Abstract
The intracellular parasite Toxoplasma gondii resides within a specialized compartment, the parasitophorous vacuole (PV), that resists fusion with host cell endocytic and lysosomal compartments. The PV is extensively modified by secretion of parasite proteins, including the dense granule protein GRA5 that is specifically targeted to the delimiting membrane of the PV (PVM). We show here that GRA5 is present both in a soluble form and in hydrophobic aggregates. GRA5 is secreted as a soluble form into the PV after which it becomes stably associated with the PVM. Topological studies demonstrated that GRA5 was inserted into the PVM as a transmembrane protein with its N-terminal domain extending into the cytoplasm and its C terminus in the vacuole lumen. Deletion of 8 of the 18 hydrophobic amino acids of the single predicted transmembrane domain resulted in the failure of GRA5 to associate with the PVM; yet it remained correctly packaged in the dense granules and was secreted as a soluble protein into the PV. Collectively, these studies demonstrate that the secretory pathway in Toxoplasma is unusual in two regards; it allows soluble export of proteins containing typical transmembrane domains and provides a mechanism for their insertion into a host cell membrane after secretion from the parasite.
INTRODUCTION
Among the varied lifestyles adopted by intracellular parasites is the residence in host cell vacuoles that do not fuse with lysosomes, a strategy exhibited by the protozoan parasite Toxoplasma and the bacterial pathogens Legionella, Chlamydia, and Mycobacteria (Garcia del-Portillo and Finlay, 1995). The Toxoplasma-containing vacuole, called the parasitophorous vacuole (PV)1, forms by active penetration of the parasite causing invagination of the plasma membrane bilayer (Suss-Toby et al., 1996). This compartment subsequently resists fusion with endosomes and lysosomes by a mechanism that is poorly understood; however, it is clear that the fate of the vacuole is determined at the time of formation (Sibley et al., 1985; Joiner, 1991; Mordue and Sibley, 1997). As the parasite grows and replicates within the cell, it forms a network of tubular membranes that are continuous with the delimiting membrane of the vacuole (Nichols et al., 1983; Sibley et al., 1995).
Secretion of proteins stored in apical organelles (micronemes, rhoptries, and dense granules) is a prominent feature of Toxoplasma invasion and presumably contributes both to the nonfusigenic status of the PV and subsequent nutrient uptake by the parasite (Carruthers and Sibley, 1997). Segregated from the endocytic system and cut off from the extracellular fluid, the parasite likely obtains its nutrients from the host cell cytoplasm by altering the permeability of the PV that is capable of bidirectional transport of metabolites of <1200 Da (Schwab et al., 1994). The parasite proteins that form this pore are presently unknown, but candidate molecules include rhoptry proteins that are inserted into the PV membrane (PVM) (Beckers et al., 1994) and various dense granule proteins (GRA proteins) that are targeted to the PVM or the intravacuolar network (reviewed in Cesbron-Delauw, 1994).
After their release within the vacuole, the GRA proteins are selectively targeted to specific locations of the PV where they are found either, like GRA1, as a soluble protein in the lumen of the vacuole or, like GRA2, as both soluble and membrane forms, the latter being closely associated with a reticulum of membranous tubules called the intravacuolar network (Cesbron-Delauw, 1994; Sibley et al., 1995). Others, like GRA3 and GRA5 are specifically targeted to the PVM (Achbarou et al., 1991; Lecordier et al., 1993). The mechanisms underlying the secretion of the GRA proteins and the basis of their targeting to specific locations in the PV are not known. Amino acid sequence analysis of several GRA proteins predicts that they may associate with membranes either via a transmembrane domain (GRA4, GRA5, GRA6, and GRA7) (Mévelec et al., 1992; Lecordier et al., 1993, 1995; Fischer et al., 1998; Jacobs et al., 1998), via two amphipathic α helices (GRA2) (Mercier et al., 1993, 1998), or via short hydrophobic regions (GRA3) (Ossorio et al., 1994). The primary amino acid sequence of GRA5 is composed of 120 amino acids consisting of an N-terminal hydrophobic leader, a hydrophilic stretch, and a single hydrophobic region of 18 amino acids, followed by a C-terminal hydrophilic region (Lecordier et al., 1993). This domain structure suggests that GRA5 is present in the secretory pathway as a membrane protein, although its membrane association or topology have not been reported previously.
Here, we examined the mechanism of GRA5 association with the PVM using an epitope tag strategy. We demonstrate that despite having an internal hydrophobic domain, GRA5 is secreted as a soluble protein and only then does it become stably associated with the PVM. Differential permeabilization experiments demonstrated that GRA5 is inserted into the PVM as a transmembrane protein with its N-terminal domain extending into the host cell and that this topology is acquired only after secretion.
MATERIALS AND METHODS
Reagents, Parasites, and Cell Culture
Enzymes were purchased from Boehringer Mannheim (Mannheim, Germany) and Amersham (Les Ulis, France); cell culture products and fetal bovine serum (FBS) were obtained from Life Technologies (Eragny, France). The RH strain of Toxoplasma gondii was grown in HFF (human foreskin fibroblasts) or 3T3 cells in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% FBS, 1% glutamine, and 20 μg/ml gentamicin. For experiments, infected cultures were scraped and forced through a 27-gauge needle, and parasites were filtered through a 3-μm polycarbonate membrane (Costar, Cambridge, MA) to eliminate cell debris.
Antibodies
Toxoplasma GRA proteins were detected using the mAbs TG17–43 (anti-GRA1), TG17–179 (anti-GRA2), and TG17–113 (anti-GRA5) (Charif et al., 1990). The mAb DG52, kindly provided by Dr. J. C. Boothroyd (Stanford University, Stanford, CA), or the mAb TG 05-54 was used to detect the Toxoplasma SAG1 surface protein. The anti-HA9 mAb 12CA5 and the anti-HA11 rabbit serum that also recognizes HA9 were purchased from Babco (Berkeley, CA).
To produce the mouse serum (anti-GRA5 Nt) that specifically reacts with the N-terminal domain of GRA5 that flanks its central hydrophobic domain, we isolated a fragment encoding the amino acids 30–67 of GRA5 by MaeIII digestion and subcloned the fragment into the expression vector pGEX-3X (Pharmacia, Uppsala, Sweden). The recombinant N-terminal fragment of GRA5 was expressed as a fusion with the Sj26 GST, purified by affinity on glutathione-agarose beads, and eluted by competition with free glutathione (Smith and Johnson, 1988). Mice were immunized by three injections of 10 μg of fusion protein. Specificity of the serum was compared with that of the anti-GRA5 mAb TG17–113 (Charif et al., 1990) by Western blot against parasite lysates and by immunofluorescence staining of infected cells.
Plasmid Construction
GRA5–HA9.
