were stained as described in Materials and methods for CD80, CD86, major histocompatibility complex (MHC) class II and ICAM-1 and analysed by FACS. FACS analysis showed that CD80 and ICAM-1 were upregulated on PEC from LNFPIII-dex injected mice compared to PEC from Dex-injected mice (Fig. 2). MHC class II and CD86 were not altered after LNFPIII-dex stimulation. These data clearly show that LNFPIII-dex induces macrophage activation via selective upregulation of maturation markers CD80 and ICAM-1.
Figure 2.
Selective induction of maturation markers by LNFPIII-dex injection. Groups of four mice were injected with 50 μg/ml of Dex or LNFPIII-dex for 20 hr (a) and 48 hr (b). PEC were isolated and stained for F4/80, CD80, CD86, MHC II, ICAM-1, PD-L1 and ICOS-L. Expression of costimulatory molecules was analysed for gated F4/80+ cells. The thin line depicts the expression of surface markers from PEC of Dex injected mice and the thick line depicts the expression from LNFPIII-dex injected mice. At least three separate experiments with similar results were performed.
In addition to CD80 and CD86, the co-stimulatory molecules PD-L1, PD-L2 and ICOS-L have been reported to play a significant role in macrophage function in schistosome infections.37–39 Characterization of peritoneal cells 20 hr after LNFPIII-dex injection revealed that the numbers of F4/80+ cells co-expressing PD-L1 but not PD-L2 was constantly increased on PEC from LNFPIII injected mice compared to Dex-injected mice. Further, we also detected upregulation of ICOS-L on PEC from LNFPIII-dex injected macrophages in each of four experiments (Fig. 2a). CD80 and PD-L1 expression were similarly elevated on macrophages 48 hr after LNFPIII-dex injection (Fig. 2b). These data strongly indicate that LNFPIII-dex induces macrophage activation with specific upregulation of costimulatory molecules and selective markers of APC activation.
Functional activity of LNFPIII-dex stimulated macrophages
Finally, because our data showed that LNFPIII-dex injection initiated alternative macrophage activation, we asked if LNFPIII-dex activated macrophages would display function in vivo. Prior to performing adoptive transfer experiments we tested the spontaneous production of cytokines from in vivo LNFPIII-dex activated macrophages by plating them in vitro for 48 hr. Interestingly high level of TGF-β was detected in all macrophage cultures (control and LNFPIII-dex activated). Further, the concentrations of CCL-17 and IL-10 were below the detectable levels (data not shown). Thus, there was no distinct LNFPIII induced cytokine/chemokine profile based on the mediators we assayed for.
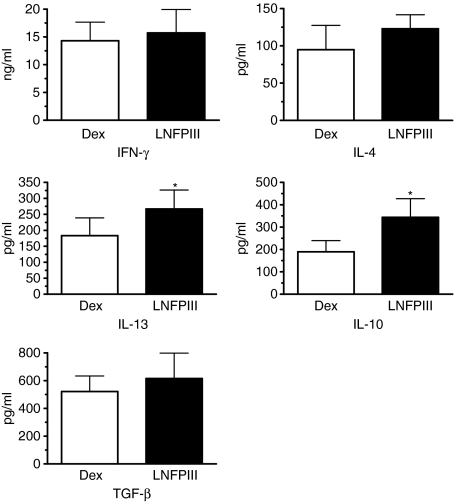
To determine if LNFIII-dex activated macrophages were capable of mediating immunomodulatory capacity, we performed adoptive transfer experiments using DO.11.10 OVA T-cell receptor transgenic mice. PEC from LNFPIII-dex or Dex-injected mice were harvested, pulsed with OVA peptide for 1 hr and then adherent cells were adoptively transferred by injection into the peritoneal cavity of DO.11.10 mice. Five to 6 days post adoptive transfer splenocytes were harvested and analysed for OVA peptide-specific cytokine responses. As shown in Fig. 3, adoptive transfer of LNFPIII activated PEC induced a different antigen-specific recall response as compared to mice that received Dex-activated macrophages. In each of four separate experiments, we found that secretion of IL-10 and IL-13 was significantly increased in mice that received LNFPIII-dex macrophages. We also observed that secretion of IL-4 was increased in each of the four experiments, but that increase though consistent, was statistically not significant. Surprisingly, IFN-γ secretion was not significantly decreased in splenocyte cultures from mice that received LNFPIII-dex activated PEC but was at the same level as in control group. Thus, the ratio of IL-13/IFN-γ in the supernatants of splenocytes from mice injected with LNFPIII-treated macrophages was higher than in mice injected with Dex-treated macrophages. It is important to mention that proliferation of isolated T cells from spleens of both groups was not altered, indicating that adoptively transferred macrophages did not suppress the proliferative capacity of recipient T cells.
Figure 3.
Functional activity of LNFPIII-dex stimulated macrophages in vivo. Groups of four to six mice were injected with 50 μg/ml of Dex or LNFPIII-dex for 20 hr. PEC were isolated, pooled and plated for 1 hr with 1000 nm of OVA peptide (OVA323–339). Adherent cells were collected and 1 million cells were injected i.p. to groups of four DO.11.10 mice. In 5–6 days splenocytes from individual mice were isolated and plated in the presence of OVA peptide (100 and 1000 nm) for 72 hr. Cytokines were measured by ELISA. Four independent experiments were performed. Summarized data are shown as mean ± SD. A statistical significance between groups was determined by Student's t-test (P < 0·05), and significant differences are indicated with an asterisk.
