Abstract
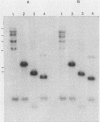
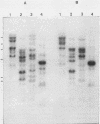
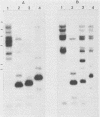
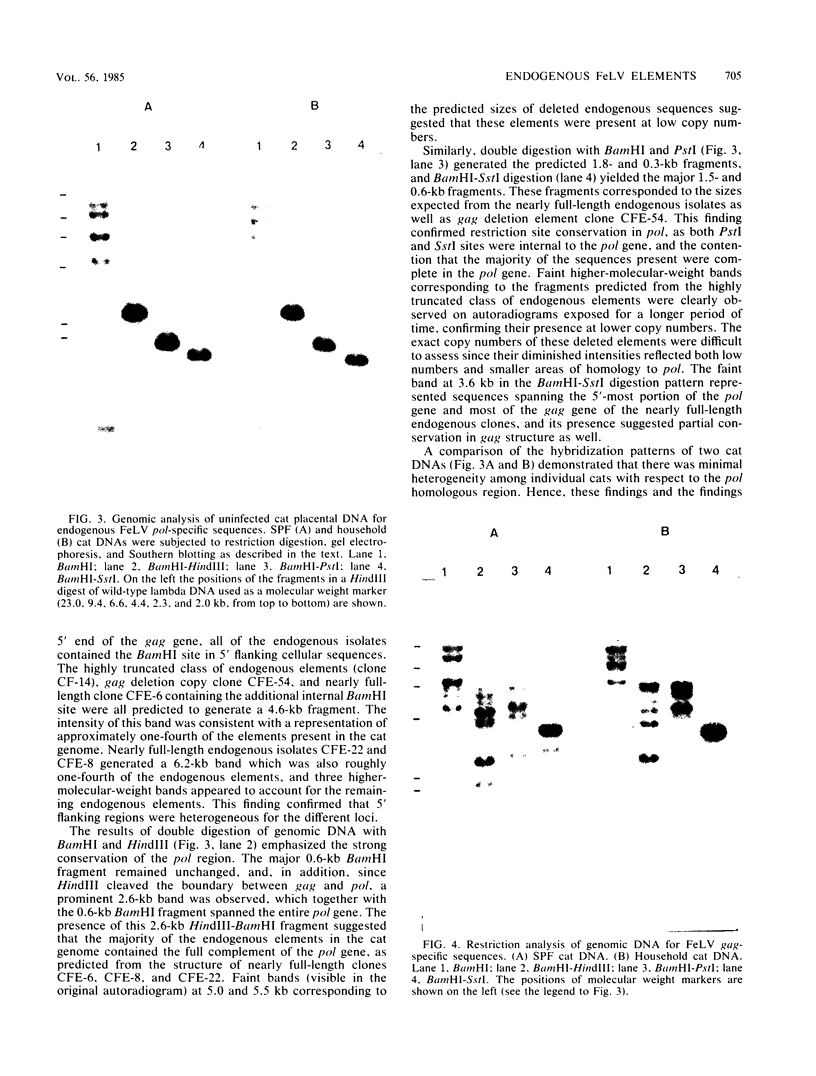
Five recombinant DNA clones of endogenous feline leukemia virus-related DNA sequences were isolated by screening a lambda phage genomic library of cat placental DNA with a probe specific to the gag-pol region of infectious feline leukemia virus. The clones containing retroviral long terminal repeat-like sequences demonstrated the existence of different size classes of endogenous elements in the cat genome, including those of nearly full length in which the gag region is heterogeneous but all of pol and most of env are highly conserved. Other size classes included elements with major deletions in gag or pol. A genomic DNA analysis suggested that the majority of endogenous elements were close to full length in size and that the highly truncated sequences which we described previously (Soe et al., J. Virol. 46:829-840, 1983) represented only a subset of the elements present. A restriction analysis of genomic DNA suggested a high degree of conservation in pol and the 5' portion of env among the various endogenous sequences present in the cat genome. We also found by using DNA transfection that while all of the endogenous clones were noninfectious, there was differential expression of the elements which we examined. These findings correlate with the subgenomic expression of endogenous feline leukemia virus sequences in cat placental tissue.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baluda M. A., Roy-Burman P. Partial characterization of RD114 virus by DNA-RNA hybridization studies. Nat New Biol. 1973 Jul 11;244(132):59–62. doi: 10.1038/newbio244059a0. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Sherr C. J., Todaro G. J. Evolution of type C viral genes: origin of feline leukemia virus. Science. 1975 Nov 28;190(4217):886–888. doi: 10.1126/science.52892. [DOI] [PubMed] [Google Scholar]
- Busch M. P., Devi B. G., Soe L. H., Perbal B., Baluda M. A., Roy-Burman P. Characterization of the expression of cellular retrovirus genes and oncogenes in feline cells. Hematol Oncol. 1983 Jan-Mar;1(1):61–75. doi: 10.1002/hon.2900010108. [DOI] [PubMed] [Google Scholar]
- Casey J. W., Roach A., Mullins J. I., Burck K. B., Nicolson M. O., Gardner M. B., Davidson N. The U3 portion of feline leukemia virus DNA identifies horizontally acquired proviruses in leukemic cats. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7778–7782. doi: 10.1073/pnas.78.12.7778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang E. H., Furth M. E., Scolnick E. M., Lowy D. R. Tumorigenic transformation of mammalian cells induced by a normal human gene homologous to the oncogene of Harvey murine sarcoma virus. Nature. 1982 Jun 10;297(5866):479–483. doi: 10.1038/297479a0. [DOI] [PubMed] [Google Scholar]
- Chiu I. M., Callahan R., Tronick S. R., Schlom J., Aaronson S. A. Major pol gene progenitors in the evolution of oncoviruses. Science. 1984 Jan 27;223(4634):364–370. doi: 10.1126/science.6197754. [DOI] [PubMed] [Google Scholar]
