Abstract
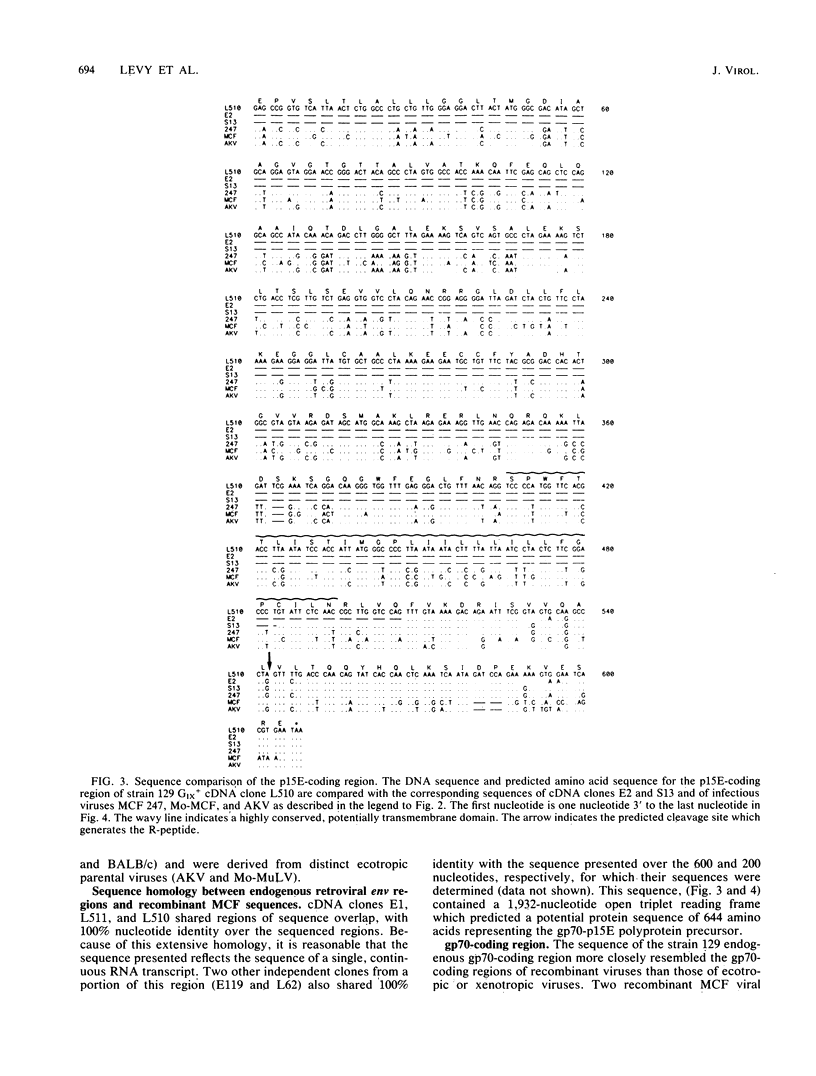
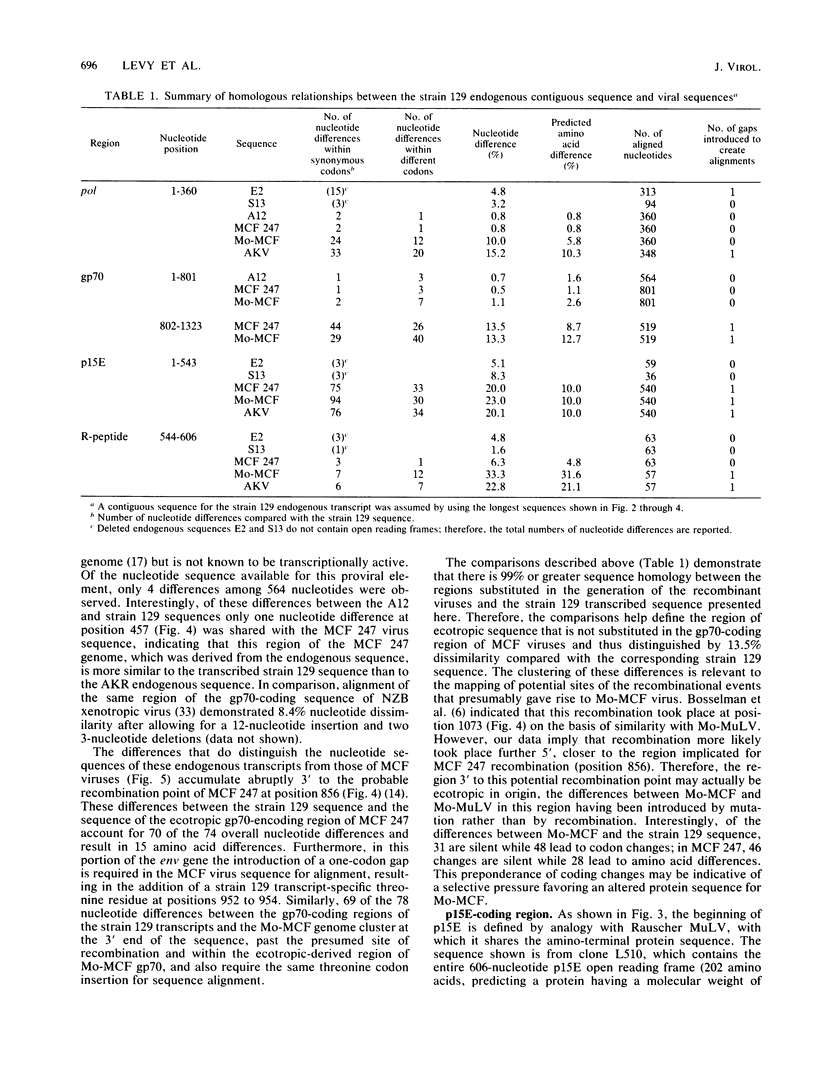
In the absence of infectious virus, strains of mice express polyadenylated RNA transcripts homologous to the genome of murine leukemia virus. In addition to transcripts consistent with full-length and spliced env retroviral RNAs, several unique RNA species which lack the env sequence accumulate in a tissue-specific manner. These RNA species are presumed to be transcribed from endogenous retroviral sequences that constitute the bulk of the murine leukemia virus-related sequences in the murine genome. To determine the relationship of these RNA transcripts to infectious murine leukemia virus and the precise structural basis of the heterogeneity observed for the env-lacking transcripts, we isolated and sequenced cDNA recombinants representing the RNAs expressed in strain 129 GIX+ mice. Comparisons of the nucleotide sequences demonstrated that the endogenous retroviral transcripts differed in pol, p15E, and R-peptide regions by single nucleotide changes. In contrast, the gp70-coding regions of two cDNA clones derived from epididymis and liver were completely homologous over a 599-nucleotide overlapping sequence. The structures of env-lacking transcripts were examined in two independent cDNA clones, and each was found to contain a different deletion that was potentially mediated by seven-base pair direct repeats in the intact sequence. The extensive sequence homology between cDNAs allowed construction of a cumulative sequence map of the 3' end of an intact endogenous retroviral transcript. A comparison of this sequence with infectious ecotropic and mink cell focus-forming viruses revealed that the endogenous transcripts are highly homologous with the substituted portions of leukemogenic mink cell focus-forming viruses and therefore further define the boundaries of recombination required to generate these viruses.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amanuma H., Katori A., Obata M., Sagata N., Ikawa Y. Complete nucleotide sequence of the gene for the specific glycoprotein (gp55) of Friend spleen focus-forming virus. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3913–3917. doi: 10.1073/pnas.80.13.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassin R. H., Ruscetti S., Ali I., Haapala D. K., Rein A. Normal DBA/2 mouse cells synthesize a glycoprotein which interferes with MCF virus infection. Virology. 1982 Nov;123(1):139–151. doi: 10.1016/0042-6822(82)90301-4. [DOI] [PubMed] [Google Scholar]
- Berns A. J., Lai M. H., Bosselman R. A., McKennett M. A., Bacheler L. T., Fan H., Maandag E. C., van der Putten H. V., Verma I. M. Molecular cloning of unintegrated and a portion of integrated moloney murine leukemia viral DNA in bacteriophage lambda. J Virol. 1980 Oct;36(1):254–263. doi: 10.1128/jvi.36.1.254-263.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestwick R. K., Boswell B. A., Kabat D. Molecular cloning of biologically active Rauscher spleen focus-forming virus and the sequences of its env gene and long terminal repeat. J Virol. 1984 Sep;51(3):695–705. doi: 10.1128/jvi.51.3.695-705.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosselman R. A., van Straaten F., Van Beveren C., Verma I. M., Vogt M. Analysis of the env gene of a molecularly cloned and biologically active Moloney mink cell focus-forming proviral DNA. J Virol. 1982 Oct;44(1):19–31. doi: 10.1128/jvi.44.1.19-31.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Lander M. R., Gupta S., Rands E., Lowy D. R. Origin of mink cytopathic focus-forming (MCF) viruses:comparison with ecotropic and xenotropic murine leukemia virus genomes. Virology. 1981 Sep;113(2):465–483. doi: 10.1016/0042-6822(81)90175-6. [DOI] [PubMed] [Google Scholar]