Role of IL-4 and IL-13 on the ability of LNFPIII to activate macrophages
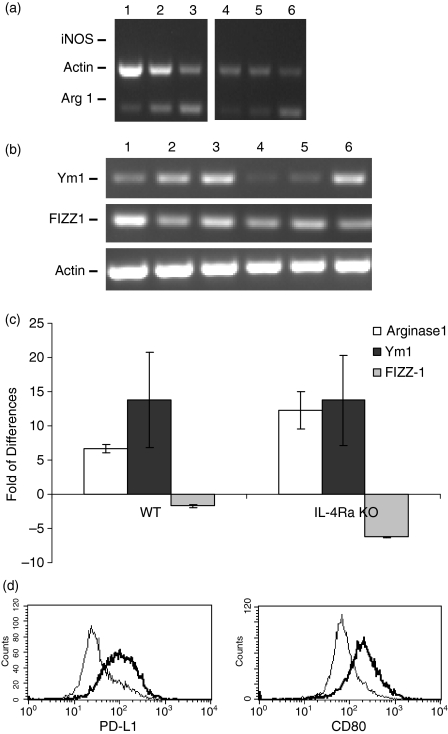
It has been reported that the appearance of AAMph was IL-4/IL-13 dependent.7,40,41 To verify the dependence of Arginase 1 and Ym1 expression on the presence of IL-4 and IL-13 we utilized IL-4Rα−/− mice, which are deficient in IL-4 and IL-13 signalling.42,43 We found that i.p.injection of LNFPIII-dex upregulated the expression of both Arginase 1 and Ym1 on PEC from IL-4Rα−/−, and wild type mice to similar levels (Fig. 4a,b). To confirm these data, we performed a quantitative real-time PCR (qRT–PCR) analysis. qRT–PCR demonstrated that LNFPIII injection significantly increased the expression of both Arginase 1 and Ym1 on PEC from both wild type (BALB/c) and IL-4Rα−/− mice (Fig. 4c). Arginase 1 expression was 6·6- and 9·5-fold higher (wild type and IL-4Rα KO, respectively) than in the control (uninjected) while Ym1 was 13-fold higher in both strains of mice. Thus, no significant differences were observed in the ability of LNFPIII to upregulate Arginase 1 or Ym1 between wild type and IL-4Rα KO mice. In both strains LNFPIII-dex injection resulted in a slight decrease in FIZZ-1 expression. These data show that in our experimental conditions the ability of LNFPIII-Dex to upregulate Arginase 1 and Ym1 expression on macrophages was IL-4/IL-13-independent. Based on this data we also examined whether IL-4/IL-13 were required for LNFPIII mediated upregulation of co-stimulatory molecules. Similar to wild type mice (Fig. 3), we detected upregulated expression of PD-L1 and CD80 on the surfaces of LNFPIII activated macrophages (Fig. 4d) from IL-4RαΚΟ mice.
Figure 4.
Role of IL-4 and IL-13 on LNFPIII-elicited macrophages. Groups of wild type mice (WT) or IL-4Rα KO mice were left unjected (lane 1, WT; lane 4 KO), injected with 50 μg/ml of Dex (lane 2, WT; lane 5 KO), LNFPIII-dex for 20 hr (lane 3, WT; lane 6 KO). Total RNA was isolated using TRIzol reagent, and gene expression was determined by RT–PCR for iNOS and Argiase 1 (a), and Ym1 and FIZZ-1 (b). (a) and (b) RT-PCR figures are representative of at least three independent experiments. (c) Real-time PCR was performed for Arginase 1, Ym1, and FIZZ-1 expression in PECs from wild type and IL-4Rα KO mice. Total RNA was isolated using Qiagen RNeasy Kit. GAPDH was used to normalize loading. Results of two independent experiments are summarized and presented as a fold of differences over the control (uninjected). Data are shown as mean ± standard error. (d) PEC from the same mice were stained for F4/80, CD80 and PD-L1. Expression of CD80 and PD-L1 was analysed for gated F4/80+ cells. The thin line depicts the expression of surface markers from PEC of Dex-injected mice and the thick line depicts the expression from LNFPIII-dex injected mice. The results for one representative of two independent experiments are shown.
