Abstract
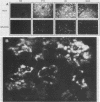
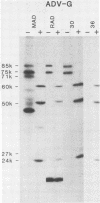
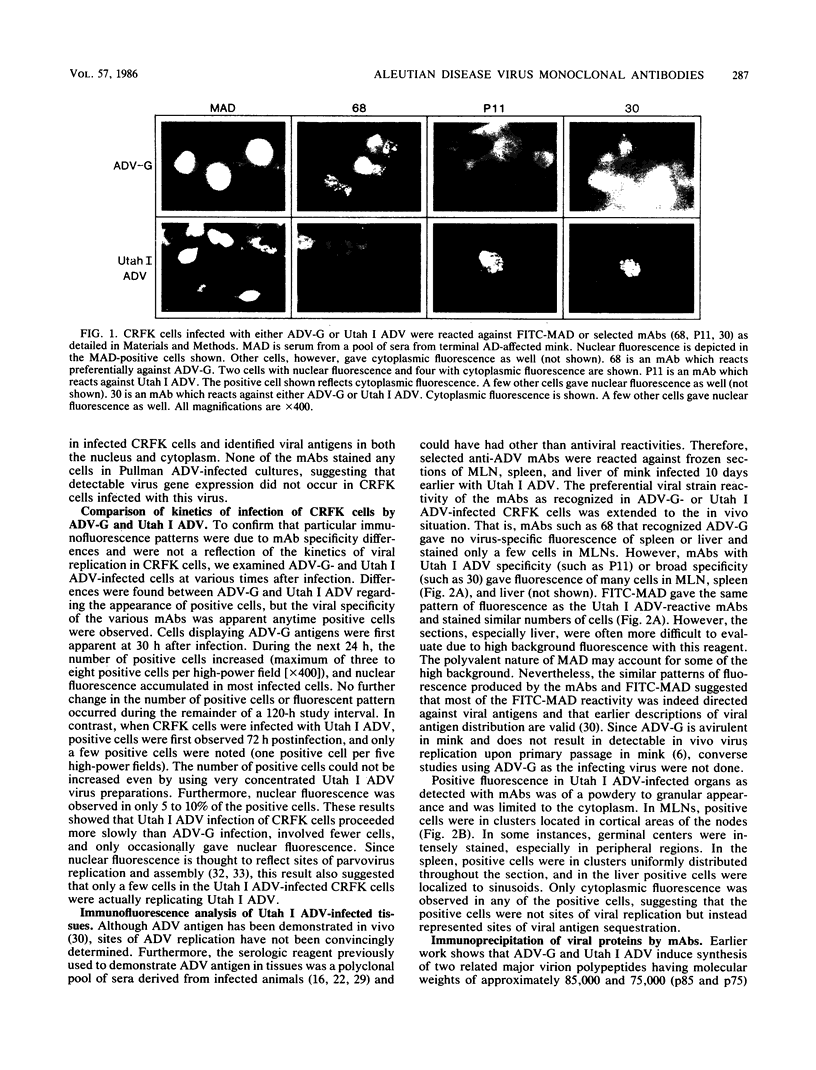
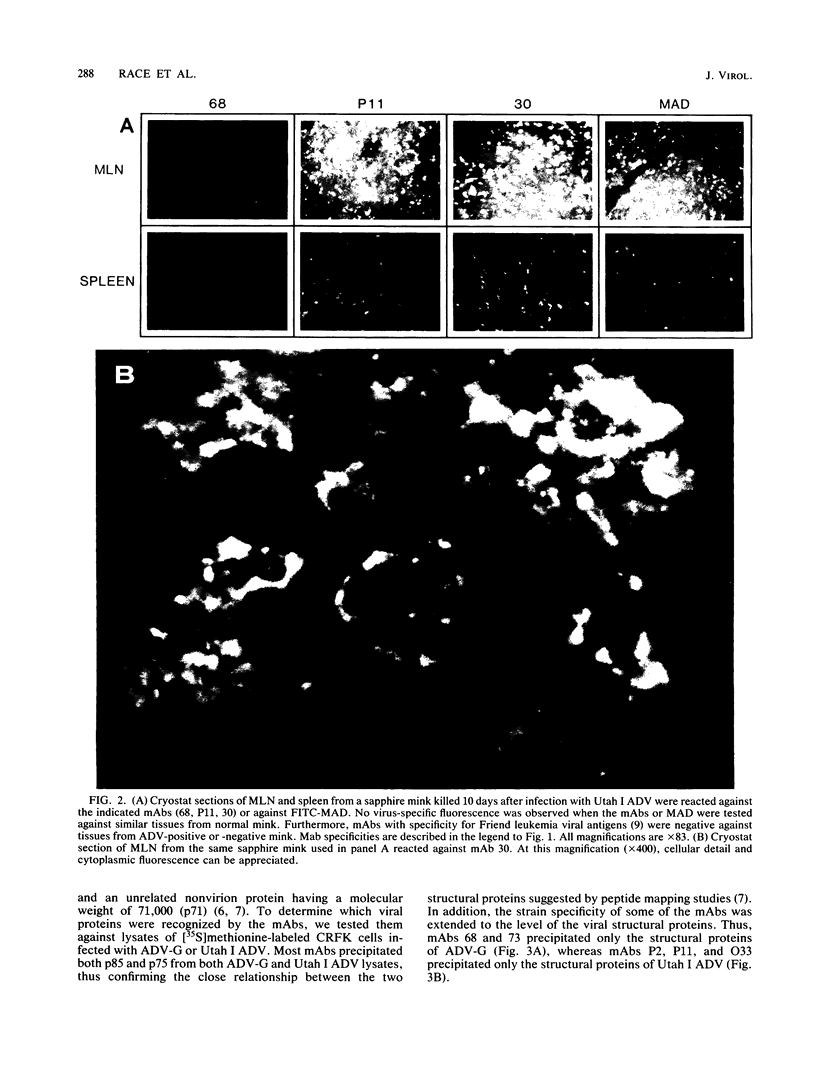
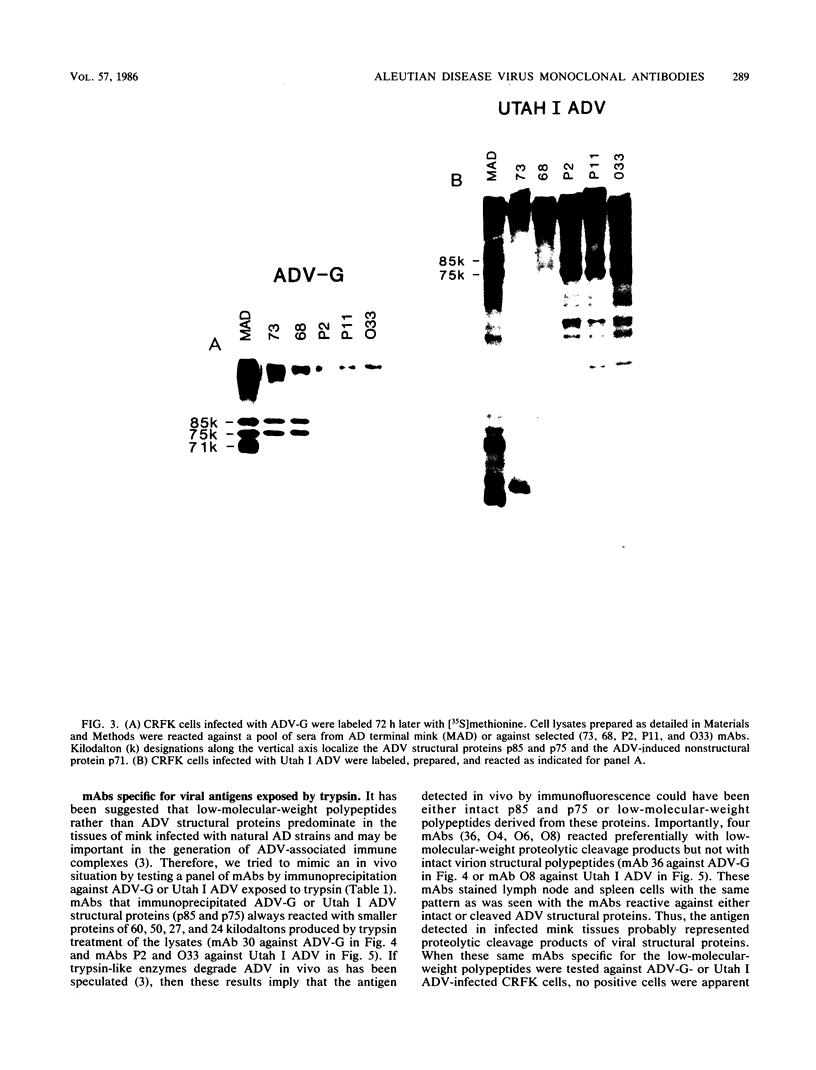
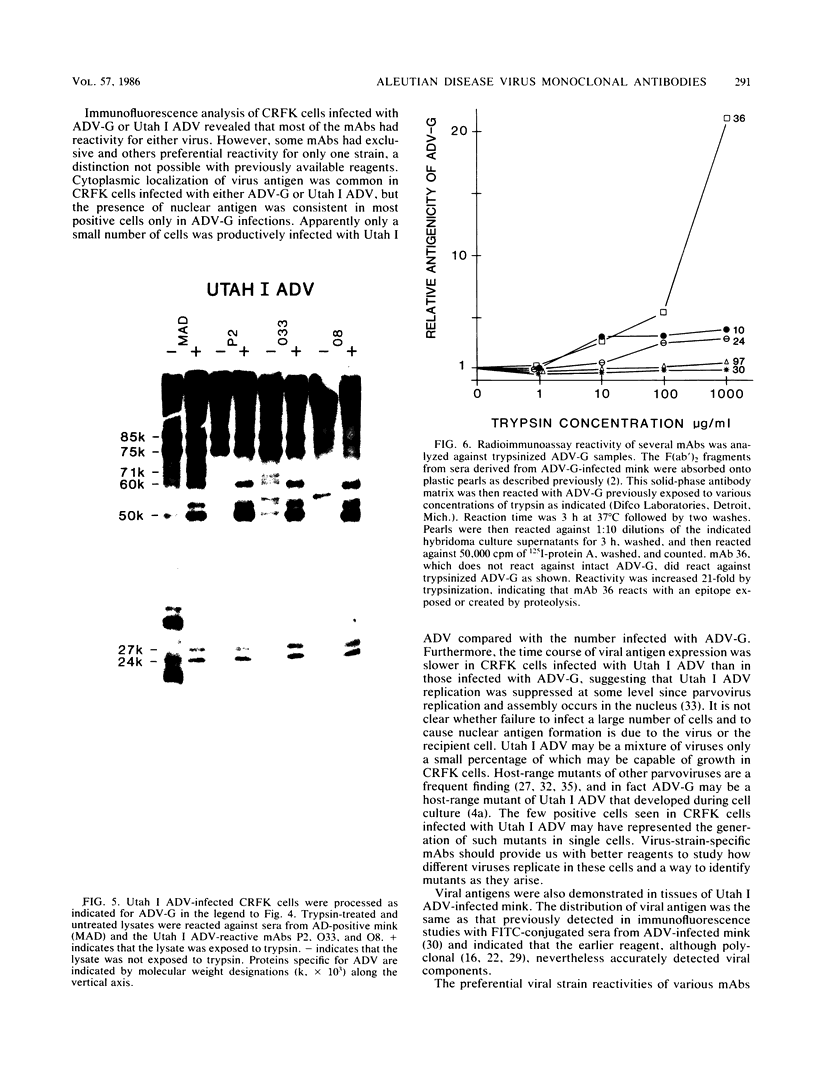
Monoclonal antibodies (mAbs) were used to study antigenic differences among strains of Aleutian disease virus (ADV) and to characterize viral proteins in vitro and in vivo. A number of ADV field strains could be discriminated, and highly virulent Utah I ADV was clearly delineated from the tissue culture-adapted avirulent ADV-G strain. This specificity could be demonstrated by indirect immunofluorescence against infected cultures of Crandell feline kidney cells or against tissues of Utah I ADV-infected mink. Viral antigens were demonstrated in both the nuclei and the cytoplasm of infected tissue culture cells. However, in mink mesenteric lymph node, spleen, and liver, viral antigen was observed only in the cytoplasm. Absence of nuclear fluorescence suggested that the detected antigen represented phagocytized viral antigens rather than replicating virus. This conclusion was supported by the finding that mAbs reactive only against low-molecular-weight polypeptides derived from intact viral proteins gave the same pattern of in vivo fluorescence as mAbs with broad reactivity for large or small (or both) viral polypeptides. The distribution of infected cells was the same as that described for macrophages in these tissues and suggested that cells of the reticuloendothelial system had sequestered viral antigens.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aasted B., Avery B., Cohn A. Serological analyses of different mink Aleutian disease virus strains. Arch Virol. 1984;80(1):11–22. doi: 10.1007/BF01315290. [DOI] [PubMed] [Google Scholar]
