Abstract
Recombinant adenoviruses (rAd) have been widely used as gene transfer vectors both in the laboratory and in human clinical trials. In the present study, we investigated the effects of adenoviral-mediated gene transfer in primary bovine adrenal chromaffin cells (BACC) and a murine pheochromocytoma cell line (MPC). Cells were infected with one of three nonreplicating E1/E3-deleted (E1-/E3-) rAd vectors: Ad.GFP, expressing a green fluorescent protein (GFP); Ad.null, expressing no transgene; or Ad.C2.TK, expressing the herpes simplex virus-1 thymidine kinase gene (TK). Forty-eight hours after exposure to Ad.GFP, the percentage of GFP-expressing BACC ranged from 23.5-97% in a dose-dependent manner and similarly from 1.06 - 84.4% in the MPC, indicating that adrenomedullary cells are a potentially valuable target for adenoviral-mediated gene transfer. Ultrastructural analysis, however, revealed profound changes in the nucleus and mitochondria of cells infected with rAd. Furthermore, infection of BACC with Ad.null was accompanied by a time- and dose-dependent decrease in cell survival due to the vector alone. Specific whole-cell norepinephrine uptake was also decreased in a time- and dose-dependent fashion in BACC. Infection of MPC cells with the Ad.C2.TK vector sensitized them to the cytotoxic effect of the antiviral drug ganciclovir, in direct proportion to the fraction of cells infected with the virus. We conclude that rAd may alter the structural and functional integrity of adrenomedullary cells, potentially interfering with the normal stress response. At the same time, in light of their ability to effectively deliver and express genes in pheochromocytoma cells, they may be applicable to the gene therapy of adrenomedullary tumors.
ADENOVIRUSES ARE COMMON infectious agents that are responsible for a wide range of mostly benign respiratory, gastrointestinal, and urinary illnesses in humans. Serious complications from adenoviral infections have occasionally been reported in newborns, immunocompromised individuals, and military recruits (1-4). Recombinant adenovirus-based vectors, generated through engineering of the wild-type viral genome, have been effectively used to deliver foreign genes (transgenes) into a variety of tissues, both in vitro and in vivo (5-7). Indeed, the use of recombinant adenoviruses (rAd) for anticancer gene therapy, including the treatment of endocrine cancers, has shown promising results in preclinical studies (8, 9). At the same time, however, there have been some reports of significant toxicity associated with the administration of these vectors to primates and humans (10-12).
The adrenal gland appears to be a major target for both wild-type adenoviruses and recombinant vectors. Profound morphological and ultrastructural changes were described in the adrenals of calves and rodents after experimental adenovirus infections (13, 14). Similar findings were observed in the adrenal glands of infants that died from disseminated adenoviral infections (15). Persistent transgene expression and functional gene activity in adrenal tissue of animals undergoing gene transfer experiments using rAd has been reported by a number of investigators (16-18). Additionally, a single intraadrenal injection of an adenoviral vector encoding the cytochrome P450 21-hydroxylase gene was able to transiently compensate the endocrine and histological alterations in a mouse model of 21-hydroxylase deficiency, a congenital adrenal disease (19). Our group has previously shown that adenovirus-mediated gene transfer into bovine adrenal cortical cells is highly efficient but can alter cellular ultrastructure and steroidogenesis, potentially interfering with the normal adrenal stress response (20). On the basis of these findings, we proposed that, if appropriately engineered, rAd might be suitable for clinical gene therapy of adrenocortical cancers (20, 21). Little is known about the tropism of rAd for the adrenal medulla.
Pheochromocytomas are neuroendocrine tumors arising from the adrenal medulla, often characterized by persistently elevated blood pressure or paroxysms of hypertension, due to excessive catecholamine production by the tumor. Whether sustained or paroxysmal, undiagnosed or un-treated, endocrine hypertension can lead to death as a result of accelerated cardiovascular disease or stroke. Although most pheochromocytomas are benign and can be surgically cured, one third are malignant, often highly metastatic, and refractory to current treatments (22, 23). Patients affected by these tumors could potentially benefit from alternative therapies, including anticancer gene therapy (21).
In the present study, we tested the efficiency of gene transfer by nonreplicating E1-/E3- rAd in bovine adrenal chromaffin cells, and its effects on cellular ultrastructure, cell survival, and catecholamine uptake. In addition, we evaluated in vitro the efficacy of a prototypical adenoviral-mediated enzyme-prodrug gene therapy in a mouse pheochromocytoma cell line, using the herpes simplex virus-1 thymidine kinase gene (HSV-TK)/ganciclovir (GCV) system.
Materials and Methods
Chemical and biological reagents
DMEM, Ham F-12, and RPMI 1640 media, HEPES, penicillin, streptomycin, and collagenase type 1 were obtained from Invitrogen Life Technologies (Carlsbad, CA). Gentamicin and PBS were purchased from BioSource (Rockville, MD). Heat-inactivated fetal bovine serum (FBS) and donor horse serum were obtained from Gemini BioProducts (Woodland, CA). [3H]Norepinephrine (NE), levo (ring-2,5,6), was purchased from NEN Life Science Products (Boston, MA), and Triton X-100 from Fisher Scientific (Pittsburgh, PA).