Discussion
Macrophages are important innate immune cells whose activation status can directly or indirectly influence the ensuing adaptive immune responses to pathogens. Depending on the nature of the disease or the pathogen macrophages exhibit a plasticity that allows them to respond in a pro-inflammatory or anti-inflammatory manner.17 For example, during the early and acute phases of Schistosoma mansoni infection, macrophages produce pro-inflammatory mediators and drive CD4+ T cells towards the Th1 type. Once eggs begin to be deposited in tissues, the phenotype of tissue macrophages switches to alternatively activated and these macrophages either regulate T cells or drive them towards the Th2 type.44,45 LNFPIII is an immunomodulatory pentasaccharide which contains the Lewis X trisaccharide, and is found in schistosome soluble egg antigen. LNFPIII was previously shown to induce the expansion of F4/80+ Gr1+ macrophages with immune suppressive capacity in the peritoneal cavities of otherwise naive mice.30 However, our previous study did not thoroughly examine the phenotype of LNFPIII stimulated macrophages or their in vivo effect on T cells. To answer these questions, we initially examined the activation phenotype of peritoneal macrophages 20 hr post-injection of LNFPIII-dex neo-glycoconjugates. Our initial observation indicated that 2 hr post-injection was insufficient time for LNFPIII to have induced measurable effects on peritoneal macrophages. However, by 20 hr post-injection we found that LNFPIII-dex stimulated macrophages had significantly upregulated expression and activity of Arginase 1 as well as expression of Ym1, both markers of alternatively activated macrophages. Examination of other alternative activation markers demonstrated that LNFPIII-dex stimulation did not induce upregulated expression of FIZZ-1, MGL-1 or MMR. This finding suggests that as soon as 20 hr post-injection LNFPIII-dex stimulation was driving peritoneal macrophages to develop towards AAMphs. LNFPIII induced upregulation of Arginase 1 is in agreement with results of other investigators studying Arginase activity in S. mansoni infected mice.46 During S. mansoni infection of mice granulomas form around tissue-trapped eggs and this is accompanied by production of IL-13.47,48 High levels of IL-13 in addition to IL-4 can drive macrophage maturation towards alternatively activated, including induction of Arginase 1 expression.7,40,41,49 The innate source of IL-4 and IL-13 at an early time after infection, and presumably 20 hr after LNFPIII-dex injection, could be eosinophils and mast cells.50,51 However, Arginase 1 upregulation has been shown to occur independent of IL-4/IL-13 expression in Trypanosoma congolense-infected mice.21 To rule out the possible influence of both IL-4 and IL-13 in macrophage activation 20 hr after LNFPIII-dex injection we used IL-4Rα−/− mice in our experiments. Interestingly, our data clearly show that the ability of LNFPIII-dex to induce upregulation of Arginase 1 and Ym1 expression is independent of IL-4 and IL-13.
It is known that LNFPIII binds to at least three different C type lectins,52,53 and leads to alternative nuclear factor-κB activation.54 Binding of LNFPIII to C type lectins on the surface of macrophages may induce the upregulation of Arginase 1 and Ym1 directly without the need of IL-4/IL-13. Further, it is possible that in vivo injection of LNFPIII induces the production of mediators such as IL-10 by other cells in the peritoneum that may play a role in macrophage activation. However, at this time, the exact mechanism by which LNFPIII leads to alternative activation of macrophages is unknown.
Schistosoma mansoni eggs trapped in the liver induce fibrosis. During S. mansoni infection AAMph play a role in tissue remodelling and fibrosis34 and are associated with production of proline.55,56 Thus, tissue-trapped eggs, which secrete glycolipids and glycoproteins or injection with the immunomodulatory glycan LNFPIII, both induce macrophages to upregulate Arginase 1 expression and suggests that egg glycans are involved in in vivo response to infection. Macrophages obtained from S. mansoni-infected mice or LNFPIII-dex injected mice have similar surface phenotypes and suppressor activity. Thus, we expected similar to macrophages from infected mice macrophages from LNFPIII-injected mice will upregulate expression of AAMph markers Ym1 and FIZZ-1. Ym1 and FIZZ-1 expression is upregulated on macrophages during chronic helminth infections.34,35,49 Interestingly, Loke et al.7 recently found that surgical trauma leads to rapid elevation of both of these markers. In our study we found that LNFPIII-dex stimulation only upregulated Ym1 expression at 20–48 hr post-injection. Although LNFPIII did not upregulate FIZZ-1 by 20–48 hr post-injection, similar to filarial infection, S. mansoni infection has been shown to upregulate both Ym1 and FIZZ-1 expression (our unpublished observations and34). Examining AAMphs in schistosome-infected mice, Herbert et al.57 concluded that alternative macrophage activation was not required for granuloma formation during acute schistosomiasis, but that they were essential for downregulation of pro-inflammatory Th1 immune responses and survival. Our results show that a single injection of LNFPIII-dex does drive peritoneal macrophages to initiate alternative activation, but is insufficient to completely mimic the changes to macrophages that occur during S. mansoni infection.
In contrast to Arginase 1 and Ym1 which expression was upregulated at 20 and 48 hr post-injection of LNFPIII-dex, other markers of AAMph FIZZ-1, MGL-1 and MMR were expressed on peritoneal macrophages but were not upregulated by LNFPIII-dex at different time points. Thus, similar to the results with Arginase 1, the finding that Ym1 is rapidly upregulated after LNFPIII stimulation supports the observation that LNFPIII-dex is driving peritoneal macrophages towards development of AAMphs.