- Devare S. G., Rapp U. R., Todaro G. J., Stephenson J. R. Acquisition of oncogenicity by endogenous mouse type C viruses: effects of variations in env and gag genes. J Virol. 1978 Nov;28(2):457–465. doi: 10.1128/jvi.28.2.457-465.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder J. H., Mullins J. I. Nucleotide sequence of the envelope gene of Gardner-Arnstein feline leukemia virus B reveals unique sequence homologies with a murine mink cell focus-forming virus. J Virol. 1983 Jun;46(3):871–880. doi: 10.1128/jvi.46.3.871-880.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green N., Shinnick T. M., Witte O., Ponticelli A., Sutcliffe J. G., Lerner R. A. Sequence-specific antibodies show that maturation of Moloney leukemia virus envelope polyprotein involves removal of a COOH-terminal peptide. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6023–6027. doi: 10.1073/pnas.78.10.6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara I., Izui S., Dixon F. J. Murine serum glycoprotein gp70 behaves as an acute phase reactant. J Exp Med. 1982 Feb 1;155(2):345–357. doi: 10.1084/jem.155.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr W. Nucleotide sequence of AKV murine leukemia virus. J Virol. 1984 Feb;49(2):471–478. doi: 10.1128/jvi.49.2.471-478.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland C. A., Hartley J. W., Rowe W. P., Hopkins N. At least four viral genes contribute to the leukemogenicity of murine retrovirus MCF 247 in AKR mice. J Virol. 1985 Jan;53(1):158–165. doi: 10.1128/jvi.53.1.158-165.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland C. A., Wozney J., Hopkins N. Nucleotide sequence of the gp70 gene of murine retrovirus MCF 247. J Virol. 1983 Sep;47(3):413–420. doi: 10.1128/jvi.47.3.413-420.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz J. M., Risser R. A locus that enhances the induction of endogenous ecotropic murine leukemia viruses is distinct from genome-length ecotropic proviruses. J Virol. 1982 Dec;44(3):950–957. doi: 10.1128/jvi.44.3.950-957.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly M., Holland C. A., Lung M. L., Chattopadhyay S. K., Lowy D. R., Hopkins N. H. Nucleotide sequence of the 3' end of MCF 247 murine leukemia virus. J Virol. 1983 Jan;45(1):291–298. doi: 10.1128/jvi.45.1.291-298.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A. S., Martin M. A. Endogenous murine leukemia proviral long terminal repeats contain a unique 190-base-pair insert. Proc Natl Acad Sci U S A. 1983 May;80(9):2699–2703. doi: 10.1073/pnas.80.9.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A. S. Nucleotide sequence analysis establishes the role of endogenous murine leukemia virus DNA segments in formation of recombinant mink cell focus-forming murine leukemia viruses. J Virol. 1984 Jun;50(3):864–871. doi: 10.1128/jvi.50.3.864-871.