Cell isolation and culture
Primary bovine adrenal chromaffin cells (BACC) were prepared from freshly obtained adrenal glands and transported to our laboratory on ice from a local slaughterhouse. Adrenal chromaffin cells were isolated as previously described (24). Briefly, intact adrenal glands were immersed in 70% ethanol for 10 sec and then dissected free of surrounding fat and connective tissue. Glands were perfused with 1× Locke’s buffer (154 mm NaCl, 5.6 mm KCl, 3.6 mm NaHCO3, 5.6 mm glucose, and 5.0 mm HEPES buffer) to clear glands of blood, perfused twice with 0.2% collagenase type 1, and incubated for 15 min at 37 C. Next, glands were dissected longitudinally, and the medullary tissue was removed, minced, and digested in 0.2% collagenase for 30 min at 37 C. After digestion, the cells were separated by centrifugation and multiple filtration steps using 250-μm nylon mesh (PGC Scientific, Frederick, MD), followed by 100- and 40-μm cell strainers (BD Falcon, Bedford, MA). Erythrocytes were removed by incubating the cells for 3 min with erythrocyte lysis buffer (0.15 m NH4Cl, 0.1 mm Na2EDTA, 12 mm NaHCO3). Lysis was stopped with ice-cold PBS. Cells were then centrifuged and resuspended in DMEM/F12 (1:1) (supplemented with 10% FBS, 200 U/ml penicillin, 200 μg/ml streptomycin, 50 μg/ml gentamicin, 2.438 mg/ml NaHCO3, and 1% of 1 m HEPES). Cells were seeded in glass Petri dishes for differential plating, a method that exploits the different adhesiveness of chromaffin and nonchromaffin cells (25). After isolation, cell viability, verified by the trypan blue exclusion, was greater than 90%. Purified chromaffin cells were grown in DMEM/F12 medium. Culture medium was replaced every 24 h.
MPC cells (cell line 4/30/PRR), established from heterozygous neurofibromatosis knockout mice (26), were kindly provided by Dr. Arthur S. Tischler (Tufts-New England Medical Center, Boston, MA). Cells were grown in RPMI 1640 medium, supplemented with 10% donor horse serum, 5% FBS, penicillin, and streptomycin.
HEK 293 cells were obtained from Microbix (Toronto, Canada) and grown in DMEM supplemented with 10% FBS and gentamicin. All cells were maintained at 37 C in 5% CO2.
Adenoviral vectors
Ad.GFP, a nonreplicating E1/E3-deleted rAd expressing an enhanced green fluorescent protein (GFP), was obtained from Quantum Biotechnologies (Montreal, Quebec, Canada). Ad.null, an E1-/E3- adenovirus expressing no transgene was generated by calcium phosphate precipitation and homologous recombination using the AdMax system (Microbix). Ad.C2.TK, expressing the herpes simplex virus-1 thymidine kinase (TK) gene under the control of the cytomegalovirus immediate-early promoter, was generated as previously reported (27) and was a kind gift of Walter J Ramsey (NewLink Genetics, Ames, IA). Viruses were doubleplaque isolated, amplified, and expanded in HEK 293 cells, purified by double cesium chloride density gradient centrifugation, titered by serial dilution as plaque forming units (PFU) per milliliter, and stored at -70 C in 10% glycerol-PBS.
Fluorescence microscopy
BACC were seeded at 2 × 106 cells per well in six-well polystyrene plates and transduced with Ad.GFP at a multiplicity of infection (MOI) of 1, 10, or 30 PFU/cell. Twenty-four hours after infection, the cells were examined with a fluorescence DM IRB microscope system (Leica Microsystems Inc., Allendale, NJ). Images were captured with a digital CCD camera (Shimazu Corp., Kyoto, Japan) and analyzed using the OpenLab software (Improvision Inc., Lexington, MA).
Electron microscopy
BACC or MPC plated at 1 × 107 cells/75-cm2 flask were infected with Ad.null at MOI of 1000 or 5000 PFU/cell for 90 min at 37 C and then washed three times with PBS and fixed in 2.5% glutaraldehyde for 1 h. Cells were harvested by scraping and centrifuged at 2500 rpm for 10 min. Afterward, they were double-fixed in osmium tetroxide (0.5%), dehydrated, and embedded into Spurr’s epoxy resin. Ultrathin sections (90 nm) were made, double-stained with uranyl acetate and lead citrate, and examined with a Philips CM10 transmission electron microscope (Electronic Instruments, Mahwah, NJ).
Flow cytometry
BACC and MPC were plated overnight at 5 × 105 cells per well in 24-well dishes. The next morning, cells were infected with Ad.GFP at MOI of 1, 10, 30, 100, or 300 PFU/cell in 1 ml culture medium. The medium was replaced 4 h later. Forty-eight hours after infection, cells were trypsinized, suspended, washed twice with PBS, and examined for expression of GFP using a FACSort flow cytometer (Becton Dickinson, San Jose, CA). Experiments were repeated twice.
[3H]NE uptake
[3H]NE uptake studies were performed as described by Jacques and co-workers (28). Briefly, BACC or MPC (2.5 × 105 cells/well) were plated in 24-well plates overnight and were then infected with increasing amounts of Ad.null (MOI = 10, 30, 100, or 300 PFU/ml). Forty-eight hours after infection, cells were washed three times with H-KRG buffer (125 mm NaCl, 4.8 mm KCl, 2.6 mm CaCl2, 1.2 mm MgSO4, 5.6 mm glucose, 25 mm HEPES, 1 mm ascorbic acid). After a 10-min preincubation in H-KRG buffer, cells were incubated with [3H]NE (50 nm/well) for 10 additional minutes at 37 C (total whole-cell uptake) or 4 C (nonspecific whole-cell uptake). [3H]NE uptake was stopped by chilling the plates on ice for 2 min. The medium was aspirated, and each well was washed once with ice-cold 0.5% BSA in PBS and then twice with 0.1% BSA in PBS at 4 C while shaking. Cells were then lysed in 0.1% Triton X-100 for 30 min at room temperature with gentle shaking. Finally, aliquots of the cell lysate were collected into scintillation vials, the Bio-Safe II counting cocktail (Research Products International Corp., Mount Prospect, IL) was added, and radioactivity was counted with the LS 6000IC scintillation counter (Beckman Coulter Inc., Fullerton, CA). The specific whole-cell [3H]NE uptake was calculated as the difference between total whole-cell uptake and nonspecific whole-cell uptake. Results were corrected for intracellular total protein concentration, measured using the Bradford method (Bio-Rad, Richmond, CA). Experiments were performed in quadruplicate and repeated four times.
