Abstract
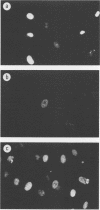
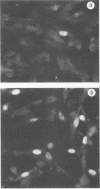
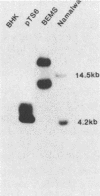
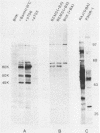
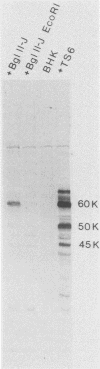
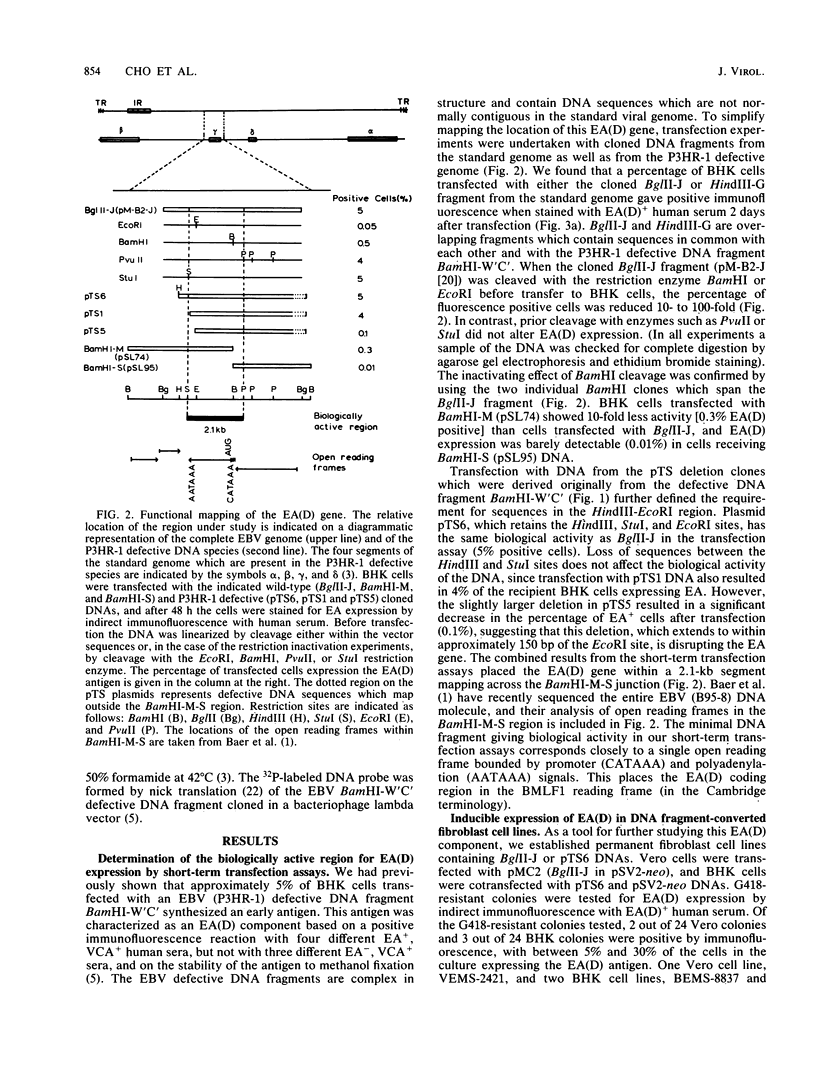
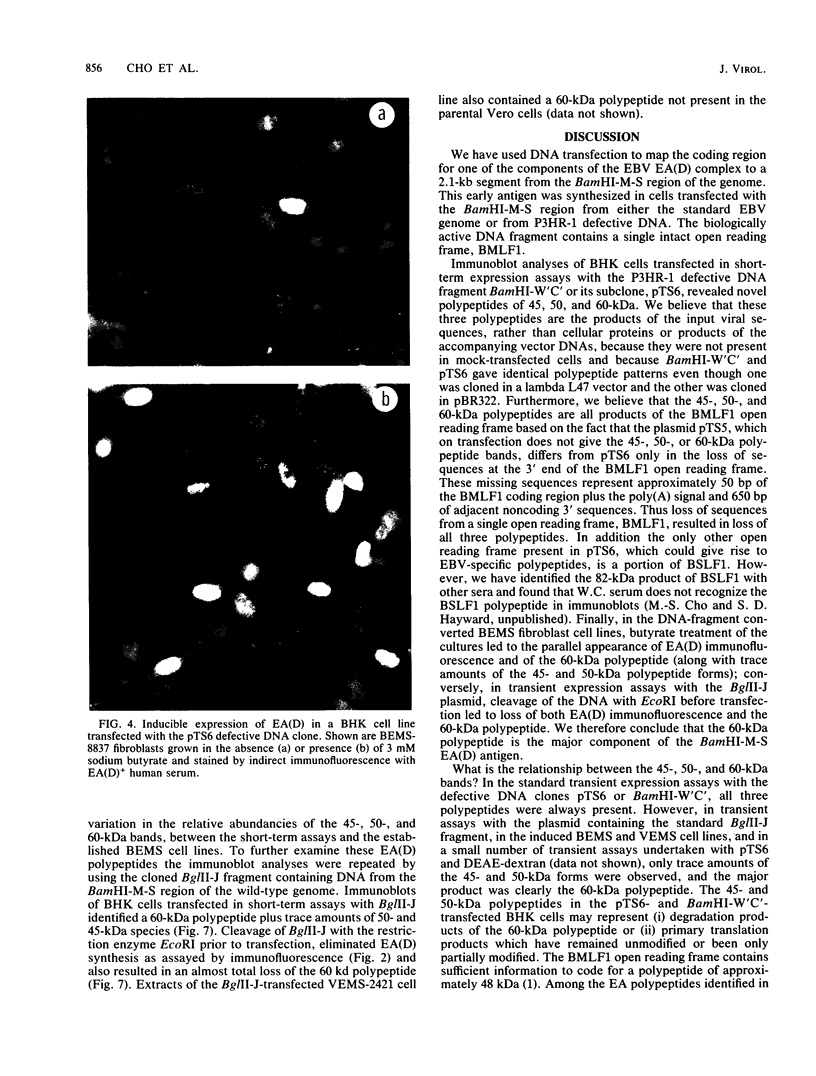
Expression of a component of the Epstein-Barr virus early antigen (EA) complex has been studied in fibroblast cells transfected with both wild-type and P3HR-1 defective DNA fragments covering the BamHI-M-S region of the Epstein-Barr virus genome. Baby hamster kidney (BHK) cells transfected with the BglII-J fragment and stained with human serum that was positive for the diffuse component of EA [EA(D)] in an indirect immunofluorescence assay exhibited positive nuclear staining in 5% of the cell population. Cleavage of BglII-J before transfection with the restriction enzyme BglII, StuI, HindIII, or PvuII did not affect EA expression, whereas prior cleavage with BamHI or EcoRI reduced or eliminated synthesis of EA. These observations were confirmed by using individual cloned subfragments. A Bal 31 deletion clone (pTS1) in which the HindIII and StuI sites were eliminated retained activity, whereas a clone (pTS5) in which the deletion extended closer to the EcoRI site had greatly reduced activity. Transfection of the individual BamHI-M or BamHI-S fragments, which span BglII-J, also resulted in little or no EA expression. The 2.1-kilobase biologically active region defined by these experiments corresponds precisely to the BMLF1 open reading frame. Immunoblot analyses of BHK cells transfected with either P3HR-1 defective DNA clones or the BglII-J wild-type fragment identified the product of this EA(D) coding region as a family of polypeptides consisting of a major 60-kilodalton product and minor 45- and 50-kilodalton species. In latently Epstein-Barr virus-infected lymphocytes these early antigens are not expressed, but can be induced by treatment of the cultures with sodium butyrate or phorbol esters. Using the BglII-J and pTS6 clones that were positive in transient assays, we also established Neor coselected BHK and Vero cell lines which showed similar regulated expression of the 60-kilodalton EA(D) protein. In these cell lines constitutive expression of EA(D) was limited (0.1% positive by indirect immunofluorescence and undetectable by immunoblot analysis). However, expression of EA(D) could be induced by treatment with sodium butyrate. In the induced cultures, up to 30% of the cells were EA(D) positive by immunofluorescence, and there was a concomitant appearance of the 60-kilodalton EA(D) polypeptide.