The GRA5 part of the construct comprises 355 bp upstream of the cap site, the 5′-nontranslated region, and the ORF until the stop codon (GenBank accession number: L06091). This fragment was amplified by PCR from the genomic clone containing the GRA5 gene (Lecordier et al., 1993) using a 5′ primer (sense; 5′-GACAAGGAATTCAGCCAGTAC-3′) that creates an EcoRI site. The 3′ primer (antisense; 5′-ACGTGGATCCGCATGCTAGCCTCTTCC-3′) was designed to replace the stop codon (position 464 of the previously published sequence [Lecordier et al., 1993]) with combined NheI–BamHI sites. The PCR fragment was cloned into the EcoRI and BamHI sites of the pBluescript KS+ vector (Stratagene, La Jolla, CA). The sequence encoding the HA9 epitope, followed by the 3′-nontranslated region of the GRA2 gene including the polyadenylation site (from the GRA2–HA9 construct [Mercier et al., 1998]), was inserted in-frame downstream of the GRA5 ORF at the NheI and BamHI sites.
GRA5Δ79–86–HA9.
The GRA5–HA9 construct was subcloned into the pAlterEX1 vector (Promega, Lyon, France) to perform mutagenesis. Two BstEII sites were created in-frame to allow deletion of the sequence encoding amino acids 79–86, by annealing of mutagenic oligonucleotides (5′-GTGGGGGTTACCGCAG-3′ and 5′-GGCCGTGGTGACCCTACTGC-3′) following the manufacturer’s protocols. The mutated construct was digested by BstEII and religated. Sites and deletion were confirmed by double-stranded Sanger dideoxy sequencing.
Transfection and Selection of Stable Transformants
Parasites (RH strain) were electroporated as described (Soldati and Boothroyd, 1993) with 100 μg of the GRA5–HA9 or GRA5Δ79–86–HA9 constructs linearized by BamHI and 10 μg of the selectable construct TUB5–CAT (kindly provided by Dr. D. Soldati, University of Heidelberg) linearized by Asp718. Transfected parasites were transferred to monolayers of HFF or 3T3 cells, and chloramphenicol selection was applied as described by Kim et al. (1993). Chloramphenicol-resistant parasites were cloned without drug pressure by limiting dilution in 96-well plates and were amplified in 24-well plates before analysis by SDS-PAGE and Western blotting.
Immunofluorescence
HFF cells were grown on 12-mm coverslips in 24-well plates until confluency and then infected with Toxoplasma parasites. After 24 or 48 h of culture, infected cells were washed three times with PBS and fixed with 3% formaldehyde in PBS for 30 min at room temperature. After three washes in PBS, cells were permeabilized 10 min in cold acetone and rinsed in PBS. After a 30-min saturation in 10% FBS in PBS, the first antibody was added for 1 h in 1% FBS in PBS. Cells were washed three times in PBS and incubated 30 min with the anti-species FITC-conjugated antibody (Sanofi Pasteur Diagnostics, Marnes-la-Coquette, France). After three washes in PBS, coverslips were mounted in Mowiol (Calbiochem, La Jolla, CA).
Digitonin permeabilization of infected cells was performed as described by Beckers et al. (1994). Total permeabilization after fixation was done by 10 min in cold 100% acetone.
Cell Fractionation
Extracellular Parasites.
Purified extracellular parasites were washed and resuspended in cold PBS without calcium and containing 1 mM EGTA and protease inhibitors (10 μg/ml Na-p-tosyl-l-lysine chloromethyl ketone, 10 μg/ml p-aminophenylmethylsulfonyl fluoride (a-PMSF), 1 μg/ml leupeptin, 10 μg/ml E-64). Parasites were lysed by three freeze/thaw (F/T) cycles, and parasite ghosts were separated by low-speed spin (10 min at 2500 × g). The supernatant was fractionated into soluble and membrane-associated fractions by high-speed spin (2 h at 100,000 × g in a Beckman [Fullerton, CA] TL-100 ultracentrifuge [TL-100.2 rotor]). To analyze the proteins not released by F/T, the low-speed pellet (LSP) containing parasite ghosts was resuspended in 50 mM Tris, pH 8.0, containing protease inhibitors and either submitted to Triton X-114 partitioning (Bordier, 1981) or treated with denaturing agents (6 M urea, 1% Nonidet P-40 [NP-40]) for 30 min at 4°C. After a second low-speed spin, soluble and membrane-associated fractions were fractionated 2 h at 100,000 × g. In some experiments, the LSP was subjected to sonication for three 15-sec pulses at an intensity of 5 using a microtip probe (Labsonic U; Braun, Melsungen, Germany), followed by removal of debris by centrifugation at 2500 × g for 10 min and fractionation of the supernatant by high-speed centrifugation (as described above).
Intracellular Parasites.
Infected cells were scraped in cold PBS without calcium and containing 1 mM EGTA and protease inhibitors (10 μg/ml Na-p-tosyl-l-lysine chloromethyl ketone, 10 μg/ml a-PMSF, 1 μg/ml leupeptin, 10 μg/ml E-64). Cells were disrupted by passage through 27-gauge needles, and parasites were eliminated by low-speed spin for 10 min at 2500 × g. The resulting supernatant, which contains the PV components, was separated into soluble and membrane-associated fractions by centrifugation for 2 h at 100,000 × g. To release membrane-associated proteins, we washed the high-speed pellet (HSP) in 50 mM Tris, pH 8.0, containing protease inhibitors and resuspended the pellet in the same buffer by sonication on ice. This sonicate was submitted either to Triton X-114 partitioning or to denaturing agents (1% NP-40, 6 M urea, 0.5 M KCl, or 0.1 M carbonate, pH 11.0) for 30 min at 4°C. Soluble and membrane-associated fractions were separated at 100,000 × g. Before analysis by SDS-PAGE and Western blotting, high-speed membrane pellets were washed with 50 mM Tris-HCl, pH 7.6, 1 mM EGTA, 100 mM sucrose, and protease inhibitors. Soluble fractions were concentrated either by centrifugation through Centricon-10 microconcentrators (Amicon, Beverly, MA) or by acetone or trichloroacetic acid precipitation.
Fractionation of Products Released from Extracellular Parasites.
The previously described assay for serum-stimulated secretion of dense granule proteins was used to induce GRA5 release by extracellular parasites (Darcy et al., 1988) (Coppens and Cesbron-Delauw, unpublished results). Extracellular tachyzoites (1.2 × 108) were incubated in 1 ml of RPMI medium supplemented with 10% (vol/vol) heat-inactivated FCS, under mild agitation, for 3 h at 37°C. Parasites were sedimented by centrifugation at 1300 × g, and the resulting LSP was resuspended in 1 ml of PBS. The low-speed supernatant (LSS) was supplemented in protease inhibitors and clarified by centrifugation at 20,800 × g. A fraction of the LSS was further separated into 100,000 × g pellet and supernatant.
Gel Electrophoresis and Western Blotting
Toxoplasma cells or membrane and soluble fractions were boiled for 3 min in SDS-PAGE sample buffer and separated by electrophoresis on 13% polyacrylamide gels (Laemmli, 1970). Gels were transferred to nitrocellulose membranes (Towbin et al., 1979) and blocked in 5% nonfat dry milk in PBS. Membranes were incubated with primary antibodies and then with anti-species peroxidase conjugates (Sanofi Pasteur Diagnostics), both diluted in 1% nonfat dry milk in PBS. Membranes were incubated 1 min with ECL reagents (Dupont New England Nuclear, Les Ulis, France) and exposed to Hyperfilm-ECL (Amersham).