Cell viability assessment
The effect of Ad.null and Ad.C2.TK on BACC or MPC survival, in the absence or presence of increasing concentrations of the antiviral nucleoside GCV was evaluated using the XTT assay (Roche Diagnostics Co., Indianapolis, IN). BACC, seeded in 96-well plates at 5 × 104 cells per well, were incubated with increasing amounts of Ad.null (MOI = 1, 10, 30, 100, or 300 PFU/cell) at 37 C for 48 and 72 h. The XTT labeling mixture was then added in accordance with the manufacturer’s instructions, and the plates were incubated for an additional 24 h. After incubation, spectrophotometric absorbance was measured with a microplate reader (Bio-Rad Laboratories, Philadelphia, PA). Similarly, MPC cells were initially plated in 25-cm2 flasks and infected with Ad.null or Ad.C2.TK (MOI = 100 PFU/cell) for 6 h at 37 C. Cells were then washed three times with PBS, trypsinized, and plated at 2 × 104 cells per well in 96-well plates. The following day, GCV (Roche, Inc., Nutley, NJ) was added in series to triplicate wells at concentrations ranging from 0.01-100 μm in ½-log increments. Seventy-two hours later, cell viability was assessed as described above. All experiments were performed four times.
Data analysis
All data were tested for normal distribution using the Kolmogorov-Smirnov test. Statistical significance was determined by ANOVA or ANOVA on Rank (if the normal distribution assumption was not met), followed by Student-Newman-Keuls test for group comparison using the SigmaStat version 3.11 software (Systat Software Inc., Point Richmond, CA). Results are presented as mean ± sem. Differences were considered significant at P < 0.05.
Results
High levels of adenoviral-mediated transgene expression in BACC and MPC cells
Twenty-four hours after transduction with the Ad.GFP vector, fluorescence microscopy revealed high levels of GFP expression in BACC at relatively low MOI of the vector (Fig. 1). Forty-eight hours after infection, the percentage of BACC cells showing fluorescence, as assessed by fluorescence-activated cell sorter, was 23.5, 63.7, 72.1, 96, and 97% at MOI of 1, 10, 30 100, and 300 PFU/cell, respectively (Fig. 2A). Adenoviral transduction in MPC cells was less efficient but still quite considerable, with 1.06, 18.7, 45.5, 70.4, and 84.4% of the cells showing a fluorescence signal at MOI of 1, 10, 30 100, and 300 PFU/cell, respectively (Fig. 2B).
FIG. 1.
Fluorescence microscopy images of primary BACC 24 h after transduction at MOI of 1-30 PFU/cell of an E1-/E3- rAd expressing GFP (Ad.GFP). GFP was readily observed in the BACC even at relatively low MOI. Magnification, ×4.
FIG. 2.

Vector dose-dependent increase in the percentage of BACC (A) and MPC cells (B) expressing GFP, assessed by flow cytometry 48 h after infection with Ad.GFP.
Ultrastructural changes in BACC and MPC after infection with rAd
Ultrastructural abnormalities in the nuclei, chromatin, and mitochondria of BACC were documented by electron microscopy after infection with Ad.null. In BACC, the nuclei exhibited distorted morphology and loss of nuclear membranes, and the chromatin was highly pyknotic (Fig. 3A). Mitochondrial abnormalities included change in mitochondrial shape from elongated (Fig. 3B) to spherical (Fig. 3C), disintegration of the inner membrane, and in some cases, complete destruction of the organelle (Fig. 3, C and D). Viral particles were visible adjacent to the plasma membrane (Fig. 3C), inside the nucleus (Fig. 3A), and in close proximity to (Fig. 3D) and inside (Fig. 3E) the mitochondria. Similar findings were observed in the mitochondria of MPC infected with Ad.null (Fig. 3F).
FIG. 3.
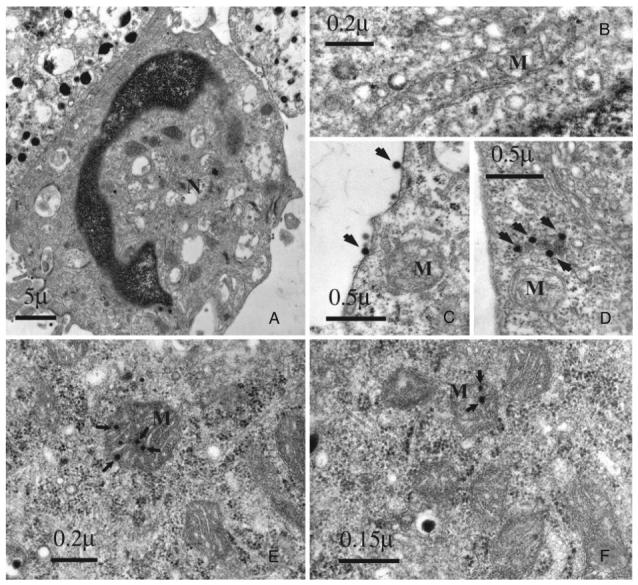
Ultrastructural changes in BACC and MPC cells after infection with Ad.null, an E1-/E3- rAd expressing no transgene. A, Nuclear abnormalities in infected BACC; B, a normal elongated mitochondrion in uninfected BACC cells; C and D, distorted mitochondrial shape in infected BACC. Viral particles (arrows) are visible adjacent to the plasma membrane and in close proximity to the organelle. Viral particles (arrows) inside the mitochondria of BACC (E) and MPC cells (F) at different stages of degeneration. M, Mitochondria; N, nucleus.
