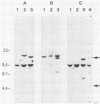
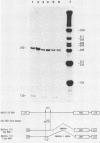
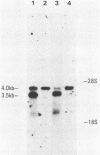
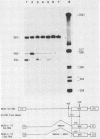
Abstract
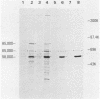
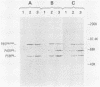
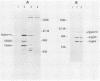
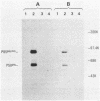
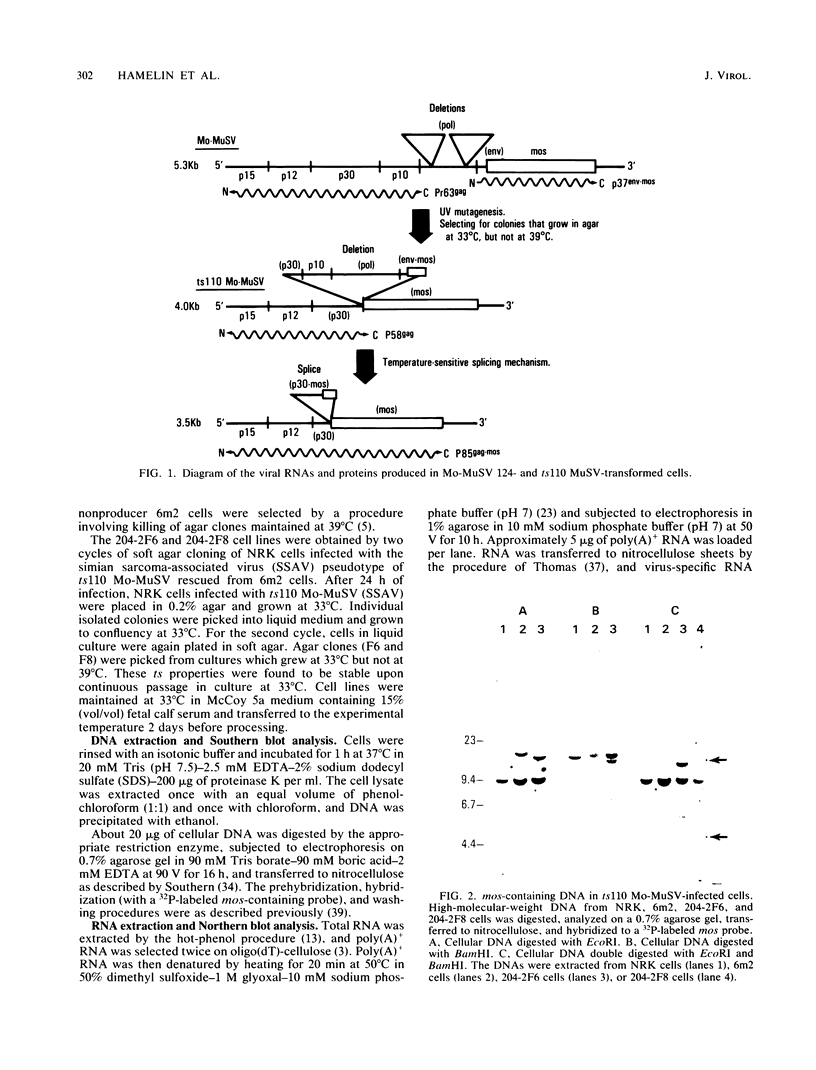
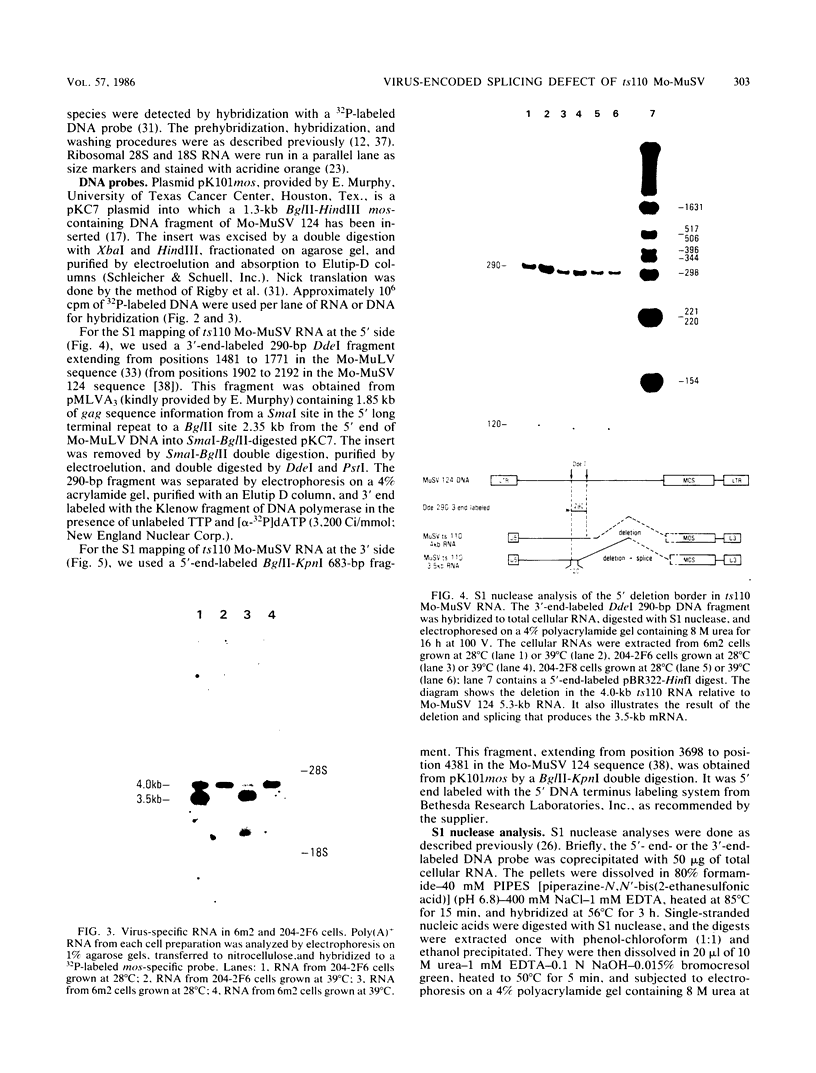
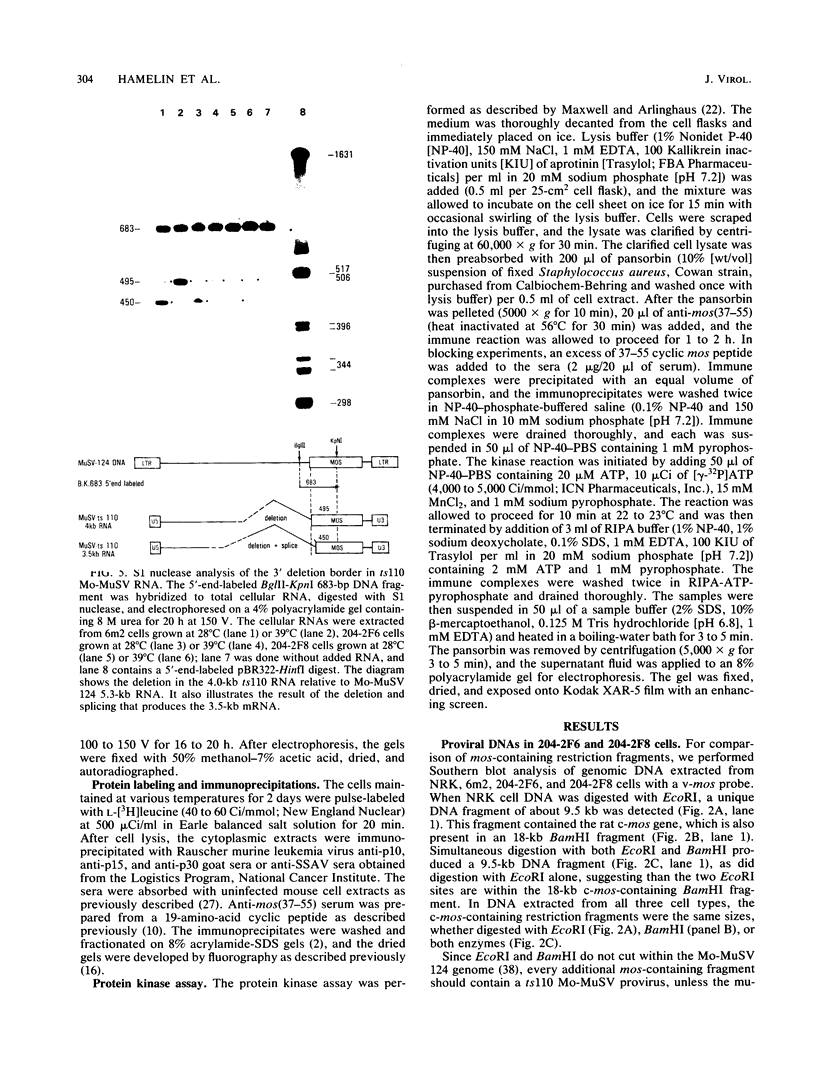
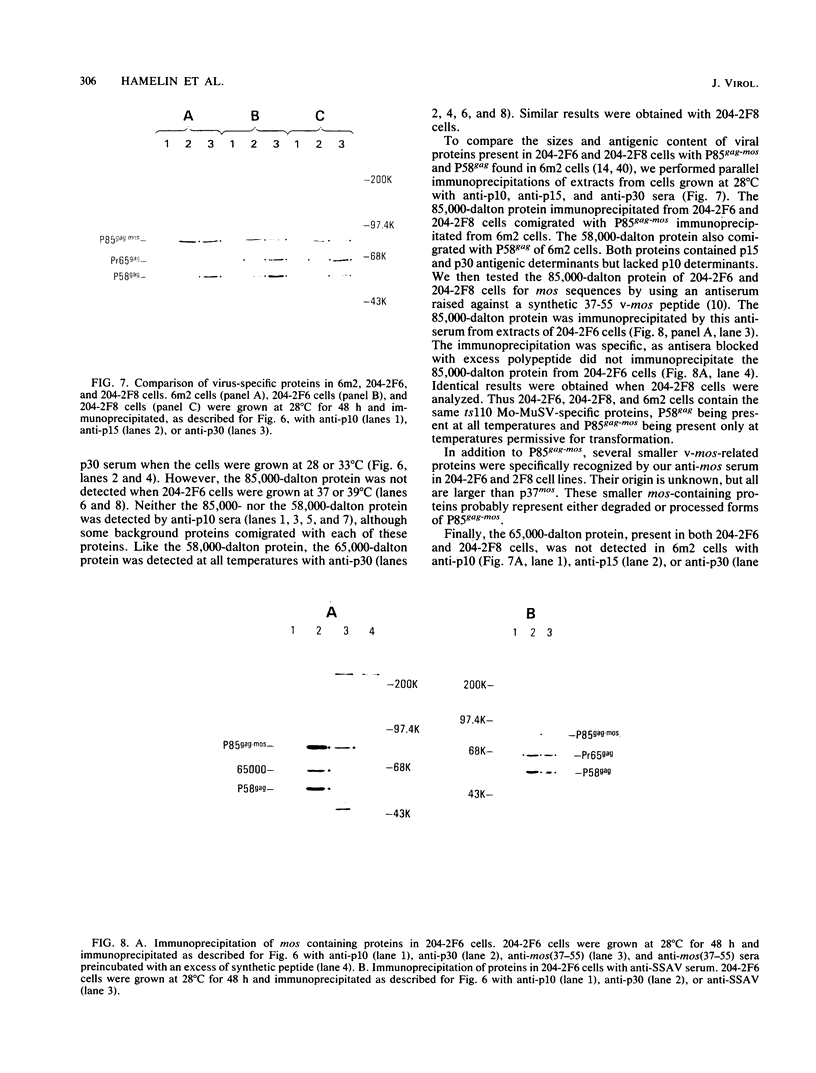
ts110 Moloney murine sarcoma virus (Mo-MuSV)-nonproductively infected cells (6m2) have a transformed phenotype at 28 to 33 degrees C and a normal phenotype at 39 degrees C. At temperatures permissive for transformation, 6m2 cells contain P58gag produced from the 4.0-kilobase (kb) viral RNA genome and P85gag-mos translated from a 3.5-kb spliced mRNA. At 39 degrees C, only the 4.0-kb RNA and its product P58gag are detected. Two temperature-sensitive defects have been observed in ts110-infected 6m2 cells: (i) the splicing of the 4.0-kb RNA to the 3.5-kb RNA; and (ii) the thermolability of P85gag-mos and its kinase activity relative to the wild-type revertant protein, termed P100gag-mos (R.B. Arlinghaus, J. Gen. Virol. 66:1845-1853, 1985). In the present study, we examined the mos gene products of two cell lines (204-2F6 and 204-2F8) obtained by infection of normal rat kidney cells with ts110 Mo-MuSV as a simian sarcoma-associated virus pseudotype to see whether the temperature-sensitive splicing defect could be transferred by viral infection. Southern blot analysis of these two cell lines showed that viral DNAs containing restriction fragments from cellular DNA are different from those in 6m2 cells, indicating that 204-2F6 and 204-2F8 cells have different ts110 provirus integration sites from those of 6m2 cells. Northern blots, S1 mapping analyses, and immunoprecipitation experiments showed unequivocally that the splicing defect of ts110 Mo-MuSV is virus encoded and is independent of host cell factors.