RESULTS
GRA5 Is Packaged within Dense-Core Secretory Granules and Becomes a Transmembrane Protein after Its Secretion into the PV
Previous electron microscopy studies demonstrated that GRA5 is stored within dense granules in Toxoplasma cells and is released into the PV where it is found closely associated with the PVM (Charif et al., 1990; Lecordier et al., 1993). To explore the behavior of GRA5 during its secretion, we examined the soluble and/or membrane distribution of GRA5 by cell fractionation experiments of both extracellular Toxoplasma cell extracts and parasite-free vacuolar fractions.
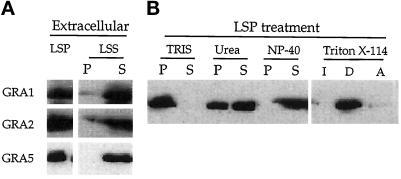
In F/T Toxoplasma cell lysates, GRA5 was detected in the soluble fraction as were two other dense granule proteins, GRA1 and GRA2 (Figure 1A). These results indicate that GRA5 exists in a soluble form in the dense granules. However, GRA5 was also detected in the LSP containing parasite ghosts, indicating that another fraction of the protein was not released by this treatment. To study the nature of this insoluble form of GRA5, we submitted the LSP to more disruptive treatments using denaturing agents or Triton X-114 partitioning. GRA5 was totally released by NP-40 and partially removed from the pellet by 6 M urea (Figure 1B). In contrast, SAG1, a Toxoplasma glycosylphosphatidylinositolanchored membrane protein, behaves as expected, being both fully solubilized by NP-40 and efficiently pelleted in urea (our unpublished results). This suggests that GRA5 may be contained in both hydrophobic aggregates and/or membranes that remain in the granule core after F/T treatment. Consistent with this, GRA5 that remained trapped in the LSP after F/T was detected in the detergent phase after Triton X-114 partitioning (Figure 1B). In contrast, GRA1 partitioned exclusively in the aqueous phase (our unpublished results). The presence of GRA5 in both a soluble and an insoluble form suggests that the granule contents are stabilized by hydrophilic and hydrophobic interactions as described for other dense-core secretory granules in various mammalian cells (Colomer et al., 1996).
Figure 1.
GRA5 is found both in a soluble form and in hydrophobic aggregates within extracellular parasites. (A) Parasites were disrupted by F/T cycles and separated by low-speed centrifugation into cell ghosts (LSP) and a soluble fraction (LSS). The LSS was further fractionated by a high-speed spin into a membrane pellet (P) and a soluble fraction (S). Fractions were separated by SDS-PAGE, and the GRA1 (24 kDa), GRA2 (28 kDa), and GRA5 (21 kDa) proteins were revealed by Western blot using the TG17–43, TG17–179, and TG17–113 monoclonal antibodies, respectively. (B) The LSP obtained in A was resuspended in 50 mM Tris, pH 8.0, incubated with denaturing agents, and fractionated by high-speed centrifugation into a membrane pellet (P) and a soluble fraction (S). Fractions were analyzed by SDS-PAGE and Western blotting using the anti-GRA5 mAb TG17–113. GRA5 remaining in the LSP was partially solubilized by urea, completely released from the membrane pellet by NP-40, and partitioned into the detergent phase (D) after treatment with Triton X-114.
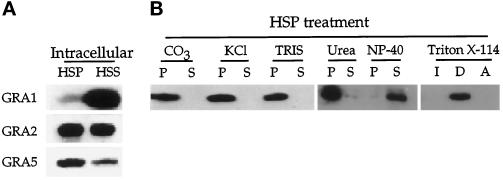
To determine which form of the protein is present in the PV, we examined the partitioning of GRA5 after mechanical disruption of infected cells by needle passages (Figure 2). This treatment, followed by low-speed centrifugation, allows separation of intact parasites from the host cell lysate that was further sedimented at 100,000 × g to obtain a soluble phase and a membrane pellet (Figure 2A). Lack of contamination in these fractions by intact parasites was assessed by the absence of the SAG1 surface protein (our unpublished results). As described previously (Sibley et al., 1995), GRA1 remained exclusively soluble in the PV, and GRA2 occurred in both soluble and membrane-associated forms. In contrast, the GRA5 protein was preferentially detected in the HSP, reflecting a predominant membrane association of this protein with the PVM (Figure 2A).
Figure 2.
GRA5 is a membrane-associated protein within the PV. (A) Infected cells were disrupted by passages through needles, and parasites and cell debris were removed by low-speed centrifugation. The LSS was further fractionated by high-speed centrifugation into a membrane pellet (HSP) and a soluble fraction (HSS), separated by SDS-PAGE, and probed with the anti-GRA1, -GRA2, and -GRA5 monoclonal antibodies as described in Figure 1. Whereas GRA1 was found almost exclusively in the soluble fraction, GRA2 was detected in both the soluble and pellet fractions. GRA5 was predominantly detected as a membrane-associated form in the HSP. (B) The membrane pellet obtained in A was resuspended in 50 mM Tris, pH 8.0, incubated with different denaturing agents, and submitted to a high-speed spin. Membrane pellets (P) and soluble fractions (S) were analyzed by SDS-PAGE and Western blotting using the anti-GRA5 mAb. GRA5 behaved as an integral membrane protein, remaining in the membrane pellet after high pH (CO3), high salt concentration (KCl), or urea treatment but was released by NP-40 and partitioned in the detergent phase after treatment with Triton X-114.
To examine the nature of the GRA5 membrane interaction, HSPs were submitted to either Triton X-114 partitioning or denaturing agents (Figure 2B). The membrane association of GRA5 was not disrupted by treatments capable of releasing peripheral membrane proteins such as high pH or high salt concentration (1 M KCl). Additionally, and in contrast to what was observed within extracellular parasites, vacuolar GRA5 was not solubilized by 6 M urea but only by NP-40. After Triton X-114 partitioning, vacuolar GRA5 was found exclusively in the detergent phase. Taken together, these experiments indicated that GRA5 behaves as an integral membrane protein after its secretion into the PV.
GRA5 Is Released from the Dense Granules Exclusively as a Soluble Form
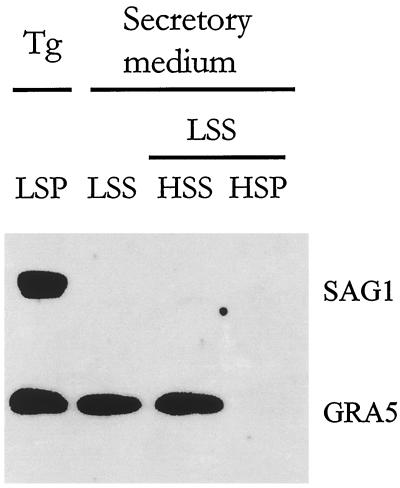
The above results suggested that GRA5 is secreted as a soluble form and that its membrane association is a postsecretory event. We therefore examined the soluble release of GRA5 using an in vitro assay for dense granule secretion (Darcy et al., 1988) (Coppens and Cesbron-Delauw, unpublished results). Extracellular parasites were incubated in a secretory medium for 3 h. Intact parasites were removed by low-speed centrifugation (LSP), and the secreted proteins were recovered in the supernatant (LSS). Western blot analysis of these fractions revealed that ∼50% of GRA5 has been released into the secretory medium (LSS) (Figure 3). After fractionation of the LSS by high-speed centrifugation, the totality of GRA5 was recovered in the high-speed supernatant (HSS). These data confirmed that GRA5 is released exclusively as a soluble form.