Full text
PDF

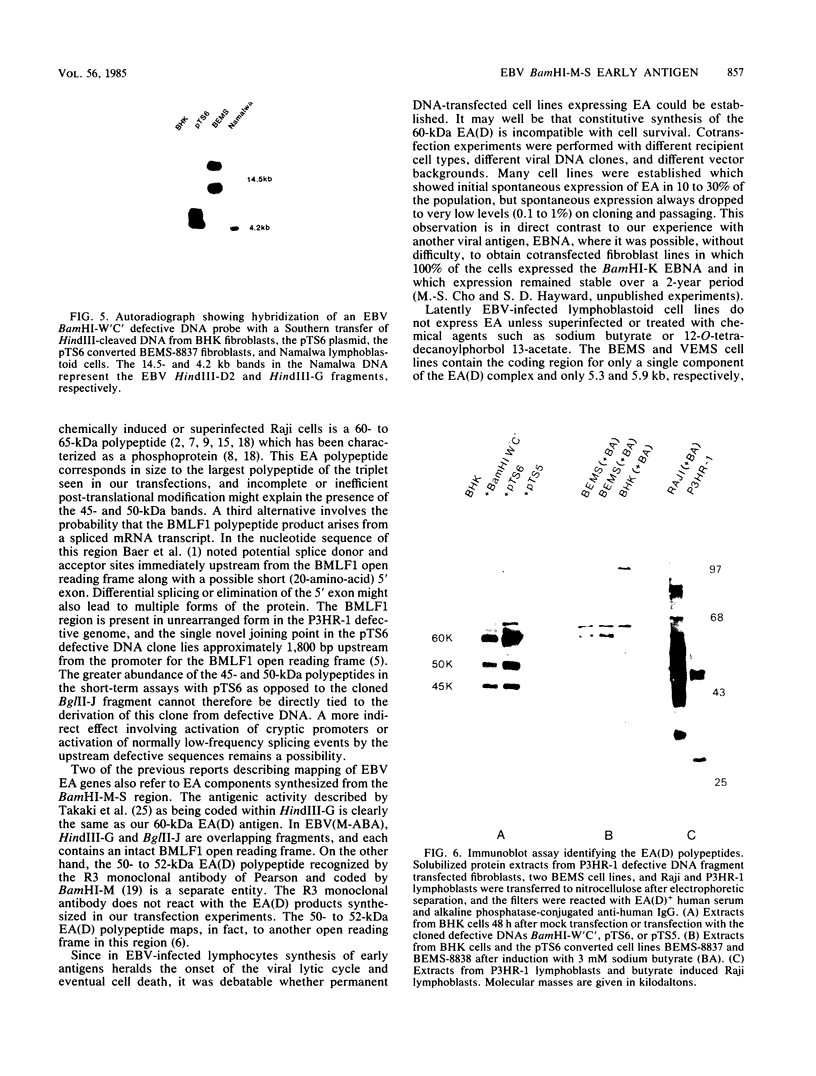


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baer R., Bankier A. T., Biggin M. D., Deininger P. L., Farrell P. J., Gibson T. J., Hatfull G., Hudson G. S., Satchwell S. C., Séguin C. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature. 1984 Jul 19;310(5974):207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- Bayliss G. J., Wolf H. The regulated expression of Epstein-Barr virus. III. Proteins specified by EBV during the lytic cycle. J Gen Virol. 1981 Sep;56(Pt 1):105–118. doi: 10.1099/0022-1317-56-1-105. [DOI] [PubMed] [Google Scholar]
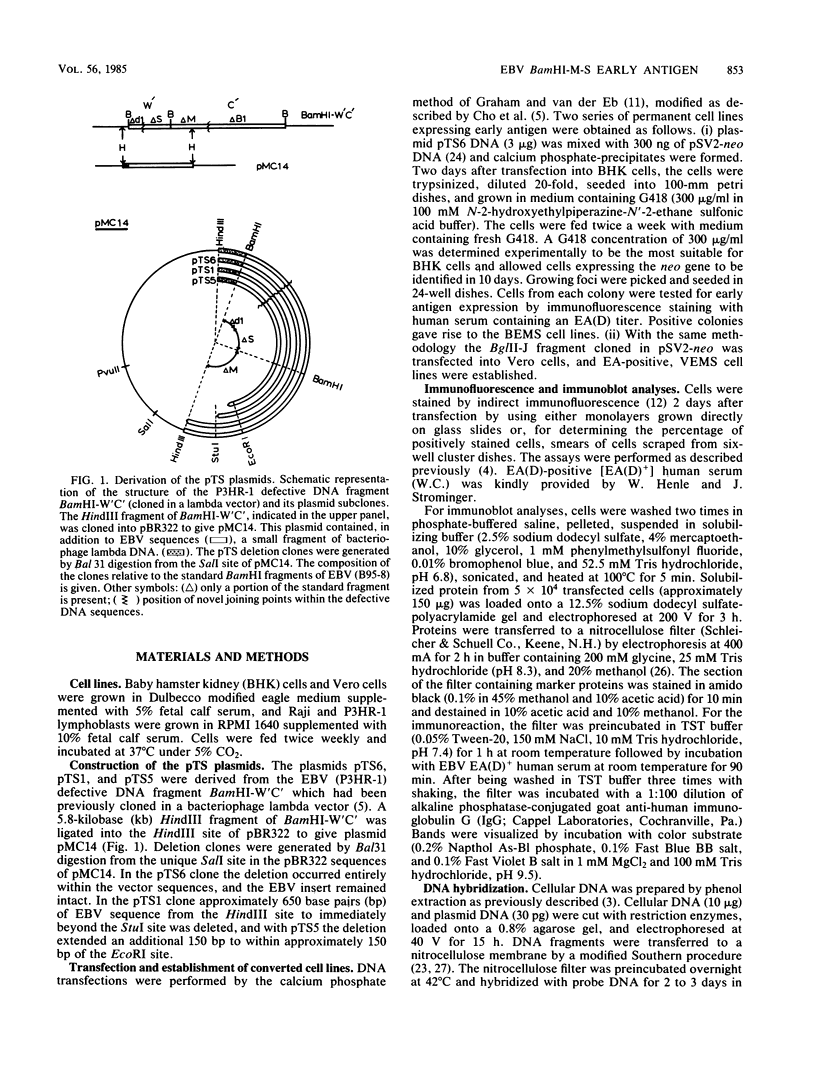
- Cho M. S., Bornkamm G. W., zur Hausen H. Structure of defective DNA molecules in Epstein-Barr virus preparations from P3HR-1 cells. J Virol. 1984 Jul;51(1):199–207. doi: 10.1128/jvi.51.1.199-207.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho M. S., Fresen K. O., zur Hausen H. Multiplicity-dependent biological and biochemical properties of Epstein-Barr virus (EBV) rescued from non-producer lines after superinfection with P3HR-1 EBV. Int J Cancer. 1980 Sep 15;26(3):357–363. doi: 10.1002/ijc.2910260316. [DOI] [PubMed] [Google Scholar]
- Cho M. S., Gissmann L., Hayward S. D. Epstein-Barr virus (P3HR-1) defective DNA codes for components of both the early antigen and viral capsid antigen complexes. Virology. 1984 Aug;137(1):9–19. doi: 10.1016/0042-6822(84)90003-5. [DOI] [PubMed] [Google Scholar]
- Cho M. S., Milman G., Hayward S. D. A second Epstein-Barr virus early antigen gene in BamHI fragment M encodes a 48- to 50-kilodalton nuclear protein. J Virol. 1985 Dec;56(3):860–866. doi: 10.1128/jvi.56.3.860-866.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dölken G., Lange W., Weitzmann U., Hirsch F. W., Löhr G. W. Purification of a protein (60K/58K) associated with the Epstein-Barr virus-induced early antigen complex in Raji cells. Int J Cancer. 1983 Sep 15;32(3):307–314. doi: 10.1002/ijc.2910320308. [DOI] [PubMed] [Google Scholar]