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Rowe S. P. Nonproducer clones of murine sarcoma virus transformed BALB-3T3 cells. Virology. 1970 Sep;42(1):9–19. doi: 10.1016/0042-6822(70)90233-3. [DOI] [PubMed] [Google Scholar]
- Arcement L. J., Karshin W. L., Naso R. B., Jamjoom G., Arlinghaus R. B. Biosynthesis of Rauscher leukemia viral proteins: presence of p30 and envelope p15 sequences in precursor polypeptides. Virology. 1976 Feb;69(2):763–774. doi: 10.1016/0042-6822(76)90504-3. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbacid M., Stephenson J. R., Aaronson S. A. Evolutionary relationships between gag gene-coded proteins of murine and primate endogenous type C RNA viruses. Cell. 1977 Apr;10(4):641–648. doi: 10.1016/0092-8674(77)90097-6. [DOI] [PubMed] [Google Scholar]
- Blair D. G., Hull M. A., Finch E. A. The isolation and preliminary characterization of temperature-sensitive transformation mutants of Moloney sarcoma virus. Virology. 1979 Jun;95(2):303–316. doi: 10.1016/0042-6822(79)90486-0. [DOI] [PubMed] [Google Scholar]
- Devare S. G., Reddy E. P., Law J. D., Robbins K. C., Aaronson S. A. Nucleotide sequence of the simian sarcoma virus genome: demonstration that its acquired cellular sequences encode the transforming gene product p28sis. Proc Natl Acad Sci U S A. 1983 Feb;80(3):731–735. doi: 10.1073/pnas.80.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue D. J., Sharp P. A., Weinberg R. A. An MSV-specific subgenomic mRNA in MSV-transformed G8-124 cells. Cell. 1979 May;17(1):53–63. doi: 10.1016/0092-8674(79)90294-0. [DOI] [PubMed] [Google Scholar]