Figure 3.
GRA5 is exocytosed in a soluble form by extracellular parasites. Extracellular tachyzoites were incubated in a secretory medium for 3 h. Parasites (Tg) were sedimented by centrifugation at 1300 × g (LSP). A fraction of the LSS was further separated into 100,000 × g pellet (HSP) and supernatant (HSS). Equal percentages of the LSP, LSS, HSS, and HSP fractions were analyzed by Western blotting probed with both the anti-SAG1 and the anti-GRA5 mAbs. The fraction of GRA5 released in the supernatant (LSS) was found exclusively associated with the soluble fraction (HSS), whereas, in control, the SAG1 surface protein remained exclusively associated with the parasite fraction (LSP).
GRA5 Tagged with the HA9 Epitope Behaves as the Wild-Type GRA5
To examine the basis of membrane association by GRA5, we constructed an epitope-tagged form of GRA5 using HA9, a peptide of influenza virus hemagglutinin (Wilson et al., 1984). The HA9 epitope tag–coding sequence (YPYDVPDYA) was fused to the C terminus of GRA5 and expressed under the control of the GRA5 gene promoter (Mercier et al., 1996). Stably transformed Toxoplasma lines expressing GRA5–HA9 were obtained using chloramphenicol selection (Kim et al., 1993).
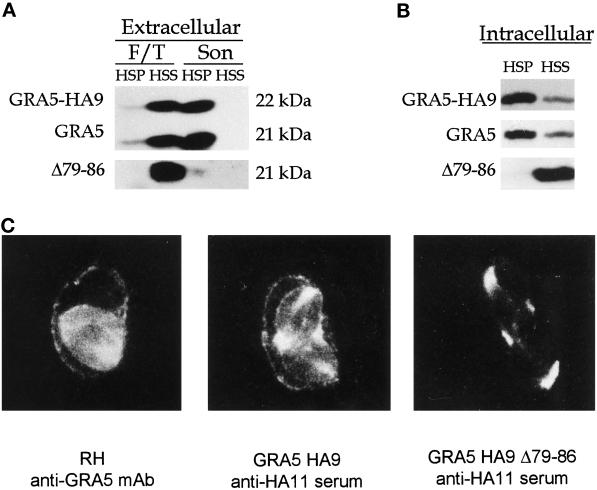
The GRA5–HA9 protein migrated with an apparent molecular weight of 22 kDa compared with that (21 kDa) of the endogenous GRA5 as detected by Western blot (Figure 4A). The distributions of wild-type and epitope-tagged GRA5 were examined using a slightly different cell fractionation procedure from that described in Figure 1. Initially, extracellular parasites were subjected to F/T lysis, the lysate was cleared from remaining cellular debris by low-speed centrifugation, and the resulting supernatant was further fractionated by sedimentation at 100,000 × g. Analysis of these fractions revealed that, like the wild-type protein, epitope-tagged GRA5 released by F/T was completely soluble (Figures 1A and 4A). The LSP was then further subjected to sonication and recentrifugation. This treatment released both the wild-type and epitope-tagged forms of GRA5 from the LSP; the majority of the proteins pelleted at 100,000 × g, indicating they were present within aggregates or membrane inclusions (Figure 4A, Son). Like the wild-type protein, GRA5–HA9 formed a stable association with the membranes within the PV (Figure 4B) that was resistant to 1 M KCl, pH 11 carbonate, and 6 M urea but fully extracted by NP-40 detergent (our unpublished results).
Figure 4.
Analysis of wild-type GRA5, GRA5–HA9, and the deletion mutant GRA5Δ79–86–HA9 by cell fractionation in both extracellular parasites (A) and the PV (B) and by immunofluorescence (C). (A) Extracellular parasites from the wild-type RH strain and from transgenic RH lines expressing either GRA5–HA9 or GRA5Δ79–86–HA9 were subjected to F/T treatment followed by low-speed centrifugation and fractionation of the supernatant into a 100,000 × g pellet (F/T, HSP) and supernatant (F/T, HSS). The wild-type GRA5 and GRA5–HA9 (both revealed by the anti-GRA5 mAb TG17–113) and GRA5Δ79–86–HA9 (revealed by anti-HA11 serum) were primarily detected in the soluble fraction. The LSP was further treated by sonication followed by fractionation of the LSS into a 100,000 × g pellet (Son, HSP) and supernatant (Son, HSS). The wild-type and epitope-tagged forms of the protein behaved as hydrophobic aggregates, being sedimented at 100,000 × g. In contrast, the deletion mutant (Δ79–86) was exclusively detected as a soluble protein. (B) PV proteins were fractionated as described in Figure 2. GRA5–HA9 and GRA5 were predominantly present as membrane-associated forms, whereas the Δ79–86 deletion mutant was released exclusively as a soluble form. The tagged proteins were both revealed by the anti-HA11 serum, whereas wild-type GRA5 was revealed by the TG17–113 mAb. (C) Immunolocalization of GRA5, GRA5–HA9, and GRA5Δ79–86–HA9 in infected cells is shown. Immunofluorescence was performed on cells infected with the wild-type RH strain (left), the full-length GRA5–HA9 clone (middle), and the deletion mutant GRA5Δ79–86–HA9 (right). The GRA5 and GRA5–HA9 proteins were both observed between intracellular parasites and associated with the vacuole membrane. In contrast, the deletion mutant was exclusively detected between parasites and was not found associated with the PVM or cytoplasmic extensions.
GRA5 and GRA5–HA9 Are Targeted to the PV Membrane
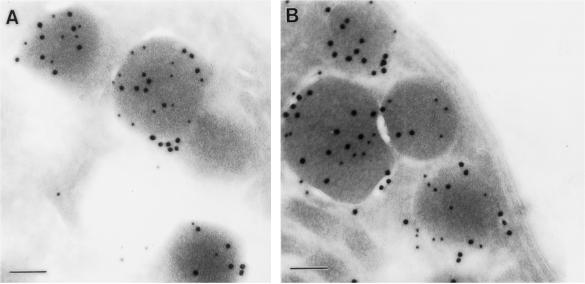
Immunofluorescence microscopy of cells infected with the wild-type strain showed that GRA5 accumulates between the parasites and associates with both the PVM (Figure 4C) and its extension in the host cell cytoplasm (our unpublished results). The accumulation between the parasites likely reflects the presence of the protein within the vacuole lumen that likely corresponds to the soluble form of the protein observed in cell fractionation experiments (Figure 2A). In cells infected with the GRA5–HA9 Toxoplasma line, similar staining was observed using the anti-HA11 rabbit serum (Figure 4C), indicating that the tagged protein has the same overall distribution as the wild-type GRA5. Immunoelectron microscopy confirmed that epitope-tagged GRA5 colocalized with GRA1 in dense granules (Figure 5A). Within the PV, GRA5 was primarily associated with the limiting membrane of the vacuole and to a lesser extent was detected within the lumen of the vacuole, associated with the intravacuolar network and the parasite cell surface (Figure 6, A and B). Previous studies based on plastic-embedded tissue reported the prominent PVM staining of GRA5 (Lecordier et al., 1993); however, the lumenal staining is newly described and presumably represents the increased sensitivity of cryoelectron microscopy (cryoEM) used here.