- Cooper G. M., Temin H. M. Lack of infectivity of the endogenous avian leukosis virus-related genes in the DNA of uninfected chicken cells. J Virol. 1976 Feb;17(2):422–430. doi: 10.1128/jvi.17.2.422-430.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder J. H., Mullins J. I. Nucleotide sequence of the envelope gene of Gardner-Arnstein feline leukemia virus B reveals unique sequence homologies with a murine mink cell focus-forming virus. J Virol. 1983 Jun;46(3):871–880. doi: 10.1128/jvi.46.3.871-880.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essex M. Horizontally and vertically transmitted oncornaviruses of cats. Adv Cancer Res. 1975;21:175–248. doi: 10.1016/s0065-230x(08)60973-2. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hampe A., Gobet M., Even J., Sherr C. J., Galibert F. Nucleotide sequences of feline sarcoma virus long terminal repeats and 5' leaders show extensive homology to those of other mammalian retroviruses. J Virol. 1983 Jan;45(1):466–472. doi: 10.1128/jvi.45.1.466-472.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy W. D., Jr, Old L. J., Hess P. W., Essex M., Cotter S. Horizontal transmission of feline leukaemia virus. Nature. 1973 Aug 3;244(5414):266–269. doi: 10.1038/244266a0. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Wolford N. K., Old L. J., Rowe W. P. A new class of murine leukemia virus associated with development of spontaneous lymphomas. Proc Natl Acad Sci U S A. 1977 Feb;74(2):789–792. doi: 10.1073/pnas.74.2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenisch R. Endogenous retroviruses. Cell. 1983 Jan;32(1):5–6. doi: 10.1016/0092-8674(83)90491-9. [DOI] [PubMed] [Google Scholar]
- Jarrett W., Jarrett O., Mackey L., Laird H., Hardy W., Jr, Essex M. Horizontal transmission of leukemia virus and leukemia in the cat. J Natl Cancer Inst. 1973 Sep;51(3):833–841. doi: 10.1093/jnci/51.3.833. [DOI] [PubMed] [Google Scholar]
- Khan A. S., Rowe W. P., Martin M. A. Cloning of endogenous murine leukemia virus-related sequences from chromosomal DNA of BALB/c and AKR/J mice: identification of an env progenitor of AKR-247 mink cell focus-forming proviral DNA. J Virol. 1982 Nov;44(2):625–636. doi: 10.1128/jvi.44.2.625-636.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury G., Gruss P. Enhancer elements. Cell. 1983 Jun;33(2):313–314. doi: 10.1016/0092-8674(83)90410-5. [DOI] [PubMed] [Google Scholar]
- Koshy R., Gallo R. C., Wong-Staal F. Characterization of the endogenous feline leukemia virus-related DNA sequences in cats and attempts to identify exogenous viral sequences in tissues of virus-negative leukemic animals. Virology. 1980 Jun;103(2):434–445. doi: 10.1016/0042-6822(80)90202-0. [DOI] [PubMed] [Google Scholar]
- Laprevotte I., Hampe A., Sherr C. J., Galibert F. Nucleotide sequence of the gag gene and gag-pol junction of feline leukemia virus. J Virol. 1984 Jun;50(3):884–894. doi: 10.1128/jvi.50.3.884-894.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutchan J. H., Pagano J. S. Enchancement of the infectivity of simian virus 40 deoxyribonucleic acid with diethylaminoethyl-dextran. J Natl Cancer Inst. 1968 Aug;41(2):351–357. [PubMed] [Google Scholar]