- Aasted B., Bloom M. E. Sensitive radioimmune assay for measuring Aleutian disease virus antigen and antibody. J Clin Microbiol. 1983 Sep;18(3):637–644. doi: 10.1128/jcm.18.3.637-644.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aasted B., Race R. E., Bloom M. E. Aleutian disease virus, a parvovirus, is proteolytically degraded during in vivo infection in mink. J Virol. 1984 Jul;51(1):7–13. doi: 10.1128/jvi.51.1.7-13.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom M. E., Mayer L. W., Garon C. F. Characterization of the Aleutian disease virus genome and its intracellular forms. J Virol. 1983 Mar;45(3):977–984. doi: 10.1128/jvi.45.3.977-984.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom M. E., Race R. E., Aasted B., Wolfinbarger J. B. Analysis of Aleutian disease virus infection in vitro and in vivo: demonstration of Aleutian disease virus DNA in tissues of infected mink. J Virol. 1985 Sep;55(3):696–703. doi: 10.1128/jvi.55.3.696-703.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom M. E., Race R. E., Hadlow W. J., Chesebro B. Aleutian disease of mink: the antibody response of sapphire and pastel mink to Aleutian disease virus. J Immunol. 1975 Oct;115(4):1034–1037. [PubMed] [Google Scholar]
- Bloom M. E., Race R. E., Wolfinbarger J. B. Characterization of Aleutian disease virus as a parvovirus. J Virol. 1980 Sep;35(3):836–843. doi: 10.1128/jvi.35.3.836-843.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom M. E., Race R. E., Wolfinbarger J. B. Identification of a nonvirion protein of Aleutian disease virus: mink with Aleutian disease have antibody to both virion and nonvirion proteins. J Virol. 1982 Aug;43(2):608–616. doi: 10.1128/jvi.43.2.608-616.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesebro B., Bloom M., Hadlow W., Race R. Purification and ultrastructure of Aleutian disease virus of mink. Nature. 1975 Apr 3;254(5499):456–457. doi: 10.1038/254456a0. [DOI] [PubMed] [Google Scholar]