Figure 5.
ImmunoEM localization of GRA5–HA9 and GRA5Δ79–86–HA9 within dense granules of Toxoplasma. (A) GRA5–HA9 (anti-HA11, 18-nm gold particles) was found concentrated within the same population of dense granules as GRA1 (TG17–43, 12-nm gold particles). (B) The deletion mutant GRA5Δ79–86–HA9 (anti-HA11, 18-nm gold particles) was also packaged within the same dense granules as GRA1 (TG17–43, 12-nm gold particles). Bar, 100 nm.
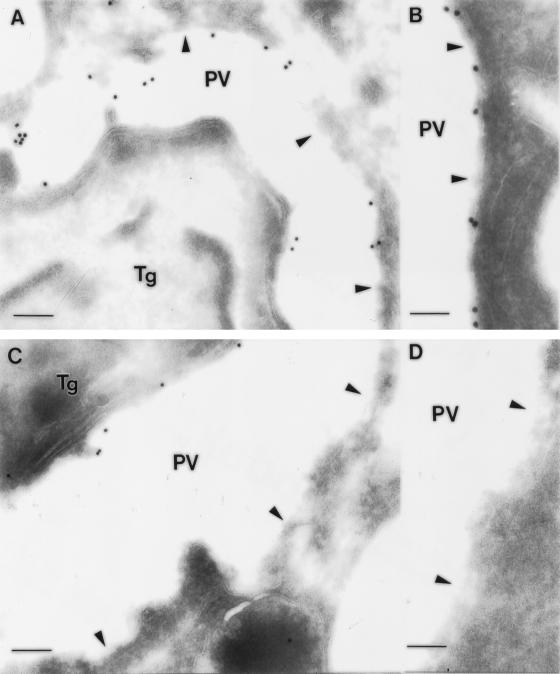
Figure 6.
ImmunoEM localization of GRA5–HA9 and GRA5Δ79–86–HA9 after secretion into the PV. (A and B) GRA5–HA9 was secreted into the PV where it became associated with the PVM (arrowheads). (C and D) The deletion mutant GRA5Δ79–86–HA9 was secreted within the PV but failed to associate with the PVM. Tg, Toxoplasma gondii cell. Bars: in A and C, 200 nm; in B and D, 100 nm.
The GRA5 Protein Interacts with the PVM through Its Central Hydrophobic Domain
GRA5 has a central hydrophobic domain (amino acids 76–93) that could form a membrane-spanning α helix (Lecordier et al., 1993). To determine whether this hydrophobic domain is involved in the membrane association of GRA5, we generated a construct in which 8 of the 18 amino acids of this domain were deleted by oligonucleotide-directed mutagenesis (GRA5Δ79–86–HA9). A Toxoplasma line expressing the GRA5Δ79–86–HA9 protein was analyzed by Western blotting, and the mutated protein was observed to comigrate with the endogenous GRA5 (our unpublished results). Immunoelectron microscopy revealed that the GRA5Δ79–86–HA9 mutant was correctly packaged in the dense granules (Figure 5B) and secreted into the PV (Figure 6C), indicating that the deletion does not alter the trafficking of the protein. However, this deletion mutant was only detected by immunofluorescence or EM within the lumen of the PV and did not decorate the PVM (Figures 4C and 6, C and D). Cell fractionation experiments also confirmed that the deletion mutant partitioned exclusively in the soluble fractions of both tachyzoites and vacuolar extracts (Figure 4, A and B, respectively). Collectively, these results demonstrate that the central hydrophobic domain is necessary for interaction of the protein within hydrophobic aggregates in dense granules and for insertion into the vacuolar membrane after secretion into the PV.
The GRA5 Protein Is Inserted in the PVM with Its N-Terminal Domain Exposed to Host Cell Cytoplasm
After secretion into the PV, GRA5 undergoes a transition from a soluble to a membrane form, and in the process, it becomes integrally associated with the PVM. Because the PVM defines the host–parasite interface, we chose to examine the topology of GRA5 within this membrane to determine whether either of the two hydrophilic ends of the protein were exposed to the host cell cytoplasm. To differentiate the C terminus from the N terminus, we used a GRA5–HA9 construct containing a C-terminal HA9 epitope tag. In addition, we generated antibodies that specifically recognized the N-terminal hydrophilic domain of GRA5 by immunizing mice with an Escherichia coli recombinant fusion protein comprising the N-terminal amino acids 30–67 of GRA5 (referred to as anti-GRA5 Nt serum). The topology of GRA5 within the PVM was determined using these domain-specific antisera to probe cells that were selectively permeabilized using low concentrations of digitonin to disrupt the host cell plasma membrane while leaving the PVM intact (Beckers et al., 1994).
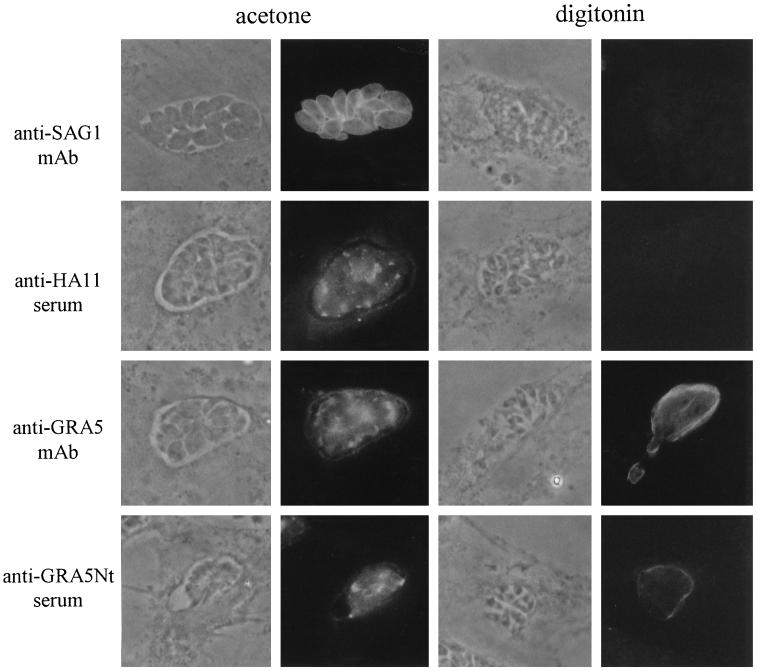
When GRA5–HA9–infected cells were fully permeabilized by acetone, the staining patterns obtained with the anti-GRA5 mAb and the anti-GRA5 Nt and anti-HA11 sera were similar (Figure 7). Both GRA5 and GRA5–HA9 were detected between the parasites and associated with the PVM. Under these conditions, the parasite surface was also readily detected by the anti-SAG1 mAb. After selective digitonin permeabilization of the plasma membrane but not the PVM (verified by the inability to detect the parasite surface with the anti-SAG1 mAb), the PV was not stained with the anti-HA11 antibody, whereas under the same conditions, prominent staining of the vacuoles was observed with both the anti-GRA5 Nt serum and the anti-GRA5 mAb. This staining was limited to the vacuole contour; GRA5 that accumulates within the PV between parasites was not detected. These results demonstrate that the GRA5 protein is a transmembrane protein with its N terminus facing the external side of the PV and exposed to the host cell cytoplasm, whereas the C terminus remains within the vacuole.