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A. S., Rowe W. P., Martin M. A. Cloning of endogenous murine leukemia virus-related sequences from chromosomal DNA of BALB/c and AKR/J mice: identification of an env progenitor of AKR-247 mink cell focus-forming proviral DNA. J Virol. 1982 Nov;44(2):625–636. doi: 10.1128/jvi.44.2.625-636.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak C. A., Rowe W. P. Genetic mapping of ecotropic murine leukemia virus-inducing loci in six inbred strains. J Exp Med. 1982 Feb 1;155(2):524–534. doi: 10.1084/jem.155.2.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak C. A., Rowe W. P. Genetic mapping of the ecotropic virus-inducing locus Akv-2 of the AKR mouse. J Exp Med. 1980 Nov 1;152(5):1419–1423. doi: 10.1084/jem.152.5.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz J., Crowther R., Straceski A., Haseltine W. Nucleotide sequence of the Akv env gene. J Virol. 1982 May;42(2):519–529. doi: 10.1128/jvi.42.2.519-529.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz J., Haseltine W. A. Localization of the leukemogenic determinants of SL3-3, an ecotropic, XC-positive murine leukemia virus of AKR mouse origin. J Virol. 1983 Aug;47(2):317–328. doi: 10.1128/jvi.47.2.317-328.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D. E., Lerner R. A., Wilson M. C. A genetic locus regulates the expression of tissue-specific mRNAs from multiple transcription units. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5823–5827. doi: 10.1073/pnas.79.19.5823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D. E., Lerner R. A., Wilson M. C. The Gv-1 locus coordinately regulates the expression of multiple endogenous murine retroviruses. Cell. 1985 May;41(1):289–299. doi: 10.1016/0092-8674(85)90082-0. [DOI] [PubMed] [Google Scholar]
- Levy J. A. Xenotropic type C viruses. Curr Top Microbiol Immunol. 1978;79:111–213. doi: 10.1007/978-3-642-66853-1_4. [DOI] [PubMed] [Google Scholar]
- Lung M. L., Hartley J. W., Rowe W. P., Hopkins N. H. Large RNase T1-resistant oligonucleotides encoding p15E and the U3 region of the long terminal repeat distinguish two biological classes of mink cell focus-forming type C viruses of inbred mice. J Virol. 1983 Jan;45(1):275–290. doi: 10.1128/jvi.45.1.275-290.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark G. E., Rapp U. R. Envelope gene sequence of two in vitro-generated mink cell focus-forming murine leukemia viruses which contain the entire gp70 sequence of the endogenous nonecotropic parent. J Virol. 1984 Feb;49(2):530–539. doi: 10.1128/jvi.49.2.530-539.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall R. D. The nature and metabolism of the carbohydrate-peptide linkages of glycoproteins. Biochem Soc Symp. 1974;(40):17–26. [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- O'Neill R. R., Buckler C. E., Theodore T. S., Martin M. A., Repaske R. Envelope and long terminal repeat sequences of a cloned infectious NZB xenotropic murine leukemia virus. J Virol. 1985 Jan;53(1):100–106. doi: 10.1128/jvi.53.1.100-106.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata Y., Ikeda H., Stockert E., Boyse E. A. Relation of GIX antigen of thymocytes to envelope glycoprotein of murine leukemia virus. J Exp Med. 1975 Jan 1;141(1):188–197. doi: 10.1084/jem.141.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old L. J., Stockert E. Immunogenetics of cell surface antigens of mouse leukemia. Annu Rev Genet. 1977;11:127–160. doi: 10.1146/annurev.ge.11.120177.001015. [DOI] [PubMed] [Google Scholar]