Decreased NE uptake in BACC after rAd infection
The specific whole-cell uptake of [3H]NE decreased in a dose- and time-dependent fashion in BACC infected with Ad.null. Compared with uninfected cells, the [3H]NE uptake in BACC infected with Ad.null at 10, 30, 100, and 300 PFU/cell was decreased by 16.9 ± 12.0% (P > 0.05), 40.9 ± 5.9% (P < 0.05), 62.7 ± 12.9%, and 75.4 ± 11.4% (P < 0.01 for both) at 48 h, respectively, and by 39.8 ± 18.8% (P > 0.05) and 62.8 ± 16.8, 80.4 ± 11.1, and 85.6 ± 6.1% (P < 0.01 for all) at 72 h, respectively (Fig. 4A). The [3H]NE uptake was not significantly affected by Ad.null in MPC cells (Fig. 4B).
FIG. 4.
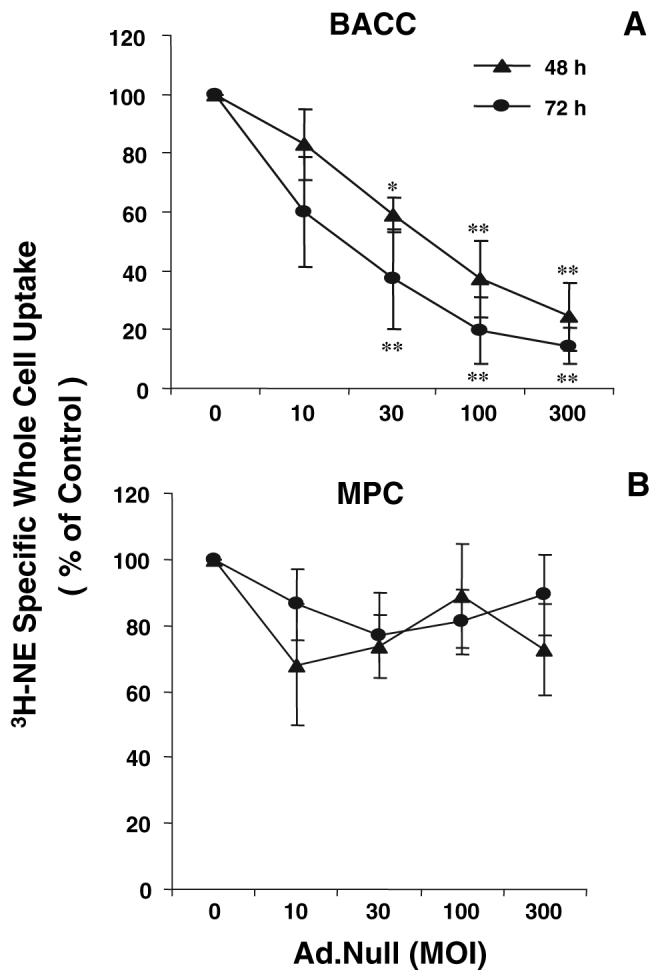
Specific whole-cell uptake of [3H]NE in BACC (A) and MPC cells (B) infected with Ad.null. Forty-eight and 72 h after infection, cells were incubated with [3H]NE (50 nm/well) for 10 min at 37 C (total whole-cell uptake) or 4 C (nonspecific whole-cell uptake). Specific whole-cell uptake was calculated as the difference between total whole-cell uptake and nonspecific whole-cell uptake. Results were corrected for intracellular protein concentration measured in the cell lysates and are presented as mean ± sem of quadruplicates from four experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Decreased viability of BACC after rAd infection
Infection of BACC with the Ad.null vector was accompanied by a significant dose- and time-dependent decrease in cell viability. At MOI of 10, 30, 100, and 300 PFU/cell, the percentage of viable cells at 48 h was 91.7 ± 1.5% (mean ± sem, P < 0.01) and 64.9 ± 1.4, 49.5 ± 1.2, and 46.1 ± 1.0% (P < 0.001 for all groups), respectively, and after 72 h, 86.8 ± 1.4, 51.0 ± 1.5, 37.7 ± 0.4, and 32.9 ± 0.9% (P < 0.001 for all groups), respectively (Fig. 5). No significant change in cell viability was observed at MOI of 1-10 (data not shown).
FIG. 5.
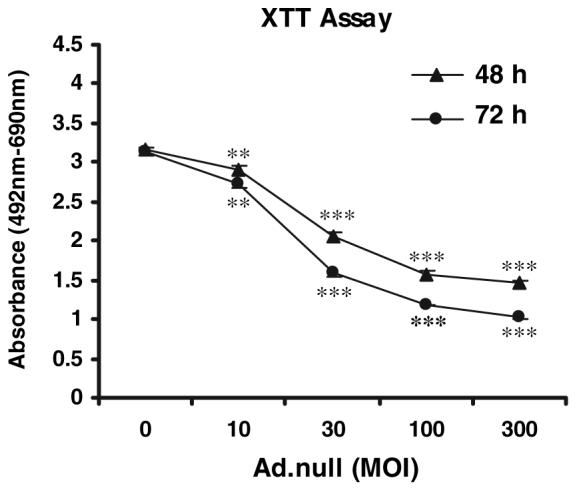
Survival of BACC 48 h after infection with Ad.null at increasing MOI. Cell viability was assessed by the XTT assay (see Materials and Methods for details). Results are the mean ± sem of quadruplicates from four experiments. **, P < 0.01; ***, P < 0.001.
Exposure to GCV induces cytotoxicity in MPC infected with Ad.C2.TK
A significant cytotoxic effect after exposure to doses of the antiviral nucleoside GCV as low as 0.03 μm was observed in MPC cells infected with the Ad.C2.TK vector, but not in MPC cells infected with the Ad.null vector, that does not express TK. Maximal cytotoxicity was achieved at 10 μm GCV, with approximately 65% killing of the infected MPC cells (Fig. 6).
FIG. 6.