Figure 7.
Orientation of the GRA5–HA9 protein within the PVM by immunofluorescence staining. Cells are visualized by phase-contrast microscopy. Immunofluorescence experiments were performed on cells infected with the GRA5–HA9 Toxoplasma clone. Cells were permeabilized either completely by cold acetone (left) or selectively by 0.004% digitonin (right). Left, when cells were permeabilized by acetone, the parasite surface was stained by the anti-SAG1 mAb. Under these conditions, the staining patterns obtained with both anti-GRA5 antibodies (the TG17–113 mAb and the anti-GRA5 Nt serum) and the anti-HA11 rabbit serum were identical; GRA5–HA9 was detected associated with the PVM and in clusters between the parasites. Right, when the host cell membrane was selectively permeabilized by digitonin, as verified by the negative staining of the parasite surface with the anti-SAG1 mAb, the C-terminal part of GRA5–HA9, recognized by the anti-HA11 serum, was not detected. In contrast, the external side of the PV was stained by both the anti-GRA5 Nt serum and the anti-GRA5 mAb.
DISCUSSION
We show here that GRA5 is secreted as a soluble protein, whereupon it undergoes a conformational change to insert into a lipid bilayer as a transmembrane protein. Within the parasite, GRA5 exists within the matrix of dense secretory granules in two forms, as a water-soluble molecule and trapped within hydrophobic aggregates. Despite the presence of a hydrophobic domain sufficient to span the membrane, it does not adopt a transmembrane configuration within the secretory granules, as shown by immunoEM and its susceptibility to extraction with urea. GRA5 is secreted by extracellular parasites as a water-soluble form, exclusively. However, after exocytosis into the PV, it becomes primarily membrane associated and is targeted to the vacuolar membrane. Within the PVM, GRA5 adopts a transmembrane topology that is fully resistant to urea extraction. Immunological approaches indicate that it inserts into the PVM with its N terminus extending into the host cell cytoplasm and its C terminus remaining within the lumen of the vacuole. Membrane insertion required the single hydrophobic region because deletion of eight hydrophobic residues from this domain specifically disrupted association with the PVM. These studies demonstrate that Toxoplasma contains a specialized secretory system designed for soluble export of proteins that are destined for insertion into host cell membranes.
Although Toxoplasma is a primitive eukaryote, it has a well-developed, although not well-studied, secretory pathway that includes prominent ER, Golgi, and three classes of secretory vesicles called rhoptries, dense granules, and micronemes. Despite the lack of specific knowledge of the secretory pathway in Toxoplasma, random cDNA-sequencing efforts indicate the presence of homologues for components of the ER translocon, including SEC61, SEC63, SEC11 (signal sequence–processing protein), and SEC23 (component of COPII coat), as well as the ER chaperones, protein disulfide isomerase, and BIP (Ajioka et al., 1998), suggesting the presence of a well-developed secretory pathway. ImmunoEM studies indicate that dense granule proteins are likely synthesized on membrane-bound ribosomes, imported into the ER, and translocated to the Golgi for packaging (Charif et al., 1990; Sibley et al., 1995) (Coppens et al., and Cesbron-Delauw, unpublished results). Dense granule secretion in Toxoplasma occurs by a process that resembles classical exocytosis with the release of amorphous material into the PV (Leriche and Dubremetz, 1990). Accordingly, the dense granules contain a mixture of proteins that are released as soluble forms in the PV and, thereafter, are targeted to their specific location in the PV.
The N-terminal hydrophobic domain of GRA5 presumably functions as a signal peptide, directing GRA5 to the ER before its cleavage, a conclusion that is indirectly supported by the relative migration on SDS-PAGE of the protein produced by in vitro translation versus metabolic-labeling studies (our unpublished results). However, the central hydrophobic domain of GRA5 does not adopt a transmembrane configuration in the ER or the dense granule as might be expected. Instead, it appears to be partially trapped in hydrophobic aggregates in the dense granules. The protein GRA3 also exists in the dense granules in primarily a soluble form, and after secretion into the PV, it forms hydrophobic aggregates that tightly associate with the PVM (Ossorio et al., 1994). GRA5 apparently undergoes a similar conformational change after secretion and, in addition, inserts into the PVM as a transmembrane protein. It is suggested that dense granules represent a default secretory pathway (Karsten et al., 1998) (our unpublished results), perhaps allowing the parasite to secrete proteins in a soluble form that would otherwise associate with membranes. In this regard, several other GRA proteins that are secreted within the PV also contain central hydrophobic domains that are predicted to mediate their interactions with membranes, including GRA4 and GRA6 (reviewed in Cesbron-Delauw, 1994). In contrast, all of the abundant cell surface proteins of Toxoplasma are linked in the membrane via glycosylphosphate inositol anchors at their C termini (Tomavo et al., 1989), an addition that may mark proteins for export to the plasma membrane (Seeber et al., 1998).
The insertion or translocation of proteins across membranes does not normally occur spontaneously but requires accessory proteins to mediate the passage of large hydrophilic protein domains across the impermeant phospholipid barrier. Protein translocation occurs via well-developed complexes including the ER translocon (von Heijne, 1997), via Sec-dependent secretion in bacteria (von Heijne, 1997), and during import into mitochondria (Stuart and Neupert, 1996). There are, however, examples of posttranslational membrane insertion of mature proteins in the absence of the ER translocon type of system. In bacteria, the M13 procoat protein inserts into the inner membrane in a Sec-independent manner via interactions between N-terminal and internal hydrophobic domains that create a hydrophobic α helix that spontaneously inserts into membranes (Ohno-Iwashita and Wickner, 1983; Gallusser and Kuhn, 1990). Membrane insertion of water-soluble proteins after their secretion occurs during translocation of the pore-forming bacterial toxins into target cell membranes (Ojcius and Young, 1991; van der Goot et al., 1992). In Yersinia, the YopB protein is released from the bacterium by a type III secretion process and then inserts in the host cell membrane and forms a protein-translocating apparatus that transfers other Yops into the host cell cytoplasm (Hakånsson et al., 1996). The specific interactions that govern the above examples are each unique, but presumably all involve conformational changes in the protein that are associated with transfer from a soluble state to insertion into the bilayer.