An exciting aspect of this study was the observation that LNFPIII-dex stimulated macrophages were functional in terms of their ability to adoptively transfer an antigen-specific Th2-type bias to recipient mice. Twenty hr post-injection of LNFPIII-dex, peritoneal macrophages were isolated and pulsed with OVA peptide then transferred into naïve DO11.10 transgenic mice. Splenic CD4+ T cells from mice that received OVA-pulsed LNFPIII-dex activated macrophages secreted significantly higher amounts of IL-13 and IL-10 in response to OVA peptide in vitro. Though not significant, we also found the level of IL-4 upregulated in each of the four experiments. We do not know at this time how LNFPIII-dex activated macrophages are modulating CD4+ T-cell antigen-specific cytokine production in recipient mice. However, it is possible that in addition to cell surface co-stimulatory molecule expression, macrophage soluble factors also play a role. In order to identify such factors, we are currently performing microarray analysis on LNFPIII-activated macrophages.
Further regarding functionality of LNFPIII-dex activated macrophages, our data show that transferred macrophages migrated from the peritoneal cavity to the spleens where they regulated OVA-specific T-cell secretory capacity. Macrophages are known to traffic to the draining lymph nodes to prime T-cell responses.58,59 Interestingly, the proliferation of cultured splenocytes from recipient mice was not suppressed by transferred LNFPIII-dex activated macrophages but rather the same as mice which received Dex stimulated macrophages, showing that neither LNFPIII-dex activated or control stimulated macrophages had T-cell suppressive activity in vivo.
The selective expression of co-stimulatory molecules on LNFPIII-dex activated macrophages might also explain the in vivo regulatory activity of these cells on antigen-specific CD4+ T-cell cytokine production. Similar to the data presented here showing that LNFPIII-dex stimulation leads to upregulation of PD-L1, the study by Smith et al., showed that F4/80+ cells from S. mansoni infected mice were PD-L1-positive but PD-L2-negative.37 The function of PD-L1/PD-1 ligation is still unclear. On one hand it has been suggested that PD-L1 may transduce negative signals to inhibit autoreactive T-cell responses,60–62 yet a clear role for PD-L1 and PD-L2 in driving stimulatory or inhibitory functions on macrophages and dendritic cells is still controversial.63–66 In schistosome-infected mice, Smith et al. suggested that PD-L1/PD-1 interactions may synergize with other inhibitory pathways to regulate the induction of T-cell non-responsiveness.37 ICOS is another co-stimulatory molecule that has been suggested to play a role in downregulating pro-inflammatory responses in schistosome infection. Rutitzky et al.38 demonstrated the protective role of ICOS-B7RP-1 co-stimulatory pathway in hepatic immunopathology by controlling levels of IFN-γ. Supporting a role for ICOS in downregulating pro-inflammatory responses, Xia et al.39 found stronger Th2 immune responses in ICOS transgenic mice infected with S. japonicum. While we did not examine function of co-stimulatory molecules on LNFPIII-dex activated macrophages we did show that ICOS-L was upregulated on PEC from mice injected with LNFPIII-dex. Taken together, we showed that injection of LNFPIII-dex upregulated expression of both PD-L1 and ICOS-L, two co-stimulatory molecules associated with down regulation of pro-inflammatory responses and/or enhanced Th2-type responses in schistosome infection. Our FACS analysis also showed upregulation of ICAM-1 and CD80 on LNFPIII-dex activated macrophages. In agreement with previous work we suggest that enhanced expression of co-stimulatory molecules such as CD80 and ICAM-1 can counterbalance the negative signals of PD-L1.66–68 Similar induction of B7-H1, B7-DC and CD80 inhibitory molecules on splenic DC in vivo and bone-marrow-derived dentritic cells from tumour-bearing mice has been recently described.69 These data together with our observations lead us to consider that LNFPIII can induce the appearance of inhibitory macrophages similar to those induced during cancer, which is consistent with our previous findings.30 Although investigation of PD-1-PD-L1 and ICOS-ICOSL co-stimulatory pathways was not the goal of this study, the observation that these molecules are upregulated suggest that additional studies be performed to examine the roles of these two molecules in regulation of T-cell functions in vivo.
In summary, our data clearly demonstrates that LNFPIII-dex is able to rapidly activate and induce phenotypic changes in peritoneal macrophages that are consistent with alternative activation. LNFPIII-dex activated macrophages were characterized by increased expression of Arginase 1 and Ym1 without upregulation of MGL-1, MMR, and FIZZ-1. This induction of Arginase 1 and Ym1 expression by LNFPIII-dex was IL-4/IL-13 independent. LNFPIII-dex activated macrophages were able to significantly change the antigen-specific T-cell immune response to promote a Th2 response (IL-10, IL-13 and IL-4 secretion) in vivo. Thus, LNFPIII-dex stimulated macrophages play an important role in downregulation of the Th1 immune response and maintenance of Th2 responses in vivo.
Acknowledgments
This work was supported by the National Institute of Health (grant 5RO1AI056484), Juvenile Diabetes Research Foundation (grant 5-2007-455) and Swiss National Science Foundation (grant PBBEB-108556) for Mirjam Walker.