- Chesebro B., Wehrly K., Cloyd M., Britt W., Portis J., Collins J., Nishio J. Characterization of mouse monoclonal antibodies specific for Friend murine leukemia virus-induced erythroleukemia cells: friend-specific and FMR-specific antigens. Virology. 1981 Jul 15;112(1):131–144. doi: 10.1016/0042-6822(81)90619-x. [DOI] [PubMed] [Google Scholar]
- Cho H. J. Purification and structure of aleutian disease virus. Front Biol. 1976;44:159–174. [PubMed] [Google Scholar]
- Cloyd M. W., Chesebro B., Portis J. L., Weir M. MCF-specific murine monoclonal antibodies made against AKR-247 MCF virus recognize a unique determinant associated with the gp70-p-15(E) complex. J Virol. 1982 Mar;41(3):1112–1117. doi: 10.1128/jvi.41.3.1112-1117.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund C. M., Hadlow W. J., Kennedy R. C., Boyle C. C., Jackson T. A. Aleutian disease of mink: properties of the etiologic agent and the host responses. J Infect Dis. 1968 Dec;118(5):510–526. doi: 10.1093/infdis/118.5.510. [DOI] [PubMed] [Google Scholar]
- Haase A. T. The slow infection caused by visna virus. Curr Top Microbiol Immunol. 1975;72:101–156. doi: 10.1007/978-3-642-66289-8_4. [DOI] [PubMed] [Google Scholar]
- Hadlow W. J., Race R. E., Kennedy R. C. Comparative pathogenicity of four strains of Aleutian disease virus for pastel and sapphire mink. Infect Immun. 1983 Sep;41(3):1016–1023. doi: 10.1128/iai.41.3.1016-1023.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadlow W. J., Race R. E., Kennedy R. C. Royal pastel mink respond variously to inoculation with Aleutian disease virus of low virulence. J Virol. 1984 Apr;50(1):38–41. doi: 10.1128/jvi.50.1.38-41.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn E. C., Hahn P. S. Autoimmunity in Aleutian disease: contribution of antiviral and anti-DNA antibody to hypergammaglobulinemia. Infect Immun. 1983 Aug;41(2):494–500. doi: 10.1128/iai.41.2.494-500.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn E. C., Kenyon A. J. Anti-deoxyribonucleic acid antibody associated with persistent infection of mink with Aleutian disease virus. Infect Immun. 