- Gallick G. E., Hamelin R., Maxwell S., Duyka D., Arlinghaus R. B. The gag-mos hybrid protein of ts110 Moloney murine sarcoma virus: variation of gene expression with temperature. Virology. 1984 Dec;139(2):366–374. doi: 10.1016/0042-6822(84)90382-9. [DOI] [PubMed] [Google Scholar]
- Gallick G. E., Sparrow J. T., Singh B., Maxwell S. A., Stanker L. H., Arlinghaus R. B. Recognition of mos-related proteins with an antiserum to a peptide of the v-mos gene product. J Gen Virol. 1985 May;66(Pt 5):945–955. doi: 10.1099/0022-1317-66-5-945. [DOI] [PubMed] [Google Scholar]
- Hamelin R., Brizzard B. L., Nash M. A., Murphy E. C., Jr, Arlinghaus R. B. Temperature-sensitive viral RNA expression in Moloney murine sarcoma virus ts110-infected cells. J Virol. 1985 Feb;53(2):616–623. doi: 10.1128/jvi.53.2.616-623.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamelin R., Devaux J., Honoré N., Auger-Buendia M. A., Tavitian A. Characterization of viral RNA in cells transformed by various isolates of Moloney murine sarcoma virus. J Gen Virol. 1983 Sep;64(Pt 9):2057–2062. doi: 10.1099/0022-1317-64-9-2057. [DOI] [PubMed] [Google Scholar]
- Hamelin R., Larsen C. J., Tavitian A. Effects of actinomycin D, toyocamycin and cycloheximide on the synthesis of low-molecular-weight nuclear RNAs in HeLa cells. Eur J Biochem. 1973 Jun;35(2):350–356. doi: 10.1111/j.1432-1033.1973.tb02846.x. [DOI] [PubMed] [Google Scholar]
- Horn J. P., Wood T. G., Blair D. G., Arlinghaus R. B. Partial characterization of a moloney murine sarcoma virus 85,000-dalton polypeptide whose expression correlates with the transformed phenotype in cells infected with a temperature-sensitive mutant virus. Virology. 1980 Sep;105(2):516–525. doi: 10.1016/0042-6822(80)90052-5. [DOI] [PubMed] [Google Scholar]