Abbreviations
- LNFPIII
lacto-N-fucopentaose III
- LeX
Lewis X, AA, alternatively activated
References
- 1.Nathan C, Shiloh MU. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc Natl Acad Sci U S A. 2000;97:8841. doi: 10.1073/pnas.97.16.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stain SC, Baer HU, Dennison AR, Blumgart LH. Current management of hilar cholangiocarcinoma. Surg Gynecol Obstet. 1992;175:579. [PubMed] [Google Scholar]
- 3.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 4.Anthony RM, Urban JF, Jr, Alem F, Hamed HA, Rozo CT, Boucher JL, Van Rooijen N, Gause WC. Memory T(H)2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nat Med. 2006;12:955. doi: 10.1038/nm1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edwards JP, Zhang X, Frauwirth KA, Mosser DM. Biochemical and functional characterization of three activated macrophage populations. J Leukoc Biol. 2006;80:1298. doi: 10.1189/jlb.0406249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loke P, MacDonald AS, Robb A, Maizels RM, Allen JE. Alternatively activated macrophages induced by nematode infection inhibit proliferation via cell-to-cell contact. Eur J Immunol. 2000;30:2669. doi: 10.1002/1521-4141(200009)30:9<2669::AID-IMMU2669>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 7.Loke P, Gallagher I, Nair MG, Zang X, Brombacher F, Mohrs M, Allison JP, Allen JE. Alternative activation is an innate response to injury that requires CD4+ T cells to be sustained during chronic infection. J Immunol. 2007;179:3926. doi: 10.4049/jimmunol.179.6.3926. [DOI] [PubMed] [Google Scholar]
- 8.Motran CC, Diaz FL, Montes CL, Bocco JL, Gruppi A. In vivo expression of recombinant pregnancy-specific glycoprotein 1a induces alternative activation of monocytes and enhances Th2-type immune response. Eur J Immunol. 2003;33:3007. doi: 10.1002/eji.200323993. [DOI] [PubMed] [Google Scholar]
- 9.Taylor MD, Harris A, Nair MG, Maizels RM, Allen JE. F4/80+ alternatively activated macrophages control CD4+ T cell hyporesponsiveness at sites peripheral to filarial infection. J Immunol. 2006;176:6918. doi: 10.4049/jimmunol.176.11.6918. [DOI] [PubMed] [Google Scholar]
- 10.Donnelly S, O’Neill SM, Sekiya M, Mulcahy G, Dalton JP. Thioredoxin peroxidase secreted by Fasciola hepatica induces the alternative activation of macrophages. Infect Immun. 2005;73:166. doi: 10.1128/IAI.73.1.166-173.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reece JJ, Siracusa MC, Scott AL. Innate immune responses to lung-stage helminth infection induce alternatively activated alveolar macrophages. Infect Immun. 2006;74:4970. doi: 10.1128/IAI.00687-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Terrazas LI, Montero D, Terrazas CA, Reyes JL, Rodriguez-Sosa M. Role of the programmed Death-1 pathway in the suppressive activity of alternatively activated macrophages in experimental cysticercosis. Int J Parasitol. 2005;35:1349. doi: 10.1016/j.ijpara.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 13.Holcomb IN, Kabakoff RC, Chan B, et al. FIZZ1, a novel cysteine-rich secreted protein associated with pulmonary inflammation, defines a new gene family. EMBO J. 2000;19:4046. doi: 10.1093/emboj/19.15.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arora S, Hernandez Y, Erb-Downward JR, McDonald RA, Toews GB, Huffnagle GB. Role of IFN-gamma in regulating T2 immunity and the development of alternatively activated macrophages during allergic bronchopulmonary mycosis. J Immunol. 2005;174:6346. doi: 10.4049/jimmunol.174.10.6346. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, Van Ginderachter JA, Brys L, De Baetselier P, Raes G, Geldhof AB. Nitric oxide-independent CTL suppression during tumor progression: association with arginase-producing (M2) myeloid cells. J Immunol. 2003;170:5064. doi: 10.4049/jimmunol.170.10.5064. [DOI] [PubMed] [Google Scholar]
- 16.Sinha P, Clements VK, Ostrand-Rosenberg S. Interleukin-13-regulated M2 macrophages in combination with myeloid suppressor cells block immune surveillance against metastasis. Cancer Res. 2005;65:11743. doi: 10.1158/0008-5472.CAN-05-0045. [DOI] [PubMed] [Google Scholar]
- 17.Porcheray F, Viaud S, Rimaniol AC, Leone C, Samah B, Dereuddre-Bosquet N, Dormont D, Gras G. Macrophage activation switching: an asset for the resolution of inflammation. Clin Exp Immunol. 2005;142:481. doi: 10.1111/j.1365-2249.2005.02934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raes G, Noel W, Beschin A, Brys L, de Baetselier P, Hassanzadeh GH. FIZZ1 and Ym as tools to discriminate between differentially activated macrophages. Dev Immunol. 2002;9:151. doi: 10.1080/1044667031000137629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raes G, Brys L, Dahal BK, et al. Macrophage galactose-type C-type lectins as novel markers for alternatively activated macrophages elicited by parasitic infections and allergic airway inflammation. J Leukoc Biol. 2005;77:321. doi: 10.1189/jlb.0304212. [DOI] [PubMed] [Google Scholar]