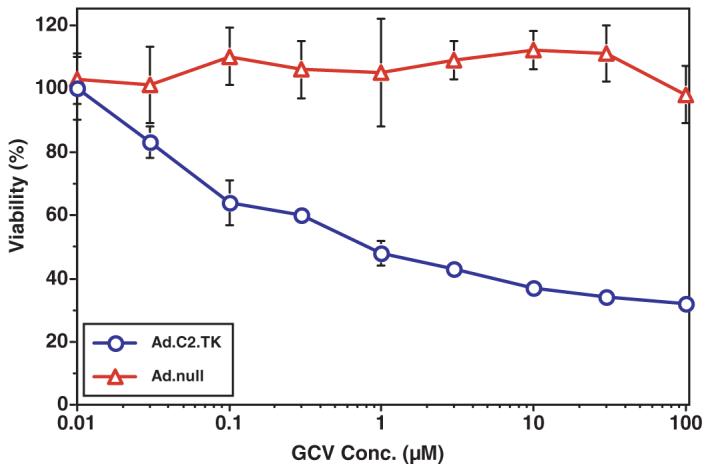
Cytotoxic effect of GCV on MPC cells infected with the Ad.C2.TK vector (circles), expressing the herpes simplex virus-1 thymidine kinase gene, compared with MPC cells infected with Ad.null (triangles). Results are the mean ± sem of triplicates from four experiments.
Discussion
The integrity of the adrenal gland is crucial for an adequate response to stress and survival in higher species (29). Recombinant adenoviruses appear to have a high degree of tropism for this endocrine organ. We have provided compelling evidence to support this in an earlier study, which explored the effects of adenoviral-mediated gene transfer on adrenocortical cells (20). In the present study, we have extended the investigation to its effects on adrenomedullary cells.
Adenovirus-mediated gene transfer in bovine chromaffin cells was highly efficient, in agreement with a recent report (30). Bovine chromaffin cells have been widely used as a model to study a variety of physiological and biochemical processes, including the synthesis, storage, and release of neuromediators, neuroendocrine transmission, and cellular exocytosis (31-34). Transfection of these cells by various methods has produced only modest results in terms of the levels of gene expression achieved (35-37). Our data indicate that rAd offer an effective gene delivery system for adrenal chromaffin cells.
Infection of BACC with the E1-/E3- nonreplicating Ad.null vector was accompanied by significant ultrastructural changes, predominantly in the nucleus and mitochondria. A sequence of nuclear alterations, featuring nucleolar hypertrophy and the appearance of multishaped inclusion bodies, was described in mouse adrenomedullary cells after experimental adenovirus infection (38, 39). We previously described mitochondrial changes in adrenocortical cells transduced with adenoviral vectors, including the development of pleiomorphic mitochondria with reduced tubular inner membranes and the appearance of intranuclear crystalline structures (20). Similar abnormalities were observed in bovine chromaffin cells after infection with Ad.null; perhaps more interesting, we were able to document the presence of adenoviral particles inside mitochondria. This observation provides additional evidence for the disruption of these intracellular organelles that may play a crucial role in the adrenal damage caused by recombinant and, possibly, wildtype adenoviruses (20).
Infection of BACC with Ad.null caused a dramatic decrease in cell viability at the highest MOI used in these experiments. Indeed, a similar finding was reported in BACC transduced with Ad.GFP (30), but it remained unclear whether it was imputable to the vector or, rather, to GFP-related cytotoxicity (40). By using a rAd expressing no transgene, we unequivocally addressed this issue. The same study had concluded, based on lack of impairment of catecholamine secretion in Ad.GFP-transduced BACC, that recombinant adenoviruses do not alter chromaffin cell function (30). Conversely, in this study, we documented an important reduction of NE uptake in BACC infected with Ad.null, which paralleled the decreased cell survival and may in part be related to it. Independent of the mechanism, which remains to be elucidated, this finding coupled with our previous data of altered steroidogenesis in BACC transduced with an E1-/E3- adenoviral vector (20) underpins the speculation that rAd may interfere with the normal adrenal stress response. This interference may have played a role in a reported fatality associated with adenoviral-mediated gene therapy (12); besides, it may be a more general determinant of viral pathogenesis during infections with wild-type adenoviruses. Coherently, abnormal circulating levels of corticosteroids and catecholamines were reported in military recruits suffering from severe adenoviral respiratory illness (41). These hypotheses bear additional investigation.
Adenovirus-mediated gene expression in mouse pheochromocytoma cells was also very efficient, albeit less so than in the primary bovine chromaffin cell model. We had theorized on the applicability of adenoviral gene therapy to the treatment of adrenal tumors, including pheochromocytomas, in particular those with high malignant potential and refractory to current therapies (21). In the present study, using a well established prototype of antitumor suicide gene therapy (42, 43), we have shown that transduction of MPC cells with the Ad.C2.TK adenoviral vector is able to exquisitely sensitize these cancer cells to the cytotoxic effect of the prodrug GCV. MPC cells exhibited little GCV bystander effect, suggesting their lack of functional gap junctions (44). Approximately 70% of the MPC cells expressed GFP when transduced with Ad.GFP at MOI of 100 PFU/cell. When infected with Ad.C2.TK at the same MOI and subsequently exposed to GCV, approximately 65% of the cells showed cytotoxicity, suggesting that only those cells actually expressing TK are affected, and little or no transfer of activated GCV from the infected cells to the HSV-TK-negative cells occurs. Activated GCV transfer is mediated via gap junction communications between cells (45). The lack of a bystander effect and less than complete cell killing could potentially be overcome by use of an enzyme-prodrug system that generates a freely diffusible activated prodrug not dependent on expression of gap junctions, which are often decreased in tumor cells (46). Recently, transcomplementing replicating adenoviral vectors were successfully used in a mouse model of adrenocortical carcinoma, where they blocked tumor growth and prolonged survival (47). Our results, together with this report, indicate that adenoviral-based suicide gene therapy may generate promising results in animal models of adrenomedullary tumors and eventually in patients with malignant pheochromocytoma refractory to the various therapeutic options presently available.