- Horn J. P., Wood T. G., Murphy E. C., Jr, Blair D. G., Arlinghaus R. B. A selective temperature-sensitive defect in viral RNA expression in cells infected with a ts transformation mutant of murine sarcoma virus. Cell. 1981 Jul;25(1):37–46. doi: 10.1016/0092-8674(81)90229-4. [DOI] [PubMed] [Google Scholar]
- Jamjoom G. A., Naso R. B., Arlinghaus R. B. Further characterization of intracellular precursor polyproteins of Rauscher leukemia virus. Virology. 1977 May 1;78(1):11–34. doi: 10.1016/0042-6822(77)90075-7. [DOI] [PubMed] [Google Scholar]
- Junghans R. P., Murphy E. C., Jr, Arlinghaus R. B. Electron microscopic analysis of ts1 10 Moloney mouse sarcoma virus, a variant of wild-type virus with two RNAs containing large deletions. J Mol Biol. 1982 Oct 25;161(2):229–250. doi: 10.1016/0022-2836(82)90150-4. [DOI] [PubMed] [Google Scholar]
- Kloetzer W. S., Maxwell S. A., Arlinghaus R. B. Further characterization of the P85gag-mos -associated protein kinase activity. Virology. 1984 Oct 15;138(1):143–155. doi: 10.1016/0042-6822(84)90154-5. [DOI] [PubMed] [Google Scholar]
- Kloetzer W. S., Maxwell S. A., Arlinghaus R. B. P85gag-mos encoded by ts110 Moloney murine sarcoma virus has an associated protein kinase activity. Proc Natl Acad Sci U S A. 1983 Jan;80(2):412–416. doi: 10.1073/pnas.80.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konarska M. M., Grabowski P. J., Padgett R. A., Sharp P. A. Characterization of the branch site in lariat RNAs produced by splicing of mRNA precursors. Nature. 1985 Feb 14;313(6003):552–557. doi: 10.1038/313552a0. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Boyle J. A., Mount S. M., Wolin S. L., Steitz J. A. Are snRNPs involved in splicing? Nature. 1980 Jan 10;283(5743):220–224. doi: 10.1038/283220a0. [DOI] [PubMed] [Google Scholar]