- Mullins J. I., Casey J. W., Nicolson M. O., Burck K. B., Davidson N. Sequence arrangement and biological activity of cloned feline leukemia virus proviruses from a virus-productive human cell line. J Virol. 1981 May;38(2):688–703. doi: 10.1128/jvi.38.2.688-703.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins J. I., Casey J. W., Nicolson M. O., Davidson N. Sequence organization of feline leukemia virus DNA in infected cells. Nucleic Acids Res. 1980 Aug 11;8(15):3287–3305. doi: 10.1093/nar/8.15.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niman H. L., Akhavi M., Gardner M. B., Stephenson J. R., Roy-Burman P. Differential expression of two distinct endogenous retrovisus genomes in developing tissues of the domestic cat. J Natl Cancer Inst. 1980 Mar;64(3):587–594. [PubMed] [Google Scholar]
- Niman H. L., Gardner M. B., Stephenson J. R., Roy-Burman P. Endogenous RD-114 virus genome expression in malignant tissues of domestic cats. J Virol. 1977 Sep;23(3):578–586. doi: 10.1128/jvi.23.3.578-586.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niman H. L., Stephenson J. R., Gardner M. B., Roy-Burman P. RD-114 and feline leukaemia virus genome expression in natural lymphomas of domestic cats. Nature. 1977 Mar 24;266(5600):357–360. doi: 10.1038/266357a0. [DOI] [PubMed] [Google Scholar]
- Pal B. K., Roy-Burman P. Phosphoproteins: structural components of oncornaviruses. J Virol. 1975 Mar;15(3):540–549. doi: 10.1128/jvi.15.3.540-549.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintrell N., Varmus H. E., Bishop J. M., Nicholson M. O., McAllister R. M. Homologies among the nucleotide sequences of the genomes of C-type viruses. Virology. 1974 Apr;58(2):568–575. doi: 10.1016/0042-6822(74)90090-7. [DOI] [PubMed] [Google Scholar]
- Rabson A. B., Steele P. E., Garon C. F., Martin M. A. mRNA transcripts related to full-length endogenous retroviral DNA in human cells. Nature. 1983 Dec 8;306(5943):604–607. doi: 10.1038/306604a0. [DOI] [PubMed] [Google Scholar]
- Reeves R. H., O'Brien S. J. Molecular genetic characterization of the RD-114 gene family of endogenous feline retroviral sequences. J Virol. 1984 Oct;52(1):164–171. doi: 10.1128/jvi.52.1.164-171.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repaske R., O'Neill R. R., Steele P. E., Martin M. A. Characterization and partial nucleotide sequence of endogenous type C retrovirus segments in human chromosomal DNA. Proc Natl Acad Sci U S A. 1983 Feb;80(3):678–682. doi: 10.1073/pnas.80.3.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roblin R., Young J. M., Mural R. J., Bell T. E., Ihle J. N. Molecular cloning and characterization of murine leukemia virus-related DNA sequences from C3H/HeN mouse DNA. J Virol. 1982 Jul;43(1):113–126. doi: 10.1128/jvi.43.1.113-126.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy-Burman P., Dougherty M., Pal B. K., Charman H. P., Klement V., Gardner M. B. Assay for type C virus in mouse sera based on particulate reverse transcriptase activity. J Virol. 1976 Sep;19(3):1107–1110. doi: 10.1128/jvi.19.3.1107-1110.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy-Burman P., Pattengale P. K., Sherwin R. P. Effect of low levels of nitrogen dioxide inhalation on endogenous retrovirus gene expression. Exp Mol Pathol. 1982 Apr;36(2):144–155. doi: 10.1016/0014-4800(82)90089-2. [DOI] [PubMed] [Google Scholar]