Poor sensitivity and specificity of currently used imaging techniques can make the diagnosis and localization of pheochromocytomas difficult. Effective identification of these tumors is essential to warrant complete surgical removal, which can cure up to 90% of the cases and prevent fatal recurrences. New genomic technologies combining delivery of a therapeutic transgene with its noninvasive visualization have been recently developed. One of these systems, for example, can monitor the expression of the herpes simplex virus TK gene by positron emission tomography (PET) (48). Several PET agents have been used to visualize primary and metastatic pheochromocytomas, including [18F]fluorodeoxy-glucose, [11C]hydroxyephedrine, and [18F]fluorodopamine (49, 50). Thus, the development of a PET-monitored adenoviral gene therapy for pheochromocytomas may be technically feasible and help improve the diagnosis and treatment of this cancer.
In conclusion, we provide new evidence that infection with nonreplicating adenoviral vectors can injure adrenal cells, interfering with their physiological role in the stress response. On the basis of this and our previous work, we propose careful monitoring of the adrenal integrity in clinical gene therapy trials, where these vectors are systemically administered at high doses. At the same time, the innate tropism and highly effective transgene delivery to the adrenal gland by recombinant adenoviruses indicate that genomic approaches based on their use may be applicable to the diagnosis and treatment of adrenal cancers. Proper engineering and extensive safety and efficacy testing in preclinical studies should be mandatory before venturing into any clinical application of these engaging technologies.
Acknowledgments
We thank Arthur S. Tischler (Tufts-New England Medical Center) and Walter J. Ramsey (NewLink Genetics, Ames, IA) for kindly providing the MPC cell line and the Ad.C2.TK vector, respectively, which were used in this study. We are also grateful to Vasiliki Michopoulos and Leah Gold for their technical assistance.
This work was supported by the Intramural Research Programs of the National Institute of Child Health and Human Development, National Institute of Mental Health, and the National Cancer Institute.
Abbreviations
- BACC
Bovine adrenal chromaffin cells
- FBS
fetal bovine serum
- GCV
ganciclovir
- GFP
green fluorescent protein
- MOI
multiplicity of infection
- NE
norepinephrine
- PET
positron emission tomography
- PFU
plaque-forming unit
- rAd
recombinant adenovirus.
References
- 1.Kolavic-Gray SA, Binn LN, Sanchez JL, Cersovsky SB, Polyak CS, Mitchell-Raymundo F, Asher LV, Vaughn DW, Feighner BH, Innis BL. Large epidemic of adenovirus type 4 infection among military trainees: epidemiological, clinical, and laboratory studies. Clin Infect Dis. 2002;35:808–818. doi: 10.1086/342573. [DOI] [PubMed] [Google Scholar]
- 2.Munoz FM, Piedra PA, Demmler GJ. Disseminated adenovirus disease in immunocompromised and immunocompetent children. Clin Infect Dis. 1998;27:1194–1200. doi: 10.1086/514978. [DOI] [PubMed] [Google Scholar]
- 3.Sanchez JL, Binn LN, Innis BL, Reynolds RD, Lee T, Mitchell-Raymundo F, Craig SC, Marquez JP, Shepherd GA, Polyak CS, Conolly J, Kohlhase KF. Epidemic of adenovirus-induced respiratory illness among US military recruits: epidemiologic and immunologic risk factors in healthy, young adults. J Med Virol. 2001;65:710–718. doi: 10.1002/jmv.2095. [DOI] [PubMed] [Google Scholar]
- 4.Zahradnik JM, Spencer MJ, Porter DD. Adenovirus infection in the immunocompromised patient. Am J Med. 1980;68:725–732. doi: 10.1016/0002-9343(80)90262-4. [DOI] [PubMed] [Google Scholar]
- 5.Ali M, Lemoine NR, Ring CJ. The use of DNA viruses as vectors for gene therapy. Gene Ther. 1994;1:367–384. [PubMed] [Google Scholar]
- 6.Barzon L, Stefani AL, Pacenti M, Palu G. Versatility of gene therapy vectors through viruses. Expert Opin Biol Ther. 2005;5:639–662. doi: 10.1517/14712598.5.5.639. [DOI] [PubMed] [Google Scholar]
- 7.Zuckerman JB, Robinson CB, McCoy KS, Shell R, Sferra TJ, Chirmule N, Magosin SA, Propert KJ, Brown-Parr EC, Hughes JV, Tazelaar J, Baker C, Goldman MJ, Wilson JM. A phase I study of adenovirus-mediated transfer of the human cystic fibrosis transmembrane conductance regulator gene to a lung segment of individuals with cystic fibrosis. Hum Gene Ther. 1999;10:2973–2985. doi: 10.1089/10430349950016384. [DOI] [PubMed] [Google Scholar]
- 8.Barzon L, Boscaro M, Palu G. Endocrine aspects of cancer gene therapy. Endocr Rev. 2004;25:1–44. doi: 10.1210/er.2002-0035. [DOI] [PubMed] [Google Scholar]
- 9.Barzon L, Pacenti M, Boscaro M, Palu G. Gene therapy for thyroid cancer. Expert Opin Biol Ther. 2004;4:1225–1239. doi: 10.1517/14712598.4.8.1225. [DOI] [PubMed] [Google Scholar]
- 10.Lozier JN, Metzger ME, Donahue RE, Morgan RA. Adenovirus-mediated expression of human coagulation factor IX in the rhesus macaque is associated with dose-limiting toxicity. Blood. 1999;94:3968–3975. [PubMed] [Google Scholar]