- Quint W., Boelens W., van Wezenbeek P., Cuypers T., Maandag E. R., Selten G., Berns A. Generation of AKR mink cell focus-forming viruses: a conserved single-copy xenotrope-like provirus provides recombinant long terminal repeat sequences. J Virol. 1984 May;50(2):432–438. doi: 10.1128/jvi.50.2.432-438.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein A., Schultz A. Different recombinant murine leukemia viruses use different cell surface receptors. Virology. 1984 Jul 15;136(1):144–152. doi: 10.1016/0042-6822(84)90255-1. [DOI] [PubMed] [Google Scholar]
- Repaske R., O'Neill R. R., Khan A. S., Martin M. A. Nucleotide sequence of the env-specific segment of NFS-Th-1 xenotropic murine leukemia virus. J Virol. 1983 Apr;46(1):204–211. doi: 10.1128/jvi.46.1.204-211.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risser R., Horowitz J. M., McCubrey J. Endogenous mouse leukemia viruses. Annu Rev Genet. 1983;17:85–121. doi: 10.1146/annurev.ge.17.120183.000505. [DOI] [PubMed] [Google Scholar]
- Ruscetti S., Davis L., Feild J., Oliff A. Friend murine leukemia virus-induced leukemia is associated with the formation of mink cell focus-inducing viruses and is blocked in mice expressing endogenous mink cell focus-inducing xenotropic viral envelope genes. J Exp Med. 1981 Sep 1;154(3):907–920. doi: 10.1084/jem.154.3.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzberg P., Colicelli J., Goff S. P. Construction and analysis of deletion mutations in the pol gene of Moloney murine leukemia virus: a new viral function required for productive infection. Cell. 1984 Jul;37(3):1043–1052. doi: 10.1016/0092-8674(84)90439-2. [DOI] [PubMed] [Google Scholar]
- Shinnick T. M., Lerner R. A., Sutcliffe J. G. Nucleotide sequence of Moloney murine leukaemia virus. Nature. 1981 Oct 15;293(5833):543–548. doi: 10.1038/293543a0. [DOI] [PubMed] [Google Scholar]
- Stockert E., Boyse E. A., Obata Y., Ikeda H., Sarkar N. H., Hoffman H. A. New mutant and congenic mouse stocks expressing the murine leukemia virus-associated thymocyte surface antigen GIX. J Exp Med. 1975 Aug 1;142(2):512–517. doi: 10.1084/jem.142.2.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockert E., DeLeo A. B., O'Donnell P. V., Obata Y., Old L. J. G(AKSL2): a new cell surface antigen of the mouse related to the dualtropic mink cell focus-inducing class of murine leukemia virus detected by naturally occurring antibody. J Exp Med. 1979 Jan 1;149(1):200–215. doi: 10.1084/jem.149.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand M., Lilly F., August J. T. Host control of endogenous murine leukemia virus gene expression: concentrations of viral proteins in high and low leukemia mouse strains. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3682–3686. doi: 10.1073/pnas.71.9.3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmus H. E. The molecular genetics of cellular oncogenes. Annu Rev Genet. 1984;18:553–612. doi: 10.1146/annurev.ge.18.120184.003005. [DOI] [PubMed] [Google Scholar]
- White J., Kielian M., Helenius A. Membrane fusion proteins of enveloped animal viruses. Q Rev Biophys. 1983 May;16(2):151–195. doi: 10.1017/s0033583500005072. [DOI] [PubMed] [Google Scholar]