- 20.Mokoena T, Gordon S. Human macrophage activation. Modulation of mannosyl, fucosyl receptor activity in vitro by lymphokines, gamma and alpha interferons, and dexamethasone. J Clin Invest. 1985;75:624. doi: 10.1172/JCI111740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noel W, Hassanzadeh G, Raes G, et al. Infection stage-dependent modulation of macrophage activation in Trypanosoma congolense-resistant and -susceptible mice. Infect Immun. 2002;70:6180. doi: 10.1128/IAI.70.11.6180-6187.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erney R, Hilty M, Pickering L, Ruiz-Palacios G, Prieto P. Human milk oligosaccharides: a novel method provides insight into human genetics. Adv Exp Med Biol. 2001;501:285. [PubMed] [Google Scholar]
- 23.Jeschke U, Stahn R, Goletz C, Wang X, Briese V, Friese K. hCG in trophoblast tumour cells of the cell line Jeg3 and hCG isolated from amniotic fluid and serum of pregnant women carry oligosaccharides of the sialyl Lewis X and sialyl Lewis a type. Anticancer Res. 2003;23:1087. [PubMed] [Google Scholar]
- 24.Jeschke U, Mylonas I, Richter DU, Streu A, Muller H, Briese V, Friese K. Human amniotic fluid glycoproteins expressing sialyl Lewis carbohydrate antigens stimulate progesterone production in human trophoblasts in vitro. Gynecol Obstet Invest. 2004;58:207. doi: 10.1159/000080073. [DOI] [PubMed] [Google Scholar]
- 25.Minas V, Mylonas I, Schiessl B, Mayr D, Schulze S, Friese K, Jeschke U, Makrigiannakis A. Expression of the blood-group-related antigens Sialyl Lewis a, Sialyl Lewis x and Lewis y in term placentas of normal, preeclampsia, IUGR- and HELLP-complicated pregnancies. Histochem Cell Biol. 2007;128:55. doi: 10.1007/s00418-007-0293-7. [DOI] [PubMed] [Google Scholar]
- 26.Elola MT, Capurro MI, Barrio MM, Coombs PJ, Taylor ME, Drickamer K, Mordoh J. Lewis x antigen mediates adhesion of human breast carcinoma cells to activated endothelium. Possible involvement of the endothelial scavenger receptor C-type lectin. Breast Cancer Res Treat. 2007;101:161. doi: 10.1007/s10549-006-9286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ko AI, Drager UC, Harn DA. A Schistosoma mansoni epitope recognized by a protective monoclonal antibody is identical to the stage-specific embryonic antigen 1. Proc Natl Acad Sci USA. 1990;87:4159. doi: 10.1073/pnas.87.11.4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hokke CH, Yazdanbakhsh M. Schistosome glycans and innate immunity. Parasite Immunol. 2005;27:257. doi: 10.1111/j.1365-3024.2005.00781.x. [DOI] [PubMed] [Google Scholar]
- 29.Bergman MP, Engering A, Smits HH, et al. Helicobacter pylori modulates the T helper cell 1/T helper cell 2 balance through phase-variable interaction between lipopolysaccharide and DC-SIGN. J Exp Med. 2004;200:979. doi: 10.1084/jem.20041061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Atochina O, Daly-Engel T, Piskorska D, McGuire E, Harn DA. A schistosome-expressed immunomodulatory glycoconjugate expands peritoneal Gr1(+) macrophages that suppress naive CD4(+) T cell proliferation via an IFN-gamma and nitric oxide-dependent mechanism. J Immunol. 2001;167:4293. doi: 10.4049/jimmunol.167.8.4293. [DOI] [PubMed] [Google Scholar]
- 31.Atochina O, Harn D. Prevention of psoriasis-like lesions development in fsn/fsn mice by helminth glycans. Exp Dermatol. 2006;15:461. doi: 10.1111/j.1600-0625.2006.00431.x. [DOI] [PubMed] [Google Scholar]
- 32.Corraliza IM, Campo ML, Soler G, Modolell M. Determination of arginase activity in macrophages: a micromethod. J Immunol Methods. 1994;174:231. doi: 10.1016/0022-1759(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 33.Raes G, Van den Bergh R, De Baetselier P, Ghassabeh GH, Scotton C, Locati M, Mantovani A, Sozzani S. Arginase-1 and Ym1 are markers for murine, but not human, alternatively activated myeloid cells. J Immunol. 2005;174:6561. doi: 10.4049/jimmunol.174.11.6561. author reply 6561. [DOI] [PubMed] [Google Scholar]