1980 Aug;29(2):452–458. doi: 10.1128/iai.29.2.452-458.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson J. B., Leader R. W., Gorham J. R., Padgett G. A. The sequential development of lesions in spontaneous Aleutian disease of mink. Pathol Vet. 1966;3(4):289–314. doi: 10.1177/030098586600300401. [DOI] [PubMed] [Google Scholar]
- Kono Y., Kobayashi K., Fukunaga Y. Antigenic drift of equine infectious anemia virus in chronically infected horses. Arch Gesamte Virusforsch. 1973;41(1):1–10. doi: 10.1007/BF01249923. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Laver W. G., Gerhard W., Webster R. G., Frankel M. E., Air G. M. Antigenic drift in type A influenza virus: peptide mapping and antigenic analysis of A/PR/8/34 (HON1) variants selected with monoclonal antibodies. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1425–1429. doi: 10.1073/pnas.76.3.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer L. W., Aasted B., Garon C. F., Bloom M. E. Molecular cloning of the Aleutian disease virus genome: expression of Aleutian disease virus antigens by a recombinant plasmid. J Virol. 1983 Dec;48(3):573–579. doi: 10.1128/jvi.48.3.573-579.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montelaro R. C., Parekh B., Orrego A., Issel C. J. Antigenic variation during persistent infection by equine infectious anemia virus, a retrovirus. J Biol Chem. 1984 Aug 25;259(16):10539–10544. [PubMed] [Google Scholar]
- Narayan O., Clements J. E., Griffin D. E., Wolinsky J. S. Neutralizing antibody spectrum determines the antigenic profiles of emerging mutants of visna virus. Infect Immun. 1981 Jun;32(3):1045–1050. doi: 10.1128/iai.32.3.1045-1050.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossal G. J., Abbot A., Mitchell J. Antigens in immunity. XIV. Electron microscopic radioautographic studies of antigen capture in the lymph node medulla. J Exp Med. 1968 Feb 1;127(2):263–276. doi: 10.1084/jem.127.