- 11.Nunes FA, Furth EE, Wilson JM, Raper SE. Gene transfer into the liver of nonhuman primates with E1-deleted recombinant adenoviral vectors: safety of readministration. Hum Gene Ther. 1999;10:2515–2526. doi: 10.1089/10430349950016852. [DOI] [PubMed] [Google Scholar]
- 12.Marshall E. Gene therapy death prompts review of adenovirus vector. Science. 1999;286:2244–2245. doi: 10.1126/science.286.5448.2244. [DOI] [PubMed] [Google Scholar]
- 13.Stauber E, Card C. Experimental intraamnionic exposure of bovine fetuses with subgroup 2, type 7 adenovirus. Can J Comp Med. 1978;42:466–472. [PMC free article] [PubMed] [Google Scholar]
- 14.Cutlip RC, McClurkin AW. Lesions and pathogenesis of disease in young calves experimentally induced by a bovine adenovirus type 5 isolated from a calf with weak calf syndrome. Am J Vet Res. 1975;36:1095–1098. [PubMed] [Google Scholar]
- 15.Medvedev N, Shastina GV. [Morphology of the adrenal cortex in infants with generalized adenovirus infections] Arkh Patol. 1978;40:32–36. Russian. [PubMed] [Google Scholar]
- 16.Schachtner S, Buck C, Bergelson J, Baldwin H. Temporally regulated expression patterns following in utero adenovirus-mediated gene transfer. Gene Ther. 1999;6:1249–1257. doi: 10.1038/sj.gt.3300939. [DOI] [PubMed] [Google Scholar]
- 17.Senoo M, Matsubara Y, Fujii K, Nagasaki Y, Hiratsuka M, Kure S, Uehara S, Okamura K, Yajima A, Narisawa K. Adenovirus-mediated in utero gene transfer in mice and guinea pigs: tissue distribution of recombinant adenovirus determined by quantitative TaqMan-polymerase chain reaction assay. Mol Genet Metab. 2000;69:269–276. doi: 10.1006/mgme.2000.2984. [DOI] [PubMed] [Google Scholar]
- 18.Yang EY, Cass DL, Sylvester KG, Wilson JM, Adzick NS. BAPS Prize-1997. Fetal gene therapy: efficacy, toxicity, and immunologic effects of early gestation recombinant adenovirus. British Association of Paediatric Surgeons. J Pediatr Surg. 1999;34:235–241. doi: 10.1016/s0022-3468(99)90181-1. [DOI] [PubMed] [Google Scholar]
- 19.Tajima T, Okada T, Ma XM, Ramsey W, Bornstein S, Aguilera G. Restoration of adrenal steroidogenesis by adenovirus-mediated transfer of human cytochromeP450 21-hydroxylase into the adrenal gland of 21-hydroxylase-deficient mice. Gene Ther. 1999;6:1898–1903. doi: 10.1038/sj.gt.3301018. [DOI] [PubMed] [Google Scholar]
- 20.Alesci S, Ramsey WJ, Bornstein SR, Chrousos GP, Hornsby PJ, Benvenga S, Trimarchi F, Ehrhart-Bornstein M. Adenoviral vectors can impair adrenocortical steroidogenesis: clinical implications for natural infections and gene therapy. Proc Natl Acad Sci USA. 2002;99:7484–7489. doi: 10.1073/pnas.062170099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alesci S, Chrousos GP, Pacak K. Genomic medicine: exploring the basis of a new approach to endocrine hypertension. Ann NY Acad Sci. 2002;970:177–192. doi: 10.1111/j.1749-6632.2002.tb04424.x. [DOI] [PubMed] [Google Scholar]
- 22.Lenders JW, Eisenhofer G, Mannelli M, Pacak K. Phaeochromocytoma. Lancet. 2005;366:665–675. doi: 10.1016/S0140-6736(05)67139-5. [DOI] [PubMed] [Google Scholar]
- 23.O’Riordain DS, Young WF, Jr, Grant CS, Carney JA, van Heerden JA. Clinical spectrum and outcome of functional extraadrenal paraganglioma. World J Surg. 1996;20:916–921. doi: 10.1007/s002689900139. discussion 922. [DOI] [PubMed] [Google Scholar]
- 24.Ehrhart-Bornstein M, Haidan A, Alesci S, Bornstein SR. Neurotransmitters and neuropeptides in the differential regulation of steroidogenesis in adrenocortical-chromaffin co-cultures. Endocr Res. 2000;26:833–842. doi: 10.3109/07435800009048606. [DOI] [PubMed] [Google Scholar]
- 25.Unsicker K, Muller TH. Purification of bovine adrenal chromaffin cells by differential plating. J Neurosci Methods. 1981;4:227–241. doi: 10.1016/0165-0270(81)90034-0. [DOI] [PubMed] [Google Scholar]
- 26.Powers JF, Evinger MJ, Tsokas P, Bedri S, Alroy J, Shahsavari M, Tischler AS. Pheochromocytoma cell lines from heterozygous neurofibromatosis knockout mice. Cell Tissue Res. 2000;302:309–320. doi: 10.1007/s004410000290. [DOI] [PubMed] [Google Scholar]
- 27.Wildner O, Morris JC, Vahanian NN, Ford H, Jr, Ramsey WJ, Blaese RM. Adenoviral vectors capable of replication improve the efficacy of HSVtk/GCV suicide gene therapy of cancer. Gene Ther. 1999;6:57–62. doi: 10.1038/sj.gt.3300810. [DOI] [PubMed] [Google Scholar]
- 28.Jaques S, Jr, Tobes MC, Sisson JC, Baker JA, Wieland DM. Comparison of the sodium dependency of uptake of meta-lodobenzylguanidine and norepinephrine into cultured bovine adrenomedullary cells. Mol Pharmacol. 1984;26:539–546. [PubMed] [Google Scholar]
- 29.Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA. 1992;267:1244–1252. [PubMed] [Google Scholar]
- 30.Li X, Drakulich DA, Zhang P, Shen M, Weber GA, Ikezu T, Hexum TD. Transduction of bovine adrenal chromaffin cells using a recombinant adenovirus expressing GFP. J Neurosci Methods. 2002;122:91–96. doi: 10.1016/s0165-0270(02)00295-9. [DOI] [PubMed] [Google Scholar]