- Sarma P. S., Log T. Subgroup classification of feline leukemia and sarcoma viruses by viral interference and neutralization tests. Virology. 1973 Jul;54(1):160–169. doi: 10.1016/0042-6822(73)90125-6. [DOI] [PubMed] [Google Scholar]
- Seeburg P. H., Shine J., Martial J. A., Ullrich A., Baxter J. D., Goodman H. M. Nucleotide sequence of part of the gene for human chorionic somatomammotropin: purification of DNA complementary to predominant mRNA species. Cell. 1977 Sep;12(1):157–165. doi: 10.1016/0092-8674(77)90193-3. [DOI] [PubMed] [Google Scholar]
- Sherr C. J., Fedele L. A., Oskarsson M., Maizel J., Vande Woude G. Molecular cloning of Snyder-Theilen feline leukemia and sarcoma viruses: comparative studies of feline sarcoma virus with its natural helper virus and with Moloney murine sarcoma virus. J Virol. 1980 Apr;34(1):200–212. doi: 10.1128/jvi.34.1.200-212.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder H. W., Jr, Singhal M. C., Zuckerman E. E., Jones F. R., Hardy W. D., Jr The feline oncornavirus-associated cell membrane antigen (FOCMA) is related to, but distinguishable from, FeLV-C gp70. Virology. 1983 Dec;131(2):315–327. doi: 10.1016/0042-6822(83)90500-7. [DOI] [PubMed] [Google Scholar]
- Soe L. H., Devi B. G., Mullins J. I., Roy-Burman P. Molecular cloning and characterization of endogenous feline leukemia virus sequences from a cat genomic library. J Virol. 1983 Jun;46(3):829–840. doi: 10.1128/jvi.46.3.829-840.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spodick D. A., Soe L. H., Roy-Burman P. Genetic analysis of the feline RD-114 retrovirus-related endogenous elements. Virus Res. 1984 Oct;1(7):543–555. doi: 10.1016/0168-1702(84)90012-1. [DOI] [PubMed] [Google Scholar]
- Steele P. E., Rabson A. B., Bryan T., Martin M. A. Distinctive termini characterize two families of human endogenous retroviral sequences. Science. 1984 Aug 31;225(4665):943–947. doi: 10.1126/science.6089336. [DOI] [PubMed] [Google Scholar]
- Stephension J. R., Reynolds R. K., Tronick S. R., Aaronson S. A. Distribution of three classes of endogenous type-C RNA viruses among inbred strains of mice. Virology. 1975 Oct;67(2):404–414. doi: 10.1016/0042-6822(75)90442-0. [DOI] [PubMed] [Google Scholar]
- Stow N. D., Wilkie N. M. An improved technique for obtaining enhanced infectivity with herpes simplex virus type 1 DNA. J Gen Virol. 1976 Dec;33(3):447–458. doi: 10.1099/0022-1317-33-3-447. [DOI] [PubMed] [Google Scholar]
- Sussman D. J., Milman G. Short-term, high-efficiency expression of transfected DNA. Mol Cell Biol. 1984 Aug;4(8):1641–1643. doi: 10.1128/mcb.4.8.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R., Lis J., Wu R. Elution of DNA from agarose gels after electrophoresis. Methods Enzymol. 1979;68:176–182. doi: 10.1016/0076-6879(79)68012-6. [DOI] [PubMed] [Google Scholar]