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish C. R., Carmichael L. E., Antczak D. F. Antigenic relationships between canine parvovirus type 2, feline panleukopenia virus and mink enteritis virus using conventional antisera and monoclonal antibodies. Arch Virol. 1982;72(4):267–278. doi: 10.1007/BF01315223. [DOI] [PubMed] [Google Scholar]
- Porter D. D., Larsen A. E., Porter H. G. The pathogenesis of Aleutian disease of mink. 3. Immune complex arteritis. Am J Pathol. 1973 May;71(2):331–344. [PMC free article] [PubMed] [Google Scholar]
- Porter D. D., Larsen A. E., Porter H. G. The pathogenesis of Aleutian disease of mink. I. In vivo viral replication and the host antibody response to viral antigen. J Exp Med. 1969 Sep 1;130(3):575–593. doi: 10.1084/jem.130.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen F., Hjort T. A simple method for the production of F(ab')2 preparations by pepsin digestion of total serum protein. Acta Pathol Microbiol Scand C. 1980 Oct;88(5):241–245. doi: 10.1111/j.1699-0463.1980.tb00101.x. [DOI] [PubMed] [Google Scholar]
- Ron D., Tattersall P., Tal J. Formation of a host range mutant of the lymphotropic strain of minute virus of mice during persistent infection in mouse L cells. J Virol. 1984 Oct;52(1):63–69. doi: 10.1128/jvi.52.1.63-69.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahrabadi M. S., Cho H. J., Marusyk R. G. Characterization of the protein and nucleic acid of Aleutian disease virus. J Virol. 1977 Aug;23(2):353–362. doi: 10.1128/jvi.23.2.353-362.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tattersall P., Bratton J. Reciprocal productive and restrictive virus-cell interactions of immunosuppressive and prototype strains of minute virus of mice. J Virol. 1983 Jun;46(3):944–955. doi: 10.1128/jvi.46.3.944-955.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]