Toxoplasma is highly specialized for protein secretion (Carruthers and Sibley, 1997) and may have more than one mechanism for insertion of proteins into host cell membranes after their secretion from the parasite. The rhoptry protein ROP2 is secreted at the time of invasion and is translocated across the PVM, exposing epitopes to the host cell cytoplasm (Beckers et al., 1994). This insertion may occur at the time of invasion because rhoptry secretion is coincident with vacuole formation (Carruthers and Sibley, 1997). In contrast, the protein studied here is secreted after invasion, suggesting a different mechanism of membrane insertion. Insertion of GRA5 into the membrane may be mediated by additional protein components within the PV, an environment enriched by extensive exocytosis of parasite proteins including a cyclophilin homologue that may participate in protein refolding (High et al., 1994). The association of GRA5 with the intravacuolar network suggests this interface may serve a function in the trafficking of secretory proteins that are destined for insertion into the PVM. Postsecretory membrane translocation also occurs in the related parasite Plasmodium that secretes proteins across the PVM within red cells via an ATP-dependent process, suggesting the presence of a specialized protein translocation apparatus (Ansorge et al., 1996). Because of the highly specialized intracellular existence of these parasites, it is likely they have evolved specific machinery for protein secretion and insertion across multiple cellular membranes. As such, these systems offer a rich territory to explore novel mechanisms of protein–membrane interactions including posttranslational bilayer insertion.
ACKNOWLEDGMENTS
The authors thank Dr. D. Soldati for the TUB5–CAT construct, Dr. J. C. Boothroyd for the DG52 monoclonal antibody, Drs. V. B. Carruthers and E. Labruyère for critical review of the manuscript, and Drs. J.-F. Dubremetz and B. Hoflack for helpful discussions. They acknowledge D. Deslée for immunizations and J. M. Merchez for illustrations. This work was supported in part by Institut Pasteur de Lille, Institut National de la Santé et de la Recherche Médicale, (CNAMTS-3AM015), EEC Biotech (BIO2CT-930238), Ministère de la Recherche (ACC-SV6), and the National Institutes of Health (AI-34036).
Abbreviations used:
- EM
electron microscopy
- FBS
fetal bovine serum
- F/T
freeze/thaw
- HFF
human foreskin fibroblasts
- HSP
high-speed pellet
- HSS
high-speed supernatant
- LSP
low-speed pellet
- LSS
low-speed supernatant
- NP-40
Nonidet P-40
- PV
parasitophorous vacuole
- PVM
PV membrane
REFERENCES
- Achbarou A, Mercereau-Puijalon O, Sadak A, Fortier B, Leriche MA, Camus D, Dubremetz JF. Differential targeting of dense granule proteins in the parasitophorous vacuole of Toxoplasma gondii. Parasitology. 1991;103:321–329. doi: 10.1017/s0031182000059837. [DOI] [PubMed] [Google Scholar]
- Ajioka JA, et al. Gene discovery by EST sequencing in Toxoplasma gondii reveals sequences restricted to the Apicomplexa. Genet Res. 1998;8:18–28. doi: 10.1101/gr.8.1.18. [DOI] [PubMed] [Google Scholar]
- Ansorge I, Benting J, Bhakdi S, Lingelbach K. Protein sorting in Plasmodium falciparum-infected red blood cells permeabilized with the pore-forming protein streptolysin O. Biochem J. 1996;315:307–314. doi: 10.1042/bj3150307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckers CJM, Dubremetz JF, Mercereau-Puijalon O, Joiner KA. The Toxoplasma gondii protein ROP2 is inserted into the parasitophorous vacuole membrane, surrounding the intracellular parasite, and is exposed to the host cell cytoplasm. J Cell Biol. 1994;127:947–961. doi: 10.1083/jcb.127.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981;256:1604–1607. [PubMed] [Google Scholar]
- Carruthers VB, Sibley LD. Sequential protein secretion from three distinct organelles of Toxoplasma gondii accompanies invasion of human fibroblasts. Eur J Cell Biol. 1997;73:114–123. [PubMed] [Google Scholar]
- Cesbron-Delauw MF. Dense granule organelles of Toxoplasma gondii: their role in the host-parasite relationship. Parasitol Today. 1994;10:293–296. doi: 10.1016/0169-4758(94)90078-7. [DOI] [PubMed] [Google Scholar]
- Charif H, Darcy F, Torpier G, Cesbron-Delauw MF, Capron A. Toxoplasma gondii: characterization and localization of antigens secreted from tachyzoites. Exp Parasitol. 1990;71:114–124. doi: 10.1016/0014-4894(90)90014-4. [DOI] [PubMed] [Google Scholar]
- Colomer V, Kicska GA, Rindler MJ. Secretory granule content proteins and the luminal domain of granule membrane proteins aggregate in vitro at mildly acidic pH. J Biol Chem. 1996;271:48–55. doi: 10.1074/jbc.271.1.48. [DOI] [PubMed] [Google Scholar]
- Darcy F, Deslée D, Santoro F, Charif H, Auriault C, Decoster A, Duquesne V, Capron A. Induction of a protective antibody-dependent response against toxoplasmosis by in vitro excreted-secreted antigens from tachyzoites of Toxoplasma gondii. Parasite Immunol. 1988;10:553–567. doi: 10.1111/j.1365-3024.1988.tb00242.x. [DOI] [PubMed] [Google Scholar]
- Fischer HG, Stachelhaus S, Sahm M, Meyer HE, Reichmann G. GRA7, an excretory 29 kDa Toxoplasma gondii dense granule antigen released by infected host-cells. Mol Biochem Parasitol. 1998;91:251–262. doi: 10.1016/s0166-6851(97)00227-2. [DOI] [PubMed] [Google Scholar]
- Gallusser A, Kuhn A. Initial steps in protein membrane insertion. Bacteriophage M13 procoat protein binds to the membrane surface by electrostatic interaction. EMBO J. 1990;9:2723–2729. doi: 10.1002/j.1460-2075.1990.tb07459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia del-Portillo F, Finlay B. The varied lifestyles of intracellular pathogens within eukaryotic vacuolar compartments. Trends Microbiol. 1995;3:373–380. doi: 10.1016/s0966-842x(00)88982-9. [DOI] [PubMed] [Google Scholar]
- Hakånsson S, Schesser K, Persson C, Galyov EE, Rosqvist R, Homble F, Wolf-Watz H. The YopB protein of Yersinia pseudotuberculosis is essential for the translocation of Yop effector proteins across the target cell plasma membrane and displays a contact-dependent membrane disrupting activity. EMBO J. 1996;15:5812–5823. [PMC free article] [PubMed] [Google Scholar]
- High KP, Joiner KA, Handschumacher RE. Isolation, cDNA sequences, and biochemical characterization of the major cyclosporin-binding proteins of Toxoplasma gondii. J Biol Chem. 1994;269:9105–9112. [PubMed] [Google Scholar]
- Jacobs D, Dubremetz JF, Loyens A, Bosman F, Saman E. Identification and heterologous expression of a new dense granule protein (GRA7) from Toxoplasma gondii. Mol Biochem Parasitol. 1998;91:237–249. doi: 10.1016/s0166-6851(97)00204-1. [DOI] [PubMed] [Google Scholar]