- Feighny R. J., Farrell M. P., Pagano J. S. Polypeptide synthesis and phosphorylation in Epstein-Barr virus-infected cells. J Virol. 1980 May;34(2):455–463. doi: 10.1128/jvi.34.2.455-463.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feighny R. J., Henry B. E., 2nd, Pagano J. S. Epstein-Barr virus polypeptides: effect of inhibition of viral DNA replication on their synthesis. J Virol. 1981 Jan;37(1):61–71. doi: 10.1128/jvi.37.1.61-71.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser R., Boyd A., Stoerker J., Holliday J. Functional mapping of the Epstein-Barr virus genome: identification of sites coding for the restricted early antigen, the diffuse early antigen, and the nuclear antigen. Virology. 1983 Aug;129(1):188–198. doi: 10.1016/0042-6822(83)90405-1. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Henle G., Henle W., Klein G. Demonstration of two distinct components in the early antigen complex of Epstein-Barr virus-infected cells. Int J Cancer. 1971 Sep 15;8(2):272–282. doi: 10.1002/ijc.2910080212. [DOI] [PubMed] [Google Scholar]
- Henle W., Henle G., Zajac B. A., Pearson G., Waubke R., Scriba M. Differential reactivity of human serums with early antigens induced by Epstein-Barr virus. Science. 1970 Jul 10;169(3941):188–190. doi: 10.1126/science.169.3941.188. [DOI] [PubMed] [Google Scholar]
- Hummel M., Kieff E. Mapping of polypeptides encoded by the Epstein-Barr virus genome in productive infection. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5698–5702. doi: 10.1073/pnas.79.18.5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallin B., Luka J., Klein G. Immunochemical characterization of Epstein-Barr virus-associated early and late antigens in n-butyrate-treated P3HR-1 cells. J Virol. 1979 Dec;32(3):710–716. doi: 10.1128/jvi.32.3.710-716.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King W., Van Santen V., Kieff E. Epstein-Barr virus RNA. VI. Viral RNA in restringently and abortively infected Raji cells. J Virol. 1981 May;38(2):649–660. doi: 10.1128/jvi.38.2.649-660.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luka J., Kallin B., Klein G. Induction of the Epstein-Barr virus (EBV) cycle in latently infected cells by n-butyrate. Virology. 1979 Apr 15;94(1):228–231. doi: 10.1016/0042-6822(79)90455-0. [DOI] [PubMed] [Google Scholar]