- 34.Reiman RM, Thompson RW, Feng CG, Hari D, Knight R, Cheever AW, Rosenberg HF, Wynn TA. Interleukin-5 (IL-5) augments the progression of liver fibrosis by regulating IL-13 activity. Infect Immun. 2006;74:1471. doi: 10.1128/IAI.74.3.1471-1479.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nair MG, Gallagher IJ, Taylor MD, Loke P, Coulson PS, Wilson RA, Maizels RM, Allen JE. Chitinase and Fizz family members are a generalized feature of nematode infection with selective upregulation of Ym1 and Fizz1 by antigen-presenting cells. Infect Immun. 2005;73:385. doi: 10.1128/IAI.73.1.385-394.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sato K, Komatsu N, Higashi N, Imai Y, Irimura T. Granulation tissue formation by nonspecific inflammatory agent occurs independently of macrophage galactose-type C-type lectin-1. Clin Immunol. 2005;115:47. doi: 10.1016/j.clim.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 37.Smith P, Walsh CM, Mangan NE, Fallon RE, Sayers JR, McKenzie AN, Fallon PG. Schistosoma mansoni worms induce anergy of T cells via selective up-regulation of programmed death ligand 1 on macrophages. J Immunol. 2004;173:1240. doi: 10.4049/jimmunol.173.2.1240. [DOI] [PubMed] [Google Scholar]
- 38.Rutitzky LI, Ozkaynak E, Rottman JB, Stadecker MJ. Disruption of the ICOS-B7RP-1 costimulatory pathway leads to enhanced hepatic immunopathology and increased gamma interferon production by CD4 T cells in murine schistosomiasis. Infect Immun. 2003;71:4040. doi: 10.1128/IAI.71.7.4040-4044.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xia CM, Pu XK, Gong W, Luo W, Zhang HQ, Deng ZB, Xue ZM. Immune response and immunopathology in inducible costimulatory molecule (ICOS) transgenic mice infected with Schistosoma japonicum. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2006;24:349. [PubMed] [Google Scholar]
- 40.Munder M, Eichmann K, Modolell M. Alternative metabolic states in murine macrophages reflected by the nitric oxide synthase/arginase balance: competitive regulation by CD4+ T cells correlates with Th1/Th2 phenotype. J Immunol. 1998;160:5347. [PubMed] [Google Scholar]
- 41.Prasse A, Germann M, Pechkovsky DV, et al. IL-10-producing monocytes differentiate to alternatively activated macrophages and are increased in atopic patients. J Allergy Clin Immunol. 2007;119:464. doi: 10.1016/j.jaci.2006.09.030. [DOI] [PubMed] [Google Scholar]
- 42.Saeftel M, Krueger A, Arriens S, Heussler V, Racz P, Fleischer B, Brombacher F, Hoerauf A. Mice deficient in interleukin-4 (IL-4) or IL-4 receptor alpha have higher resistance to sporozoite infection with Plasmodium berghei (ANKA) than do naive wild-type mice. Infect Immun. 2004;72:322. doi: 10.1128/IAI.72.1.322-331.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mohrs M, Ledermann B, Kohler G, Dorfmuller A, Gessner A, Brombacher F. Differences between IL-4- and IL-4 receptor alpha-deficient mice in chronic leishmaniasis reveal a protective role for IL-13 receptor signaling. J Immunol. 1999;162:7302. [PubMed] [Google Scholar]
- 44.Pearce EJ, MacDonald AS. The immunobiology of schistosomiasis. Nat Rev Immunol. 2002;2:499. doi: 10.1038/nri843. [DOI] [PubMed] [Google Scholar]
- 45.Wilson MS, Mentink-Kane MM, Pesce JT, Ramalingam TR, Thompson R, Wynn TA. Immunopathology of schistosomiasis. Immunol Cell Biol. 2007;85:148. doi: 10.1038/sj.icb.7100014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hesse M, Modolell M, La Flamme AC, Schito M, Fuentes JM, Cheever AW, Pearce EJ, Wynn TA. Differential regulation of nitric oxide synthase-2 and arginase-1 by type 1/type 2 cytokines in vivo: granulomatous pathology is shaped by the pattern of l-arginine metabolism. J Immunol. 2001;167:6533. doi: 10.4049/jimmunol.167.11.6533. [DOI] [PubMed] [Google Scholar]
- 47.Chiaramonte MG, Cheever AW, Malley JD, Donaldson DD, Wynn TA. Studies of murine schistosomiasis reveal interleukin-13 blockade as a treatment for established and progressive liver fibrosis. Hepatology. 2001;34:273. doi: 10.1053/jhep.2001.26376. [DOI] [PubMed] [Google Scholar]
- 48.McKenzie GJ, Fallon PG, Emson CL, Grencis RK, McKenzie AN. Simultaneous disruption of interleukin (IL)-4 and IL-13 defines individual roles in T helper cell type 2-mediated responses. J Exp Med. 1999;189:1565. doi: 10.1084/jem.189.10.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Loke P, Nair MG, Parkinson J, Guiliano D, Blaxter M, Allen JE. IL-4 dependent alternatively-activated macrophages have a distinctive in vivo gene expression phenotype. BMC Immunol. 2002;3:7. doi: 10.1186/1471-2172-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sabin EA, Kopf MA, Pearce EJ. Schistosoma mansoni egg-induced early IL-4 production is dependent upon IL-5 and eosinophils. J Exp Med. 1996;184:1871. doi: 10.1084/jem.184.5.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gessner A, Mohrs K, Mohrs M. Mast cells, basophils, and eosinophils acquire constitutive IL-4 and IL-13 transcripts during lineage differentiation that are sufficient for rapid cytokine production. J Immunol. 2005;174:1063. doi: 10.4049/jimmunol.174.2.1063. [DOI] [PubMed] [Google Scholar]