- 31.Kobayashi H, Yanagita T, Yokoo H, Wada A. Pathophysiological function of adrenomedullin and proadrenomedullin N-terminal peptides in adrenal chromaffin cells. Hypertens Res. 2003;26(Suppl):S71–S78. doi: 10.1291/hypres.26.s71. [DOI] [PubMed] [Google Scholar]
- 32.Livett BG, Boksa P, Dean DM, Mizobe F, Lindenbaum MH. Use of isolated chromaffin cells to study basic release mechanisms. J Auton Nerv Syst. 1983;7:59–86. doi: 10.1016/0165-1838(83)90069-3. [DOI] [PubMed] [Google Scholar]
- 33.Pan CY, Jeromin A, Lundstrom K, Yoo SH, Roder J, Fox AP. Alterations in exocytosis induced by neuronal Ca2+ sensor-1 in bovine chromaffin cells. J Neurosci. 2002;22:2427–2433. doi: 10.1523/JNEUROSCI.22-07-02427.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pan CY, Lee H, Chen CL. Lysophospholipids elevate [Ca2+]i and trigger exocytosis in bovine chromaffin cells. Neuropharmacology. 2006;51:18–26. doi: 10.1016/j.neuropharm.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 35.Ashery U, Betz A, Xu T, Brose N, Rettig J. An efficient method for infection of adrenal chromaffin cells using the Semliki Forest virus gene expression system. Eur J Cell Biol. 1999;78:525–532. doi: 10.1016/s0171-9335(99)80017-x. [DOI] [PubMed] [Google Scholar]
- 36.Wick PF, Senter RA, Parsels LA, Uhler MD, Holz RW. Transient transfection studies of secretion in bovine chromaffin cells and PC12 cells. Generation of kainate-sensitive chromaffin cells. J Biol Chem. 1993;268:10983–10989. [PubMed] [Google Scholar]
- 37.Wilson SP, Liu F, Wilson RE, Housley PR. Optimization of calcium phosphate transfection for bovine chromaffin cells: relationship to calcium phosphate precipitate formation. Anal Biochem. 1995;226:212–220. doi: 10.1006/abio.1995.1216. [DOI] [PubMed] [Google Scholar]
- 38.Hoenig EM, Margolis G, Kilham L. Experimental adenovirus infection of the mouse adrenal gland. II. Electron microscopic observations. Am J Pathol. 1974;75:375–394. [PMC free article] [PubMed] [Google Scholar]
- 39.Margolis G, Kilham L, Hoenig EM. Experimental adenovirus infection of the mouse adrenal gland. I. Light microscopic observations. Am J Pathol. 1974;75:363–374. [PMC free article] [PubMed] [Google Scholar]
- 40.Liu HS, Jan MS, Chou CK, Chen PH, Ke NJ. Is green fluorescent protein toxic to the living cells? Biochem Biophys Res Commun. 1999;260:712–717. doi: 10.1006/bbrc.1999.0954. [DOI] [PubMed] [Google Scholar]
- 41.Mason JW, Buescher EL, Belfer ML, Artenstein MS, Mougey EH. A prospective study of corticosteroid and catecholamine levels in relation to viral respiratory illness. J Human Stress. 1979;5:18–28. doi: 10.1080/0097840X.1979.9934524. [DOI] [PubMed] [Google Scholar]
- 42.Moolten FL. Tumor chemosensitivity conferred by inserted herpes thymidine kinase genes: paradigm for a prospective cancer control strategy. Cancer Res. 1986;46:5276–5281. [PubMed] [Google Scholar]
- 43.Moolten FL, Wells JM. Curability of tumors bearing herpes thymidine kinase genes transferred by retroviral vectors. J Natl Cancer Inst. 1990;82:297–300. doi: 10.1093/jnci/82.4.297. [DOI] [PubMed] [Google Scholar]
- 44.van Dillen IJ, Mulder NH, Vaalburg W, de Vries EF, Hospers GA. Influence of the bystander effect on HSV-tk/GCV gene therapy. A review. Curr Gene Ther. 2002;2:307–322. doi: 10.2174/1566523023347733. [DOI] [PubMed] [Google Scholar]
- 45.Mesnil M, Piccoli C, Tiraby G, Willecke K, Yamasaki H. Bystander killing of cancer cells by herpes simplex virus thymidine kinase gene is mediated by connexins. Proc Natl Acad Sci USA. 1996;93:1831–1835. doi: 10.1073/pnas.93.5.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamasaki H, Mesnil M, Omori Y, Mironov N, Krutovskikh V. Intercellular communication and carcinogenesis. Mutat Res. 1995;333:181–188. doi: 10.1016/0027-5107(95)00144-1. [DOI] [PubMed] [Google Scholar]
- 47.Wolkersdorfer GW, Bornstein SR, Higginbotham JN, Hiroi N, Vaquero JJ, Green MV, Blaese RM, Aguilera G, Chrousos GP, Ramsey WJ. A novel approach using transcomplementing adenoviral vectors for gene therapy of adrenocortical cancer. Horm Metab Res. 2002;34:279–287. doi: 10.1055/s-2002-33255. [DOI] [PubMed] [Google Scholar]
- 48.Jacobs A, Voges J, Reszka R, Lercher M, Gossmann A, Kracht L, Kaestle C, Wagner R, Wienhard K, Heiss WD. Positron-emission tomography of vector-mediated gene expression in gene therapy for gliomas. Lancet. 2001;358:727–729. doi: 10.1016/s0140-6736(01)05904-9. [DOI] [PubMed] [Google Scholar]
- 49.Ilias I, Shulkin B, Pacak K. New functional imaging modalities for chromaffin tumors, neuroblastomas and ganglioneuromas. Trends Endocrinol Metab. 2005;16:66–72. doi: 10.1016/j.tem.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 50.Pacak K, Eisenhofer G, Goldstein DS. Functional imaging of endocrine tumors: role of positron emission tomography. Endocr Rev. 2004;25:568–580. doi: 10.1210/er.2003-0032. [DOI] [PubMed] [Google Scholar]