- Maxwell S. A., Arlinghaus R. B. Serine kinase activity associated with Maloney murine sarcoma virus-124-encoded p37mos. Virology. 1985 May;143(1):321–333. doi: 10.1016/0042-6822(85)90119-9. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy E. C., Jr, Arlinghaus R. B. Comparative tryptic peptide analysis of candidate P85gag-mos of ts110 Moloney murine sarcoma virus and P38-P23 mos gene-related proteins of wild-type virus. Virology. 1982 Sep;121(2):372–383. doi: 10.1016/0042-6822(82)90175-1. [DOI] [PubMed] [Google Scholar]
- Nash M. A., Brizzard B. L., Wong J. L., Murphy E. C., Jr Murine sarcoma virus ts110 RNA transcripts: origin from a single proviral DNA and sequence of the gag-mos junctions in both the precursor and spliced viral RNAs. J Virol. 1985 Feb;53(2):624–633. doi: 10.1128/jvi.53.2.624-633.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash M., Brown N. V., Wong J. L., Arlinghaus R. B., Murphy E. C., Jr S1 nuclease mapping of viral RNAs from a temperature-sensitive transformation mutant of murine sarcoma virus. J Virol. 1984 May;50(2):478–488. doi: 10.1128/jvi.50.2.478-488.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naso R. B., Arcement L. J., Arlinghaus R. B. Biosynthesis of Rauscher leukemia viral proteins. Cell. 1975 Jan;4(1):31–36. doi: 10.1016/0092-8674(75)90130-0. [DOI] [PubMed] [Google Scholar]
- Padgett R. A., Konarska M. M., Grabowski P. J., Hardy S. F., Sharp P. A. Lariat RNA's as intermediates and products in the splicing of messenger RNA precursors. Science. 1984 Aug 31;225(4665):898–903. doi: 10.1126/science.6206566. [DOI] [PubMed] [Google Scholar]
- Papkoff J., Lai M. H., Hunter T., Verma I. M. Analysis of transforming gene products from Moloney murine sarcoma virus. Cell. 1981 Nov;27(1 Pt 2):109–119. doi: 10.1016/0092-8674(81)90365-2. [DOI] [PubMed] [Google Scholar]