- Mueller-Lantzsch N., Yamamoto N. Epstein-Barr virus-induced proteins. IV. Characterization of an EBV-associated phosphopolypeptide. J Gen Virol. 1981 Aug;55(Pt 2):333–341. doi: 10.1099/0022-1317-55-2-333. [DOI] [PubMed] [Google Scholar]
- Pearson G. R., Vroman B., Chase B., Sculley T., Hummel M., Kieff E. Identification of polypeptide components of the Epstein-Barr virus early antigen complex with monoclonal antibodies. J Virol. 1983 Jul;47(1):193–201. doi: 10.1128/jvi.47.1.193-201.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack A., Hartl G., Zimber U., Freese U. K., Laux G., Takaki K., Hohn B., Gissmann L., Bornkamm G. W. A complete set of overlapping cosmid clones of M-ABA virus derived from nasopharyngeal carcinoma and its similarity to other Epstein-Barr virus isolates. Gene. 1984 Mar;27(3):279–288. doi: 10.1016/0378-1119(84)90072-6. [DOI] [PubMed] [Google Scholar]
- Powell A. L., King W., Kieff E. Epstein-Barr virus-specific RNA. III. Mapping of DNA encoding viral RNA in restringent infection. J Virol. 1979 Jan;29(1):261–274. doi: 10.1128/jvi.29.1.261-274.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Takaki K., Polack A., Bornkamm G. W. Expression of a nuclear and a cytoplasmic Epstein-Barr virus early antigen after DNA transfer: cooperation of two distant parts of the genome for expression of the cytoplasmic antigen. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4568–4572. doi: 10.1073/pnas.81.14.4568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Hausen H., O'Neill F. J., Freese U. K., Hecker E. Persisting oncogenic herpesvirus induced by the tumour promotor TPA. Nature. 1978 Mar 23;272(5651):373–375. doi: 10.1038/272373a0. [DOI] [PubMed] [Google Scholar]