- Joiner KA. Rhoptry lipids and parasitophorous vacuole formation: a slippery issue. Parasitol Today. 1991;7:226–227. doi: 10.1016/0169-4758(91)90232-d. [DOI] [PubMed] [Google Scholar]
- Karsten V, Qi H, Beckers CJM, Reddy A, Dubremetz JF, Webster P, Joiner KA. The protozoan parasite Toxoplasma gondii targets proteins to dense granules and vacuolar space using both conserved and unusual mechanisms. J Cell Biol. 1998;141:1323–1333. doi: 10.1083/jcb.141.6.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Soldati D, Boothroyd JC. Gene replacement in Toxoplasma gondii with chloramphenicol acetyltransferase as selectable marker. Science. 1993;262:911–914. doi: 10.1126/science.8235614. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lecordier L, Mercier C, Torpier G, Tourvieille B, Darcy F, Liu JL, Maes P, Tartar A, Capron A, Cesbron-Delauw MF. Molecular structure of a Toxoplasma gondii dense granule antigen (GRA5) associated with the parasitophorous vacuole membrane. Mol Biochem Parasitol. 1993;59:143–154. doi: 10.1016/0166-6851(93)90015-p. [DOI] [PubMed] [Google Scholar]
- Lecordier L, Moleon-Borodowski I, Dubremetz JF, Tourvieille B, Mercier C, Deslée D, Capron A, Cesbron-Delauw MF. Characterization of a dense granule antigen of Toxoplasma gondii (GRA6) associated to the network of the parasitophorous vacuole. Mol Biochem Parasitol. 1995;70:85–94. doi: 10.1016/0166-6851(95)00010-x. [DOI] [PubMed] [Google Scholar]
- Leriche MA, Dubremetz JF. Exocytosis of Toxoplasma gondii dense granules into the parasitophorous vacuole after host cell invasion. Parasitol Res. 1990;76:559–562. doi: 10.1007/BF00932560. [DOI] [PubMed] [Google Scholar]
- Mercier C, Cesbron-Delauw MF, Sibley LD. The amphipathic α-helices of the Toxoplasma protein GRA2 mediate postsecretory membrane association. J Cell Sci. 1998;111:2171–2180. doi: 10.1242/jcs.111.15.2171. [DOI] [PubMed] [Google Scholar]
- Mercier C, Lecordier L, Darcy F, Deslée D, Murray A, Tourvieille B, Maes P, Capron A, Cesbron-Delauw MF. Molecular characterization of a dense granule antigen (GRA2) associated with the network of the parasitophorous vacuole in Toxoplasma gondii. Mol Biochem Parasitol. 1993;58:71–82. doi: 10.1016/0166-6851(93)90092-c. [DOI] [PubMed] [Google Scholar]
- Mercier C, Lefèvre-Van Hende S, Garber GE, Lecordier L, Capron A, Cesbron-Delauw MF. Common cis-acting elements critical for the expression of several genes of Toxoplasma gondii. Mol Microbiol. 1996;21:421–428. doi: 10.1046/j.1365-2958.1996.6501361.x. [DOI] [PubMed] [Google Scholar]
- Mévelec MN, Chardès T, Mercereau-Puijalon O, Bourguin I, Achbarou A, Dubremetz JF, Bout D. Molecular cloning of GRA4, a Toxoplasma gondii dense granule protein, recognized by mucosal IgA antibodies. Mol Biochem Parasitol. 1992;56:227–238. doi: 10.1016/0166-6851(92)90172-g. [DOI] [PubMed] [Google Scholar]
- Mordue DG, Sibley LD. Intracellular fate of vacuoles containing Toxoplasma gondii is determined at the time of formation and depends on the mechanism of entry. J Immunol. 1997;159:4452–4459. [PubMed] [Google Scholar]
- Nichols BA, Chiappino ML, O’Connor GR. Secretion from the rhoptries of Toxoplasma gondii during host-cell invasion. J Ultrastruct Res. 1983;83:85–98. doi: 10.1016/s0022-5320(83)90067-9. [DOI] [PubMed] [Google Scholar]
- Ohno-Iwashita Y, Wickner W. Reconstitution and asymmetric assembly of M13 procoat protein into liposomes which have bacterial leader peptidase. J Biol Chem. 1983;258:1895–1900. [PubMed] [Google Scholar]
- Ojcius DM, Young JD. Cytolytic pore-forming proteins and peptides: is there a common structural motif? Trends Biochem Sci. 1991;16:225–229. doi: 10.1016/0968-0004(91)90090-i. [DOI] [PubMed] [Google Scholar]
- Ossorio PN, Dubremetz JF, Joiner KA. A soluble secretory protein of the intracellular parasite Toxoplasma gondii associates with the parasitophorous vacuole membrane through hydrophobic interactions. J Biol Chem. 1994;21:15350–15357. [PubMed] [Google Scholar]
- Schwab JC, Beckers CJM, Joiner KA. The parasitophorous vacuole membrane surrounding intracellular Toxoplasma gondii functions as a molecular sieve. Proc Natl Acad Sci USA. 1994;91:509–513. doi: 10.1073/pnas.91.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeber F, Dubremetz JF, Boothroyd JC. Analysis of Toxoplasma gondii stably transfected with a transmembrane variant of its major surface protein, SAG1. J Cell Sci. 1998;111:23–29. doi: 10.1242/jcs.111.1.23. [DOI] [PubMed] [Google Scholar]
- Sibley LD, Niesman IR, Parmley SF, Cesbron-Delauw MF. Regulated secretion of multi-lamellar vesicles leads to formation of a tubulo-vesicular network in host-cells vacuoles occupied by Toxoplasma gondii. J Cell Sci. 1995;108:1669–1677. doi: 10.1242/jcs.108.4.1669. [DOI] [PubMed] [Google Scholar]
- Sibley LD, Weidner E, Krahenbuhl JL. Phagosome acidification blocked by intracellular Toxoplasma gondii. Nature. 1985;315:416–419. doi: 10.1038/315416a0. [DOI] [PubMed] [Google Scholar]
- Smith DB, Johnson KS. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione-S-transferase. Gene. 1988;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Soldati D, Boothroyd JC. Transient transfection and expression in the obligate intracellular parasite Toxoplasma gondii. Science. 1993;260:349–352. doi: 10.1126/science.8469986. [DOI] [PubMed] [Google Scholar]
- Stuart RA, Neupert W. Topogenesis of inner membrane proteins of mitochondria. Trends Biochem Sci. 1996;21:261–267. [PubMed] [Google Scholar]
- Suss-Toby L, Zimmerberg J, Ward GE. Toxoplasma invasion: the parasitophorous vacuole is formed from host cell plasma membrane and pinches off via a fission pore. Proc Natl Acad Sci USA. 1996;96:8413–8418. doi: 10.1073/pnas.93.16.8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomavo S, Schwarz RT, Dubremetz JF. Evidence for glycosyl-phosphatidylinositol anchoring of Toxoplasma gondii major surface antigens. Mol Cell Biol. 1989;9:4576–4580. doi: 10.1128/mcb.9.10.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from acrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Goote FG, Lakey JH, Pattus F. The molten globule intermediate for protein insertion or translocation through membanes. Trends Cell Biol. 1992;2:343–348. [PubMed] [Google Scholar]
- von Heijne G. Getting greasy: how transmembrane polypeptide segments integrate into the lipid bilayer. Mol Microbiol. 1997;24:249–253. doi: 10.1046/j.1365-2958.1997.3351702.x. [DOI] [PubMed] [Google Scholar]
- Wilson IA, Niman HL, Houghten RA, Cherenson AR, Connolhy ML, Lerner RA. The structure of an antigenic determinant in a protein. Cell. 1984;37:767–778. doi: 10.1016/0092-8674(84)90412-4. [DOI] [PubMed] [Google Scholar]