- 52.Van Liempt E, Imberty A, Bank CM, Van Vliet SJ, Van Kooyk Y, Geijtenbeek TB, Van Die I. Molecular basis of the differences in binding properties of the highly related C-type lectins DC-SIGN and L-SIGN to Lewis X trisaccharide and Schistosoma mansoni egg antigens. J Biol Chem. 2004;279:33161. doi: 10.1074/jbc.M404988200. [DOI] [PubMed] [Google Scholar]
- 53.van Liempt E, van Vliet SJ, Engering A, Garcia Vallejo JJ, Bank CM, Sanchez-Hernandez M, van Kooyk Y, van Die I. Schistosoma mansoni soluble egg antigens are internalized by human dendritic cells through multiple C-type lectins and suppress TLR-induced dendritic cell activation. Mol Immunol. 2007;44:2605. doi: 10.1016/j.molimm.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 54.Thomas PG, Carter MR, Da’dara AA, DeSimone TM, Harn DA. A helminth glycan induces APC maturation via alternative NF-kappa B activation independent of I kappa B alpha degradation. J Immunol. 2005;175:2082. doi: 10.4049/jimmunol.175.4.2082. [DOI] [PubMed] [Google Scholar]
- 55.Dunn MA, Rojkind M, Hait PK, Warren KS. Conversion of arginine to proline in murine schistosomiasis. Gastroenterology. 1978;75:1010. [PubMed] [Google Scholar]
- 56.Dunn MA, Seifter S, Hait PK. Proline trapping in granulomas, the site of collagen biosynthesis in murine schistosomiasis. Hepatology. 1981;1:28. doi: 10.1002/hep.1840010105. [DOI] [PubMed] [Google Scholar]
- 57.Herbert DR, Holscher C, Mohrs M, et al. Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology. Immunity. 2004;20:623. doi: 10.1016/s1074-7613(04)00107-4. [DOI] [PubMed] [Google Scholar]
- 58.Muller WA, Randolph GJ. Migration of leukocytes across endothelium and beyond: molecules involved in the transmigration and fate of monocytes. J Leukoc Biol. 1999;66:698. doi: 10.1002/jlb.66.5.698. [DOI] [PubMed] [Google Scholar]
- 59.Randolph GJ, Inaba K, Robbiani DF, Steinman RM, Muller WA. Differentiation of phagocytic monocytes into lymph node dendritic cells in vivo. Immunity. 1999;11:753. doi: 10.1016/s1074-7613(00)80149-1. [DOI] [PubMed] [Google Scholar]
- 60.Martin-Orozco N, Wang YH, Yagita H, Dong C. Cutting edge: programmed death (PD) ligand-1/PD-1 interaction is required for CD8+ T cell tolerance to tissue antigens. J Immunol. 2006;177:8291. doi: 10.4049/jimmunol.177.12.8291. [DOI] [PubMed] [Google Scholar]
- 61.Keir ME, Liang SC, Guleria I, et al. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med. 2006;203:883. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cheng X, Zhao Z, Ventura E, Gran B, Shindler KS, Rostami A. The PD-1/PD-L pathway is up-regulated during IL-12-induced suppression of EAE mediated by IFN-gamma. J Neuroimmunol. 2007;185:75. doi: 10.1016/j.jneuroim.2007.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nakanishi J, Wada Y, Matsumoto K, Azuma M, Kikuchi K, Ueda S. Overexpression of B7-H1 (PD-L1) significantly associates with tumor grade and postoperative prognosis in human urothelial cancers. Cancer Immunol Immunother. 2007;56:1173. doi: 10.1007/s00262-006-0266-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liang SC, Latchman YE, Buhlmann JE, Tomczak MF, Horwitz BH, Freeman GJ, Sharpe AH. Regulation of PD-1, PD-L1, and PD-L2 expression during normal and autoimmune responses. Eur J Immunol. 2003;33:2706. doi: 10.1002/eji.200324228. [DOI] [PubMed] [Google Scholar]
- 65.Zhao Y, Huang Z, Qi M, Lazzarini P, Mazzone T. Immune regulation of T lymphocyte by a newly characterized human umbilical cord blood stem cell. Immunol Lett. 2007;108:78. doi: 10.1016/j.imlet.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 66.Kuipers H, Muskens F, Willart M, Hijdra D, van Assema FB, Coyle AJ, Hoogsteden HC, Lambrecht BN. Contribution of the PD-1 ligands/PD-1 signaling pathway to dendritic cell-mediated CD4+ T cell activation. Eur J Immunol. 2006;36:2472. doi: 10.1002/eji.200635978. [DOI] [PubMed] [Google Scholar]
- 67.Brown JA, Dorfman DM, Ma FR, Sullivan EL, Munoz O, Wood CR, Greenfield EA, Freeman GJ. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol. 2003;170:1257. doi: 10.4049/jimmunol.170.3.1257. [DOI] [PubMed] [Google Scholar]
- 68.Ito T, Ueno T, Clarkson MR, et al. Analysis of the role of negative T cell costimulatory pathways in CD4 and CD8 T cell-mediated alloimmune responses in vivo. J Immunol. 2005;174:6648. doi: 10.4049/jimmunol.174.11.6648. [DOI] [PubMed] [Google Scholar]
- 69.Idoyaga J, Moreno J, Bonifaz L. Tumor cells prevent mouse dendritic cell maturation induced by TLR ligands. Cancer Immunol Immunother. 2007;56:1237. doi: 10.1007/s00262-006-0275-y. [DOI] [PMC free article] [PubMed] [Google Scholar]