- Papkoff J., Verma I. M., Hunter T. Detection of a transforming gene product in cells transformed by Moloney murine sarcoma virus. Cell. 1982 Jun;29(2):417–426. doi: 10.1016/0092-8674(82)90158-1. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Ruskin B., Krainer A. R., Maniatis T., Green M. R. Excision of an intact intron as a novel lariat structure during pre-mRNA splicing in vitro. Cell. 1984 Aug;38(1):317–331. doi: 10.1016/0092-8674(84)90553-1. [DOI] [PubMed] [Google Scholar]
- Shinnick T. M., Lerner R. A., Sutcliffe J. G. Nucleotide sequence of Moloney murine leukaemia virus. Nature. 1981 Oct 15;293(5833):543–548. doi: 10.1038/293543a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stanker L. H., Gallick G. E., Horn J. P., Arlinghaus R. B. P85gag-mos encoded by ts110 Moloney murine sarcoma virus: rapid at the restrictive temperature. J Gen Virol. 1983 Oct;64(Pt 10):2203–2211. doi: 10.1099/0022-1317-64-10-2203. [DOI] [PubMed] [Google Scholar]
- Stanker L. H., Gallick G. E., Kloetzer W. S., Murphy E. C., Jr, Arlinghaus R. B. P85: a gag-mos polyprotein encoded by ts110 Moloney murine sarcoma virus. J Virol. 1983 Mar;45(3):1183–1189. doi: 10.1128/jvi.45.3.1183-1189.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Beveren C., van Straaten F., Galleshaw J. A., Verma I. M. Nucleotide sequence of the genome of a murine sarcoma virus. Cell. 1981 Nov;27(1 Pt 2):97–108. doi: 10.1016/0092-8674(81)90364-0. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood T. G., Peltier-Horn J., Robey W. G., Blair D. G., Arlinghaus R. B. Characterization of virus-specified proteins present in NRK cells infected with a temperature-sensitive transformation mutant of Moloney murine sarcoma virus. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):747–754. doi: 10.1101/sqb.1980.044.01.080. [DOI] [PubMed] [Google Scholar]